Abstract
Background and purpose:
The involvement of the neuropeptide oxytocin in the control of male sexual responses is documented although its exact mechanisms of action, and especially the site(s) of action, are not fully delineated. In order to clarify this issue, we tested the effects of a peptide oxytocin antagonist delivered through different routes on sexual responses elicited, in anaesthetized male rats, by i.c.v. 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT), a dopamine agonist, preferentially active on D3 receptors.
Experimental approach:
Seminal vesicle pressure (SVP) and bulbospongiosus muscle (BS) electromyograms were recorded as physiological markers of emission and expulsion phases of ejaculation respectively and intracavernosal pressure (ICP) was monitored as a physiological marker of erection.
Key results:
When injected i.v., the oxytocin antagonist did not impair 7-OH-DPAT-induced SVP and ICP responses while BS burst frequency was diminished. When delivered i.c.v., the oxytocin antagonist dose-dependently inhibited occurrence of 7-OH-DPAT-induced sexual responses. When delivered intrathecally (i.t.) at the level of the 6th lumbar (L6) segment, but not the 13th thoracic (T13) segment, the oxytocin antagonist reduced the duration of BS responses and the occurrence of ejaculation without impairing ICP responses.
Conclusions and implications:
Brain oxytocin receptors mediate male sexual responses elicited by i.c.v. 7-OH-DPAT in anaesthetized rats whereas L6 spinal oxytocin receptors only impair the occurrence of ejaculation. Peripheral oxytocin receptors are marginally involved in 7-OH-DPAT-induced sexual responses. These findings should be considered for the development of potential pharmacological treatment of premature ejaculation in man.
Keywords: sexual functions, bulbospongiosus muscle, dopamine D3 receptors, erection, seminal vesicle, premature ejaculation
Introduction
Ejaculation comprises two successive phases, emission and expulsion, that involve different pelvi-perineal anatomical structures. The emission phase consists of secretion of the various components of semen by seminal vesicles (SVs), prostate and through release of ampullary vasa deferentia content into the prostatic urethra. The expulsion phase corresponds to forceful propulsion of sperm from the prostatic urethra to the urethral meatus through rhythmic contractions of perineal striated muscles, with a primary role for the bulbospongiosus muscle (BS). A tight coordination between sympathetic, parasympathetic and somatic divisions of the nervous system is necessary for normal anterograde ejaculation. As a centrally integrated and highly coordinated process, ejaculation involves sensory receptors and areas, afferent neural pathways, cerebral sensory and motor areas, spinal motor centres, as well as multiple efferent pathways (reviewed by Giuliano and Clement, 2005). A variety of neurotransmitters distributed in supraspinal and spinal nuclei are important for control of ejaculation. Animal studies have provided insights into the central neurochemicals involved in the control of the ejaculatory process by demonstrating the primary importance of dopamine and 5-HT (serotonin), as well as other factors such as oxytocin (for review, see Hull et al., 2004; Peeters and Giuliano, 2008).
Behavioural investigations have shown that the dopamine D3 receptor-preferring agonist, 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT), decreases the number of intromissions preceding ejaculation and the latency of ejaculation in rats (Ahlenius and Larsson, 1995; Ferrari and Giuliani, 1996). Furthermore, 7-OH-DPAT has recently been reported to trigger rhythmic contractions of BS and ejaculation when delivered centrally in anaesthetized rats (Clement et al., 2007; Kitrey et al., 2007).
The involvement of oxytocin in male sexual functions is well documented, and notably this neuropeptide was found to be one of the most potent agents to induce penile erection in various species (reviewed by Argiolas and Melis, 1995). Supraspinal and spinal sites have been found to mediate the pro-erectile effect of oxytocin (Argiolas et al., 1985; Giuliano et al., 2001). A key role for oxytocin in the control of male sexual behaviour, including ejaculation, has also been demonstrated. Infused into the cerebral ventricle of male rats free to copulate with a receptive female, oxytocin facilitates ejaculatory behaviour by shortening ejaculation latency and post-ejaculatory refractory period (Arletti et al., 1985). Administered via intracerebroventricular (i.c.v.) route, oxytocin was also found to significantly increase latencies of mount and intromission (Stoneham et al., 1985). Furthermore, in the mating test a selective oxytocin-receptor antagonist delivered via i.c.v. route to sexually vigorous male rats was reported to inhibit sexual behaviour, including ejaculation, and to reverse the pro-sexual effects of the non-selective dopamine-receptor agonist, apomorphine (Argiolas et al., 1988b). Systemic, similarly to central, administration of oxytocin was reported to shorten ejaculation latency and post-ejaculatory interval in sexually active male rats (Arletti et al., 1985; Stoneham et al., 1985).
To further understand the role of oxytocin in the expression of sexual response, the effects of a peptide antagonist of the oxytocin receptor, administered via different routes, on the pro-sexual activity of 7-OH-DPAT were investigated in anaesthetized male rats. Intracavernosal pressure, SV pressure (SVP) and BS electromyogram were monitored as physiological markers, respectively, of the erection, emission and expulsion phases of ejaculation.
Methods
Animals
All animal experiments were carried out in accordance with the European Community Council Directive (86/609/EEC) on the use of laboratory animals. All efforts were undertaken to minimize the number of animals used and their suffering. A total of 110 sexually naïve, adult, male Wistar rats (Charles-River, L'Arbresle, France) weighing 250–300 g were used in this study. Animals were housed in groups of four at 20±2 °C under a 12-h light/dark cycle, with access to food and water ad libitum. Animals were maintained in these conditions for at least 8 days prior to testing.
