Abstract
Background and purpose:
Bradycardia is a risk factor for the development of torsade de pointes (TdP). The aim of this work was to compare the importance of changes in heart rate and arterial blood pressure in the development of drug-induced TdP and to investigate the role of vagal influences.
Experimental approach:
Experiments were performed in open-chest, pentobarbital-anaesthetized, male rabbits which were given clofilium (20, 60 and 200 nmol kg−1 min−1) with rising doses of either phenylephrine (75, 150, 225 and 300 nmol kg−1 min−1), angiotensin II (0.25, 0.5, 0.75 and 1 nmol kg−1 min−1) or saline. A fourth group received phenylephrine and cloflium after bilateral vagotomy. ECGs, haemodynamics and epicardial monophasic action potentials were recorded.
Key results:
TdP occurred in 57% of rabbits given phenylephrine and clofilium. Replacement of phenylephrine with saline or angiotensin II reduced the incidence of TdP to 0 and 17%, respectively. Vagotomy prevented TdP in rabbits given phenylephrine and clofilium. Increases in blood pressure induced by phenylephrine and angiotensin II were similar. Bradycardia only occurred with phenylephrine and was reduced but not abolished by vagotomy. Neither short-term variability of repolarization nor action potential triangulation could predict TdP.
Conclusions and implications:
These results indicate that reflex activation of vagal nerve activity is essential for the induction of drug-induced TdP in α1-adrenoceptor-stimulated anaesthetized rabbits. This implies that alterations in vagal activity may also precipitate episodes of drug-induced TdP in man and that this should be considered in selecting models used in drug development.
Keywords: bradycardia, clofilium, proarrhythmia, repolarization, short-term variability, torsade de pointes, triangulation, vagotomy
Introduction
Torsade de pointes (TdP) is a potentially lethal arrhythmia that can be induced by a wide range of drugs. Many of the drugs that have been associated with the occurrence of TdP block the rapidly activating delayed rectifier potassium current (IKr). This action commonly results in prolongation of the QT interval of the ECG but it is now widely accepted that induction of TdP cannot be predicted simply from the extent of QT prolongation (Lawrence et al., 2005; Shah and Hondeghem, 2005; Hondeghem, 2006). Other parameters, such as beat-to-beat instability of repolarization or triangulation of action potentials, have been suggested to be better predictors of TdP (Hondeghem et al., 2001; Thomsen et al., 2004). Known risk factors for drug-induced TdP include female gender, hypokalaemia and bradycardia (Haverkamp et al., 2000; Gupta et al., 2007).
An in vivo model that has been used extensively to test for drug-induced TdP is the anaesthetized, α-adrenoceptor-stimulated rabbit. This model was originally described by Carlsson et al. (1990, 1993) and has also been used, either in its original form or with minor modifications, by a number of other groups (Buchanan et al., 1993; Bril et al., 1996; Farkas et al., 1998; Brooks et al., 2000; Lu et al., 2000; Batey and Coker, 2002). Conscious rabbits are particularly susceptible to the torsadogenic actions of IKr blockers (Carlsson, 2006). Anaesthesia reduces this susceptibility but it can be restored by concomitant infusion of an α1-adrenoceptor agonist (Carlsson et al., 1990; Carlsson, 2006). However, it is still not clear how addition of an α1-adrenoceptor agonist increases TdP. It has been suggested that stimulation of cardiac α1-adrenoceptors increases free cytosolic Ca2+ concentrations, which facilitates the formation of early after-depolarizations particularly in the presence of a drug that delays repolarization (Carlsson et al., 1990; Carlsson, 2006). Early after-depolarizations may be the trigger that initiates an episode of TdP, which is then perpetuated by re-entry (Belardinelli et al., 2003).