Surgical preparation
Rats were anaesthetized with urethane (1.2 g kg−1) and absence of hind-paw pinch reflex was checked. Their body temperature was maintained at 37 °C using a homeothermic blanket. The carotid artery was catheterized with polyethylene tubing (PE50) filled with heparinized saline (50 IU mL−1) to record blood pressure with a pressure transducer (EM750; Elcomatic, Glasgow, UK). For injecting via intravenous (i.v.) route, the jugular vein was catheterized with a polyethylene tubing (PE10) filled with 0.9% (w/v) NaCl. SV pressure (SVP) was measured with a catheter filled with mineral oil and inserted in the right SV through the apex and connected to a pressure transducer. For intracavernous pressure (ICP) monitoring, the penis was denuded of skin and a 25-G needle connected to a catheter was inserted into a corpus cavernosum. A pressure transducer was used for recording ICP variations. The BS was exposed with a perineal incision. Electrical activity of BS was recorded by passing a Teflon-insulated stainless steel wire laterally throughout the muscle, with two 1–2 mm pieces (separated by 1–2 mm) of insulation stripped off. Electrical signal from the BS was amplified (DP-301; Warner Instrument Corp., Hamden, CT, USA; gain, 10 000; low pass, 1 kHz; high pass, 10 Hz) before being digitized. At the end of the experiments, rats were killed with an overdose of urethane.
Intracerebroventricular cannula implantation
A guide cannula (22 G) was stereotaxically placed above the cerebral ventricle (coordinates according to Paxinos and Watson rat brain atlas: Paxinos and Watson (1998), 0.5 mm posterior to bregma, 1 mm lateral to midline and 4 mm below the skull). The internal cannula (with 0.5-mm projection below the guide cannula) was connected to a Hamilton syringe placed in a micropump allowing delivery of microvolumes. At the end of the experimental session, methylene blue dye was injected through the cannula and the brain, removed and grossly dissected, was inspected for presence of blue dye in the ventricles.
Intrathecal catheter implantation
For catheter insertion via intrathecal (i.t.) route, the rat's head was placed in a stereotaxic frame and was rotated nose downwards. The catheter was a polyethylene tubing (PE10) stretched to 150% of its original length in hot water and cut to the required length so that its distal opening reached the targeted levels of the spinal cord; that is, the thirteenth thoracic (T13) or the sixth lumbar (L6) segment. The skin and neck muscles were incised and retracted. The atlanto-occipital membrane was opened and the catheter, filled with 0.9% (w/v) NaCl, was carefully advanced in the caudal direction. The rostral free end of the catheter was secured with ligatures that closed the neck muscles and skin layers. The exact location of the caudal tip of the catheter was checked at the end of each experiment after death of the animal and exposure of the spinal cord. Only rats with a catheter tip located at the T13 or L6 spinal level were considered for the results.
Experimental design
Intravenous injection of d(CH2)51,Tyr(Me)2,Orn8-oxytocin, the oxytocin antagonist
Two doses of oxytocin antagonist (0.25 and 1 μg kg−1) were tested by delivery i.v. route in separate groups of 10 rats. After a 5-min period to obtain baseline values, the oxytocin antagonist or its vehicle was given via i.v. route (volume 1 mL kg−1), followed, 15 min later, by i.c.v. 7-OH-DPAT (10 μg in 5 μL) delivery.
Intracerebroventricular injection of d(CH2)51,Tyr(Me)2,Orn8-oxytocin
Three doses of oxytocin antagonist (0.001, 0.01 and 0.1 μg) were tested by injection via i.c.v. route in separate groups of 10 rats. After a 5-min baseline period, i.c.v. delivery of the oxytocin antagonist or its vehicle was performed (volume 5 μL), followed, 15 min later, by i.c.v. 7-OH-DPAT (10 μg in 5 μL) delivery. Each injection was given at a flow rate of 1 μL min−1.
Intrathecal injection of d(CH2)51,Tyr(Me)2,Orn8-oxytocin
One dose of the oxytocin antagonist (0.1 μg) was tested by injection via i.t. route either at T13 level, where pre-ganglionic sympathetic neurones sending projections to the anatomical structures involved in sexual response are located, or at L6 level, where pre-ganglionic parasympathetic neurones innervating the same anatomical structures and motoneurones destined to pelvi-perineal striated muscles are located, in separate groups of 10 rats. After the 5-min baseline values were obtained, i.t. delivery of oxytocin antagonist or its vehicle was performed (volume 10 μL), followed, 15 min later, by i.c.v. 7-OH-DPAT (10 μg in 5 μL) delivery.
Data analysis
Sexual responses (that is, ejaculation; ICP and SVP rises and BS rhythmic contractile responses) were counted during the recording period. The duration of the BS contractile responses and the frequency of burst within each BS contractile response, the duration and amplitude of SVP and ICP rises, were calculated for each rat displaying such responses and was averaged for each treatment group. The amplitude of ICP increases corresponded to the difference between basal ICP and ICP value at the plateau reached during the erectile response. As there is a correlation between ICP and arterial pressure, the amplitude of the ICP increase was expressed relative to the mean arterial pressure (MAP) as already described (Giuliano et al., 1993). This corresponded to the ratio of the maximal value of ICP increase reached during an erectile response and the MAP calculated for the duration of the same erectile response, and was expressed as a percentage of MAP.
Statistical procedures
Inter-group statistical comparisons of the number of sexual responses were performed using Mann–Whitney test (i.t. oxytocin antagonist) or Kruskal–Wallis test, followed, whenever P<0.05, by Dunn's post hoc test (i.v. and i.c.v. oxytocin antagonist delivery). Non-parametric tests were used, as in most of the treatment groups the values did not follow a Gaussian distribution. Statistical comparisons of the quantitative parameters characterizing sexual responses were performed between treatment groups using Student's t-test (i.t. oxytocin antagonist) or one-way analysis of variance (ANOVA), followed, whenever P<0.05, by Newman–Keuls' post hoc test (i.v. and i.c.v. oxytocin antagonist). Parametric tests were used here as, in most of the treatment groups, the values followed a Gaussian distribution.
Drugs
R(+)-7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT; Sigma-Aldrich, Saint Quentin Fallavier, France) was dissolved in 0.9% (w/v) NaCl. This solution was prepared weekly and stored at −20 °C. d(CH2)51,Tyr(Me)2,Orn8-oxytocin (Bachem, Weil am Rhein, Germany), a potent oxytocin-receptor antagonist, was dissolved in 0.9% (w/v) NaCl. This solution was prepared weekly and stored at −20 °C.
Results
Effects of i. c.v. 7-OH-DPAT on the physiological markers of sexual responses
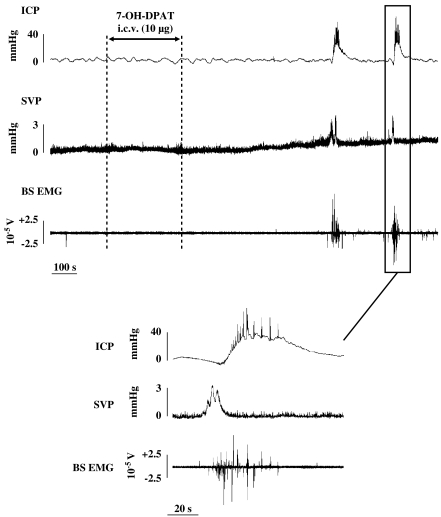
Twenty-five out of the 40 rats (63%) receiving i.c.v. 7-OH-DPAT and the vehicle of the oxytocin antagonist (NaCl (0.9%, w/v)) via i.c.v., i.t. or i.v. route (thereafter control rats) displayed at least one ejaculation (that is, expulsion of a seminal plug out of the urethra) during the 30-min period after 7-OH-DPAT delivery. Ejaculation always occurred concomitantly to coordinated increases in SVP, BS activity and ICP following a typical sequence (Figure 1). A steep rise in SVP was first followed by intense rhythmic BS contractions (on average 5.9±1.3 s after SVP rise has started, n=33), and then by a more progressive increase in ICP (on average 8.6±1.4 s after SVP rise has started, n=33). Two to four peaks were noticed during the SVP response (Figure 1, lower panel). In control rats (n=33), the maximal amplitude of the increase in SVP was 4.1±0.6 mm Hg and the mean duration was 18.5±1.3 s. BS responses (n=35) occurred in the form of burst of rhythmic contractions with a mean duration and burst frequencies, as shown in Figure 2. Increases in ICP were characterized by a plateau with superimposed brief suprasystolic peaks (Figure 1, lower panel). For control rats (n=37), the mean duration of ICP rises and the mean amplitude (as % MAP) are shown in Figure 2.
Figure 1.
Recording of ICP, SVP and BS electromyogram (BS EMG) obtained in anaesthetized rats after i.c.v. delivery of 7-OH-DPAT (10 μg). The tracing is displayed on an expanded time scale to show the temporal organization of a sexual response. BS, bulbospongiosus muscle; ICP, intracavernosal pressure; i.c.v., intracerebroventricular; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino) tetralin; SVP, seminal vesicle pressure.
Figure 2.
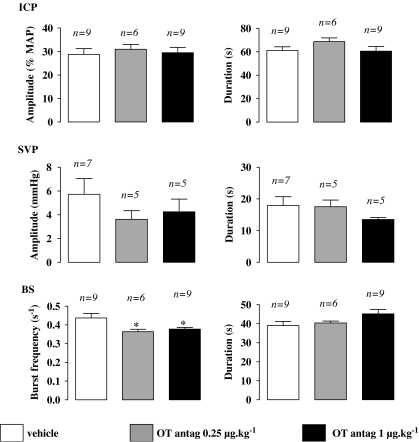
Effects of i.v. injection of oxytocin antagonist (OT antag.) on the quantitative parameters of sexual responses induced by 7-OH-DPAT. Amplitude of ICP rises, expressed as percentage of MAP measured during the corresponding ICP rise, and duration of ICP rises were determined in rats pretreated, 15 min prior to i.c.v. 7-OH-DPAT (10 μg), with i.v. oxytocin antagonist vehicle, oxytocin antagonist 0.25 or 1 μg kg−1. Amplitude and duration of SVP increases, frequency of burst within a contractile response of BS, as well as duration of the BS contractile response, were determined. Bars and error bars represent the means±s.e.mean of n number of rats. *P<0.05 versus control group; one-way ANOVA+Newman–Keuls' post hoc test. ANOVA, analysis of variance; BS, bulbospongiosus muscle; ICP, intracavernosal pressure; i.v., intravenous; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino)tetralin; MAP, mean arterial pressure.
Effects of i.v. oxytocin antagonist on 7-OH-DPAT-induced sexual responses
There was no sexual response during the 15-min period separating oxytocin i.v. antagonist delivery and 7-OH-DPAT infusion (data not shown). Pretreatment with the oxytocin antagonist had no effects on the occurrence of sexual responses elicited by 7-OH-DPAT (Tables 1 and 2). The quantitative parameters characterizing SVP and ICP increases were not significantly modified by i.v. oxytocin antagonist delivery (Figure 2). The burst frequency of BS responses, but not their duration, was significantly reduced (one-way ANOVA+Newman–Keul's post hoc test, P<0.05) in rats pre-treated via i.v. delivery of oxytocin antagonist at both doses when compared with i.v. control rats (Figure 2).
Table 1.
Effects of i.v. oxytocin antagonist on i.c.v. 7-OH-DPAT-induced sexual responses
|
OT antag., i.v. |
|||
|---|---|---|---|
| 0 | 0.25 μg kg−1 | 1 μg kg−1 | |
| Number of ejaculations | 0.9±0.2 (10) | 0.6±0.2 (10) | 0.9±0.2 (10) |
| Latency of first ejaculation(s) | 887±160 (7) | 866±76 (4) | 852±182 (7) |
| Number of SVP responses | 2.4±0.5 (10) | 2.3±0.9 (10) | 2.6±0.7 (10) |
| Latency of first SVP response(s) | 897±172 (7) | 808±195 (5) | 764±73 (7) |
| Number of BS responses | 2.6±0.4 (10) | 2.6±0.8 (10) | 2.9±0.4 (10) |
| Latency of first BS response(s) | 906±160 (7) | 818±162 (5) | 770±82 (7) |
Abbreviations: BS, bulbospongiosus muscle; i.c.v., intracerebroventricular; i.v., intravenous; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino) tetralin; OT, oxytocin; SVP, seminal vesicle pressure.
The OT antagonist (OT antag.) was injected via i.v. route 15 min prior to 7-OH-DPAT (10 μg, i.c.v.). SVP and BS EMG were recorded over 30 min following 7-OH-DPAT delivery. The number of ejaculations, and SVP and BS responses, was counted during the 30-min 7-OH-DPAT post-injection period. The latency of the first ejaculation, and SVP and BS responses after i.c.v. 7-OH-DPAT delivery, was also determined. The values are the means±s.e.mean of data from n number of rats. Statistical analysis was performed by Kruskal–Wallis+Dunn's post hoc test for comparison of the number of sexual responses. One-way ANOVA+Newman–Keuls' post hoc test for comparison of the latency of sexual responses.
Table 2.
Effects of oxytocin antagonist, administered through different routes, on ICP responses induced by 7-OH-DPAT
| Number of ICP responses | Latency of first ICP response(s) | |
|---|---|---|
| OT antag., i.v. | ||
| 0 | 2.7±0.4 (10) | 794±156 (9) |
| 0.25 μg kg−1 | 2.6±0.8 (10) | 838±163 (6) |
| 1 μg kg−1 | 2.9±0.4 (10) | 867±82 (9) |
| OT antag., i.c.v. | ||
| 0 | 3.6±1.0 (10) | 645±135 (9) |
| 0.001 μg | 3.5±0.7 (10) | 488±77 (9) |
| 0.01 μg | 1.9±0.5 (10) | 1146±124b,d (7) |
| 0.1 μg | 0.8±0.5a,c (10) | 1354±205b,d (3) |
| OT antag., i.t. | ||
| T13 | ||
| 0 | 3.2±0.8 (10) | 654±125 (8) |
| 0.1 μg | 2.7±0.5 (10) | 793±194 (9) |
| L6 | ||
| 0 | 2.5±0.5 (10) | 933±95 (9) |
| 0.1 μg | 2.3±0.7 (10) | 898±138 (6) |
Abbreviations: ICP, intracavernosal pressure; i.c.v., intracerebroventricular; i.t., intrathecal; i.v., intravenous; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino) tetralin; OT, oxytocin.
The OT antagonist (OT antag.) was injected via i.v., i.c.v. or i.t. route 15 min prior to 7-OH-DPAT (10 μg, i.c.v.). ICP was recorded over 30 min following 7-OH-DPAT delivery. The number of ICP responses was counted during the 30-min 7-OH-DPAT post-injection period. The latency of the first ICP response after i.c.v. delivery of 7-OH-DPAT was also determined. The values are the means±s.e.mean of data from n number of rats. Statistical analysis was performed by Kruskal–Wallis+Dunn's post hoc test for comparison of the number of ICP responses: aP<0.05, different from corresponding control; cP<0.05, different from OT antag., 0.001 μg. One-way ANOVA+Newman–Keuls' post hoc test for comparison of latency of the first ICP response: bP<0.01, different from corresponding control; dP<0.01, different from OT antag., 0.001 μg.
Effects of i.c.v. oxytocin antagonist on 7-OH-DPAT-induced sexual responses
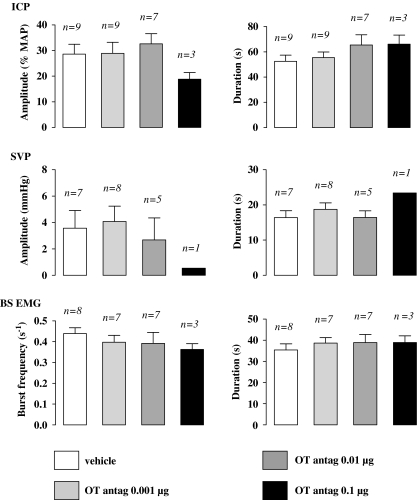
No sexual response was observed during the 15-min period following oxytocin antagonist i.c.v. injection and preceding 7-OH-DPAT delivery (data not shown). Ejaculation induced by 7-OH-DPAT was abolished in all rats pretreated with the oxytocin antagonist (0.1 μg, i.c.v.), whereas only one rat displayed one ejaculation in the group administered the oxytocin antagonist at 0.01 μg per rat (Table 3). At the highest i.c.v. dose, oxytocin antagonist produced a dose-dependent decrease in the number of 7-OH-DPAT-induced SVP, BS and ICP responses when compared with the decrease by the lowest oxytocin antagonist dose and the vehicle (Tables 2 and 3; Kruskal–Wallis+Dunn's post hoc test, P<0.05). In rats pretreated via i.c.v. route with 0.01 and 0.1 μg of oxytocin antagonist, there was a significant increase in the latency of the first SVP, BS and ICP responses after 7-OH-DPAT delivery when compared with the effects of the lowest dose of oxytocin antagonist (0.001 μg) or the vehicle (one-way ANOVA+Newman–Keuls' post hoc test, P<0.01; Tables 2 and 3). No appreciable modification of the quantitative parameters of SVP, BS and ICP responses elicited by 7-OH-DPAT was observed in rats pretreated with the i.c.v. oxytocin antagonist (Figure 3).
Table 3.
Effects of i.c.v. oxytocin antagonist on i.c.v. 7-OH-DPAT-induced sexual responses
|
OT antag., i.c.v. |
||||
|---|---|---|---|---|
| 0 | 0.001 μg | 0.01 μg | 0.1 μg | |
| Number of ejaculations | 0.6±0.2 (10) | 0.3±0.2 (10) | 0.1±0.1 (10) | 0.0±0.0a (10) |
| Latency of first ejaculation(s) | 618±215 (5) | 432±31 (4) | 1136 (1) | — (0) |
| Number of SVP responses | 3.4±1.0 (10) | 3.5±0.7 (10) | 1.5±0.5 (10) | 0.4±0.5a,c (10) |
| Latency of first SVP response(s) | 644±136 (9) | 454±53 (9) | 1156±124b,d (7) | 1355±206b,d (3) |
| Number of BS responses | 4.2±1.1 (10) | 4.0±0.7 (10) | 2.0±0.5 (10) | 0.8±0.5a,c (10) |
| Latency of first BS response(s) | 652±106 (9) | 459±81 (9) | 1166±160b,d (7) | 1361±182b,d (3) |
Abbreviations: BS, bulbospongiosus muscle; i.c.v., intracerebroventricular; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino) tetralin; OT, oxytocin; SVP, seminal vesicle pressure.
The OT antagonist (OT antag.) was injected via i.c.v. route 15 min prior to 7-OH-DPAT (10 μg, i.c.v.). SVP and BS EMG were recorded over 30 min following 7-OH-DPAT delivery. The number of ejaculations, and SVP and BS responses, was counted during the 30-min 7-OH-DPAT post-injection period. The latency of the first ejaculation, and SVP and BS responses after i.c.v. delivery of 7-OH-DPAT, was also determined. The values are the means±s.e.mean of data from n number of rats. Statistical analysis was performed by Kruskal–Wallis+Dunn's post hoc test for comparison of the number of sexual responses: aP<0.05, different from corresponding control; cP<0.05, different from OT antag., 0.001 μg. One-way ANOVA+Newman–Keuls' post hoc test for comparison of the latency of sexual responses: bP<0.01, different from corresponding control; dP<0.01, different from OT antag., 0.001 μg.
Figure 3.
Effects of i.c.v. injection of oxytocin antagonist (OT antag.) on the quantitative parameters of sexual responses induced by 7-OH-DPAT. Amplitude of ICP rises, expressed as percentage of MAP measured during the corresponding ICP rise, and duration of ICP rises were determined in rats pretreated, 15 min prior to i.c.v. 7-OH-DPAT (10 μg), with i.c.v. oxytocin antagonist vehicle, oxytocin antagonist 0.001, 0.01 or 0.1 μg. Amplitude and duration of SVP increases, frequency of burst within a contractile response of BS, as well as duration of the BS contractile response, were also determined. Bars and error bars represent the means±s.e.mean of n number of rats. BS, bulbospongiosus muscle; ICP, intracavernosal pressure; i.c.v., intracerebroventricular; MAP, mean arterial pressure; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino) tetralin.
Effects of i.t. oxytocin antagonist on 7-OH-DPAT-induced sexual responses
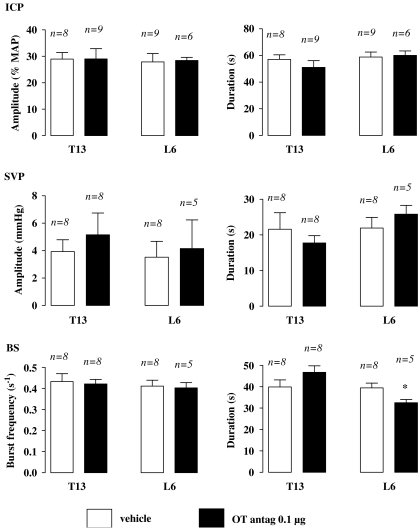
The most effective i.c.v. oxytocin antagonist dose (0.1 μg) in inhibiting ejaculation was selected for i.t. administration. There was no sexual response over the 15-min period following oxytocin antagonist i.t. (either T13 or L6 spinal level) injection and preceding 7-OH-DPAT delivery (data not shown). Occurrence of ejaculation was significantly impaired in rats i.t. delivered with oxytocin antagonist at the L6 level (Mann–Whitney, P<0.05) whereas the latency of the first ejaculation was comparable to that of the L6 i.t. control group (Table 4). In rats receiving i.t. oxytocin antagonist at the L6 level, the number of SVP responses was half that of L6 i.t. vehicle-pretreated animals, although statistical significance was not reached (Mann–Whitney, P=0.20; Table 4). There was no noticeable effect of L6 i.t. administration of oxytocin antagonist on the quantitative parameters of SVP responses (Figure 4). Neither number of BS responses nor latency of the first BS response was modified by i.t. delivery of oxytocin antagonist at L6 (Table 4). However, duration of 7-OH-DPAT-induced BS responses was significantly decreased in rats pretreated with oxytocin antagonist at L6 (i.t.) as compared with the vehicle given at L6 (Student's t-test, P<0.05). The burst frequency of BS contractions was unchanged by oxytocin antagonist given to L6 i.t. (Figure 4). ICP responses elicited by 7-OH-DPAT were not altered in rats pretreated with i.t. oxytocin antagonist at the L6 level (Table 2; Figure 4).
Table 4.
Effects of i.t. oxytocin antagonist on i.c.v. 7-OH-DPAT-induced sexual responses
|
OT antag., i.t. |
||||
|---|---|---|---|---|
|
T13 |
L6 |
|||
| 0 | 0.1 μg | 0 | 0.1 μg | |
| Number of ejaculations | 0.9±0.2 (10) | 0.9±0.3 (10) | 0.9±0.2 (10) | 0.3±0.1a (10) |
| Latency of first ejaculation(s) | 898±125 (7) | 1080±163 (7) | 1005±141 (6) | 928±375 (3) |
| Number of SVP responses | 2.6±1.0 (10) | 3.0±0.7 (10) | 2.4±0.7 (10) | 1.2±0.6 (10) |
| Latency of first SVP response(s) | 651±125 (8) | 886±171 (8) | 773±122 (8) | 766±141 (5) |
| Number of BS responses | 3.7±0.8 (10) | 3.4±0.7 (10) | 2.7±0.5 (10) | 2.3±0.7 (10) |
| Latency of first BS response(s) | 656±107 (8) | 892±189 (8) | 779±102 (8) | 774±113 (5) |
Abbreviations: BS, bulbospongiosus muscle; i.c.v., intracerebroventricular; i.t., intrathecal; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino) tetralin; OT, oxytocin; SVP, seminal vesicle pressure.
The OT antagonist (OT antag.) was injected via i.t. route 15 min prior to 7-OH-DPAT (10 μg, i.c.v.). SVP and BS EMG were recorded over 30 min following 7-OH-DPAT delivery. The number of ejaculations, and SVP and BS responses, was counted during the 30-min 7-OH-DPAT post-injection period. The latency of the first ejaculation, and SVP and BS responses after i.c.v. delivery of 7-OH-DPAT, was also determined. The values are the means±s.e.mean of data from n number of rats. Statistical analysis was performed by Man–Whitney's test for comparison of the number of sexual responses (same spinal level): aP<0.05, different from corresponding control; Student's t-test for comparison of the latency of sexual responses.
Figure 4.
Effects of i.t. injection of oxytocin antagonist (OT antag.) on the quantitative parameters of sexual responses induced by 7-OH-DPAT. Amplitude of ICP rises, expressed as percentage of MAP measured during the corresponding ICP rise, and the duration of ICP rises were determined in rats pretreated via i.t. route, 15 min prior to i.c.v. 7-OH-DPAT (10 μg), with oxytocin antagonist vehicle or oxytocin 0.1 μg of the antagonist at the level of the thirteenth thoracic (T13) or the sixth lumbar (L6) segment of the spinal cord. Amplitude and duration of SVP increases, frequency of burst within a contractile response of BS, as well as duration of the BS contractile response, were determined. Bars and error bars represent the means±s.e.mean of n rats. Statistical analysis was performed by one-way ANOVA+Newman–Keuls' post hoc test; *P<0.05 versus control group. ANOVA, analysis of variance; BS, bulbospongiosus muscle; i.c.v., intracerebroventricular; i.t., intrathecal; MAP, mean arterial pressure; 7-OH-DPAT, 7-hydroxy-2-(di-N-propylamino) tetralin.
When delivered at the T13 level, the oxytocin antagonist did not exert any effect on 7-OH-DPAT-induced sexual responses (Tables 2 and 4; Figure 4).
Discussion and conclusions
The present study demonstrates that brain oxytocin receptors are of primary importance in mediating the pro-ejaculatory and pro-erectile effects of the dopamine D3 receptor-preferring agonist, 7-OH-DPAT, in anaesthetized rats. It was also found that spinal oxytocin receptors at L6 played a modulating role in the pro-ejaculatory activity of 7-OH-DPAT.
When sexual responses are elicited in the male by 7-OH-DPAT, a significant decrease was observed in the BS burst frequency in rats given the oxytocin antagonist via i.v. route (Figure 2). The other parameters that were measured, and especially occurrence of BS responses and ejaculation, were unchanged (Tables 1, 2; Figure 2). As the oxytocin antagonist used in the present study is a peptide, it is very likely that it did not cross the blood–brain barrier. Therefore, the effects of i.v. injection of this compound are due to its peripheral actions. There are no data available in the literature that may help to explain the peripheral mode of action of the oxytocin antagonist on BS contractile activity. Oxytocin receptors have been found in the epididymis (Filippi et al., 2002) and in the testis (Nicholson et al., 1984). It has been proposed that oxytocin when bound to its peripheral receptors promotes sperm transport during the emission phase of ejaculation by increasing the contraction of seminal tract smooth muscle cells (Filippi et al., 2003). This peripheral action of oxytocin might explain the facilitation of ejaculation found in copulating rats after systemic delivery of oxytocin (Arletti et al., 1985; Stoneham et al. 1985). The present results do not support this view, since 7-OH DPAT-induced ejaculation was not affected by i.v. pretreatment with the oxytocin antagonist. Because of the high affinity of oxytocin receptors for the oxytocin antagonist used (EC50∼1 nM), we assume that the highest dose tested was sufficient to block most of the peripheral oxytocin receptors.
Oxytocinergic nerve terminals originating in the parvocellular part of the paraventricular nucleus of the hypothalamus (PVN) have been identified in the vicinity of preganglionic parasympathetic neurons in the L6–S1 spinal segments (Tang et al., 1998). Moreover, i.t. delivery of oxytocin at the L6 level, but not at the level of the thoracic sympathetic neurons (that is, T12–T13), induces ICP increase in anaesthetized rats, indicating that activation of oxytocin receptors at the L6 level exerts a pro-erectile effect (Giuliano et al., 2001). These findings are partly corroborated by the present results showing that injection of the oxytocin antagonist at either the T13 or L6 spinal level did not impair 7-OH-DPAT-induced erection (Table 2). It is not clear why injection of the oxytocin antagonist at the L6 level was without any effect on 7-OH-DPAT-induced erection, although a difference in the pro-erectile mechanisms that were recruited is a possibility. It should be noted that ICP increases elicited by 7-OH-DPAT occurred after initiation of SVP increases and BS contractions. This indicates that the ejaculatory response preceded the erectile response under the conditions of our study. This is in contradiction with the sequence of events taking place in a natural context and led us to assume that, in the 7-OH-DPAT model erections could well be reflexive responses. Reflexive erection is a spinal reflex induced by pelvic afferent inputs. Oxytocin has been reported to mediate non-contact erection (psychogenic erection) and erection elicited by centrally acting compounds (Melis et al., 1989, 1997), but involvement of oxytocin in reflexive erection has not been documented.
Impairment of 7-OH-DPAT-induced ejaculation was observed in rats injected with the oxytocin antagonist via i.t. route at the L6 level (Table 4). This may be explained by a significant decrease in the mean duration of BS responses (Figure 4). The reason for this decrease is, however, unknown. No immunohistochemical studies have reported the existence of oxytocin receptors or fibres in Onuf's nucleus, where the motoneurons controlling the BS are located. In addition, there is no clear evidence for involvement of oxytocin in mediation or modulation of somatomotor outputs. However, binding sites for oxytocin have been detected in neurons of the dorsal grey commissure in the L6–S1 spinal segments (Veronneau-Longueville et al., 1999), which send projections to lumbosacral somatic motoneurons (Sasek et al., 1984). From this, it can be suggested that, upon L6 delivery, the oxytocin antagonist, by acting on dorsal grey column neurons, modulated the activity of the somatic motoneurons controlling the BS, and this resulted in changes in BS contraction parameters.
Experimental evidence supports a role of brain oxytocin in the expression of sexual behaviour, including penile erection and ejaculation. Anatomical studies using immunohistochemistry have shown the presence of oxytocin and/or oxytocin receptors in several brain areas known to be involved in the control of erection and other aspects of male sexual behaviour, such as the PVN, the bed nucleus of the stria terminalis, the medulla oblongata and both lobes of the pituitary gland (Freund et al., 1987; Elands et al., 1988; Adan et al., 1995). Oxytocin, given centrally in nanogram amounts to male rats, elicits erection (Argiolas et al., 1985) and facilitates copulatory behaviour (Arletti et al., 1985). Conversely, i.c.v. administration of oxytocin antagonists prevents oxytocin-induced erection (Melis et al., 1989) and non-contact erection by presenting to a male rat a non-accessible oestrous female (Melis et al., 1997). Furthermore, i.c.v. delivery of oxytocin antagonists impairs the copulatory behaviour of male rat by decreasing mount, intromission and ejaculation frequencies (Argiolas et al., 1988a). A role for oxytocin receptors in mediating the pro-sexual activity of dopamine agonists has also been described. Indeed, erection and facilitation of male sexual behaviour induced by the non-selective dopamine agonist apomorphine are inhibited by blockade of brain oxytocin receptors (Argiolas et al., 1988b; Melis et al., 1989). The present reversal of 7-OH-DPAT-induced erection and ejaculation by i.c.v. delivery of the oxytocin antagonist (Tables 2 and 3) is in agreement with these previous observations. Interestingly, dopamine has been identified as one of the neurotransmitters capable of activating PVN oxytocin neurons, inducing penile erection and facilitating copulation (Argiolas and Melis, 2004; Succu et al., 2007). As a whole, these data suggest a close relationship between dopamine and oxytocin in the central control of sexual responses. We have recently found that 7-OH-DPAT delivered into the medial preoptic area elicits ejaculation in anaesthetized rats (Kitrey et al., 2007). Neuroanatomical studies have established the existence of projections from the medial preoptic area to the parvocellular part of the PVN, where oxytocin-synthesizing neurones are localized, which in turn send axons to spinal lumbosacral preganglionic neurons involved in the control of male sexual responses (Luiten et al., 1985; Simerly and Swanson, 1988; Tang et al., 1998). Therefore, it can be hypothesized that stimulation of dopamine D3 receptors in the medial preoptic area, and to a lesser extent D2 receptors, increases PVN oxytocin release, which activates spinal autonomic nuclei controlling peripheral events related to erection and ejaculation. Whether this mechanism can account for all the behavioural effects of oxytocin and synthetic ligands of oxytocin receptors is yet to be ascertained, but it is likely that interactions between oxytocin and other neurochemical systems take place. Notably, interaction between 5-hydroxytryptaminergic and oxytocinergic neurotransmissions is important in a sexual context (de Jong et al., 2007).
In conclusion, brain oxytocin receptors mediate ejaculation induced by 7-OH-DPAT, and L6 spinal oxytocin receptors have a modulating role in 7-OH-DPAT-induced ejaculation, whereas peripheral oxytocin receptors appear to be involved marginally. These findings provide further insights into the role of oxytocin in male sexual functions and might be helpful for future pharmacological research, in particular for treatment of ejaculatory dysfunctions, including premature ejaculation.
Acknowledgments
We thank M Laurin for technical assistance and A Wohlhuter for language corrections.
Abbreviations
- 7-OH-DPAT
7-hydroxy-2-(di-N-propylamino) tetralin
- BS
bulbospongiosus muscle
- ICP
intracavernosal pressure
- PVN
paraventricular nucleus of the hypothalamus
- SVP
seminal vesicle pressure
Conflict of interest
The authors state no conflict of interest.
References
- Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, et al. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022–4028. doi: 10.1210/endo.136.9.7649111. [DOI] [PubMed] [Google Scholar]
- Ahlenius S, Larsson K. Effects of the dopamine D3 receptor ligand 7-OH-DPAT on male rat ejaculatory behavior. Pharmacol Biochem Behav. 1995;51:545–547. doi: 10.1016/0091-3057(94)00390-5. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Collu M, D'Aquila P, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5-Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur J Pharmacol. 1988a;149:389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Collu M, D'Aquila P, Gessa GL, Melis MR, Serra G. Apomorphine stimulation of male copulatory behavior is prevented by the oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin in rats. Pharmacol Biochem Behav. 1988b;33:81–83. doi: 10.1016/0091-3057(89)90433-4. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. Neuromodulation of penile erection: an overview of the role of neurotransmitters and neuropeptides. Prog Neurobiol. 1995;47:235–245. [PubMed] [Google Scholar]
- Argiolas A, Melis MR. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol Behav. 2004;83:309–317. doi: 10.1016/j.physbeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Gessa GL. Intraventricular oxytocin induces yawning and penile erection in rats. Eur J Pharmacol. 1985;117:395–396. doi: 10.1016/0014-2999(85)90018-4. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bazzani C, Castelli M, Bertolini A. Oxytocin improves male copulatory performance in rats. Horm Behav. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Clement P, Bernabe J, Denys P, Alexandre L, Giuliano F. Ejaculation induced by i.c.v. injection of the preferential dopamine D3 receptor agonist 7-hydroxy-2-(di-N-propylamino)tetralin in anesthetized rats. Neuroscience. 2007;145:605–610. doi: 10.1016/j.neuroscience.2006.12.003. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Veening JG, Olivier B, Waldinger MD. Oxytocin involvement in SSRI-induced delayed ejaculation: a review of animal studies. J Sex Med. 2007;4:14–28. doi: 10.1111/j.1743-6109.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- Elands J, Beetsma A, Barberis C, de Kloet ER. Topography of the oxytocin receptor system in rat brain: an autoradiographical study with a selective radioiodinated oxytocin antagonist. J Chem Neuroanat. 1988;1:293–302. [PubMed] [Google Scholar]
- Ferrari F, Giuliani D. Behavioral effects induced by the dopamine D3 agonist 7-OH-DPAT in sexually active and inactive male rats. Neuropharmacology. 1996;35:279–284. doi: 10.1016/0028-3908(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Filippi S, Vannelli GB, Granchi S, Luconi M, Crescioli C, Mancina R, et al. Identification, localization and functional activity of oxytocin receptors in epididymis. Mol Cell Endocrinol. 2002;193:89–100. doi: 10.1016/s0303-7207(02)00101-6. [DOI] [PubMed] [Google Scholar]
- Filippi S, Vignozzi L, Vannelli GB, Ledda F, Forti G, Maggi M. Role of oxytocin in the ejaculatory process. J Endocrinol Invest. 2003;26:82–86. [PubMed] [Google Scholar]
- Freund MM, Stoeckel ME, Palacios JM, Pazos A, Reichart JM, Porte A, et al. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20:599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, Jardin A, Rousseau JP. Antierectile role of the sympathetic nervous system in rats. J Urol. 1993;150:519–524. doi: 10.1016/s0022-5347(17)35539-8. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, McKenna KE, Longueville F, Rampin O. Spinal proerectile effect of oxytocin in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:1870–1877. doi: 10.1152/ajpregu.2001.280.6.R1870. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Clement P. Neuroanatomy and physiology of ejaculation. Annu Rev Sex Res. 2005;16:190–216. [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kitrey ND, Clement P, Bernabe J, Alexandre L, Giuliano F. Microinjection of the preferential dopamine receptor D3 agonist 7-OH-DPAT into the hypothalamic medial preoptic area induced ejaculation in anesthetized rats. Neuroscience. 2007;149:636–641. doi: 10.1016/j.neuroscience.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A, Gessa GK. Evidence that apomorphine induces penile erection and yawning by releasing oxytocin in the central nervous system. Eur J Pharmacol. 1989;164:565–570. doi: 10.1016/0014-2999(89)90265-3. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Iannucci U, Argiolas A. Morphine prevention of apomorphine- and oxytocin-induced penile erection and yawning: involvement of nitric oxide. Naunyn Schmiedeberg's Arch Pharmacol. 1997;355:595–600. doi: 10.1007/pl00004989. [DOI] [PubMed] [Google Scholar]
- Nicholson HD, Swann RW, Burford GD, Wathes DC, Porter DG, Pickering BT. Identification of oxytocin and vasopressin in the testis and in adrenal tissue. Regul Pept. 1984;8:141–146. doi: 10.1016/0167-0115(84)90169-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: New York; 1998. [Google Scholar]
- Peeters M, Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci Biobehav Rev. 2008;32:438–453. doi: 10.1016/j.neubiorev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Sasek CA, Seybold VS, Elde RP. The immunohistochemical localization of nine peptides in the sacral parasympathetic nucleus and the dorsal grey commissure in rat spinal cord. Neuroscience. 1984;12:855–873. doi: 10.1016/0306-4522(84)90175-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Stoneham MD, Everitt BJ, Hansen S, Lightman SL, Todd K. Oxytocin and sexual behaviour in the male rat and rabbit. J Endocrinol. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Melis T, Boi A, Argiolas A, Melis MR. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacology. 2007;52:1034–1043. doi: 10.1016/j.neuropharm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–254. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F, Rampin O, Freund-Mercier M-J, Tang Y, Calas A, Marson L, et al. Oxitocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience. 1999;93:1437–1447. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]