Another possibility is that the haemodynamic changes induced by α1-adrenoceptor stimulation are important in the generation of TdP. It has been suggested that elevated blood pressure and slow ventricular rate may be prerequisites for the generation of TdP in α1-adrenoceptor-stimulated anaesthetized rabbits (Farkas et al., 2002), or that increased ventricular stretch resulting from α1-adrenoceptor-mediated peripheral vasoconstriction may be important (Farkas et al., 2006). Also, during development of a modification of the original model, it was noted that TdP often developed when changes in heart rate or blood pressure were occurring (Batey and Coker, 2002). The main aim of the present studies was therefore to investigate whether increases in arterial blood pressure or reductions in heart rate were critical for the development of TdP in anaesthetized rabbits. A secondary aim was to explore whether beat-to-beat instability of repolarization or action potential triangulation could predict TdP in this model.
Methods
Animal preparation
The experimental work was carried out in accordance with the Guidance on the Operation of the Animals (Scientific Procedures) Act 1986, London, UK, under the authority of Project Licence no. 40/1702 and approved by the University of Liverpool Animal Welfare Committee. Male New Zealand White rabbits (2.7–3.2 kg) purchased from Charles River, Margate, UK, were used for this study.
Local anaesthetic cream (EMLA 5%) was applied to both marginal ear veins and 15 min later general anaesthesia was induced by i.v. administration of sodium pentobarbital (∼30 mg kg−1) through a cannula inserted in one ear vein. Additional doses of sodium pentobarbital (3–6 mg kg−1) were given via this cannula when necessary. Most rabbits required additional anaesthetic during the surgical preparation, especially before performing the thoracotomy or during the stabilization period. Approximately half of the rabbits also required further doses of pentobarbital during the breaks in the drug administration protocol at the end of each cycle. Care was taken to ensure that there was no pedal withdrawal reflex at any time. A midline incision was made in the neck and the trachea was isolated, then cannulated to permit artificial ventilation, when required (38 strokes min−1, 6–7 mL kg−1; Bioscience pump; Harvard Apparatus, Edenbridge, Kent, UK). Through this incision both vagal nerves were located and loose sutures placed around them. The left carotid artery was also located and cannulated with the cannula being advanced into the lumen of the left ventricle for pressure measurement. The right femoral artery and vein were cannulated to allow measurement of blood pressure and drug administration, respectively. The remaining ear vein was also cannulated for drug infusion. A thoracotomy was performed by splitting the sternum from the xiphoid cartilage to a level just above the atria. The pericardium was opened to expose the anterior surface of the left ventricle and allow placement of a monophasic action potential (MAP) electrode (EP Technologies model no. 225; Linton Instrumentation, Diss, Norfolk, UK) on the epicardium approximately half-way between the base and the apex of the ventricle. Immediately after opening the chest the rabbits were ventilated (if not already on the pump) and a positive end-expiratory pressure of 1–2 cm water was applied. A digital thermocouple was inserted into the abdominal cavity just below the diaphragm to record body temperature, which was maintained by means of a heated table. After completing the surgical preparation, there was a stabilization period of at least 20 min. During this time blood gases, pH and K+ were measured (Ciba Corning 850 analyser; Siemens Medical Solutions, Newbury, Berks, UK) and ventilation volume was adjusted if necessary to maintain PO2 above 80 mm Hg and PCO2 between 35 and 40 mm Hg. In one group, the sutures around both vagal nerves were lifted to allow the vagi to be cut.
Leads I, II and III of the ECG were recorded simultaneously from subcutaneous needle electrodes attached to Gould 6615-65 ECG or 6615-58 Bioelectric amplifiers. The MAP electrode was also connected to a Gould 6615-58 Bioelectric amplifier. Arterial and left ventricular pressures were detected via Bell and Howell-type 4-422 transducers attached to Gould 6615-30 DC bridge amplifiers. The signals from the amplifiers were recorded at a sampling rate of 1000 Hz (ECGs and MAP) or 250 Hz (blood pressures) using a Po-Ne-Mah data acquisition and analysis system (Linton Instrumentation).
Experimental protocol
The standard arrhythmia induction protocol involved combined administration of rising doses of the α-adrenoceptor agonist, phenylephrine, and the IKr blocking drug, clofilium. The protocol consisted of three cycles of drug administration. In each cycle, phenylephrine was infused at a rate of 75 nmol kg−1 min−1 for 15 min, then the dose of phenylephrine was increased to 150 nmol kg−1 min−1 for 3 min followed by further increases to 225 then 300 nmol kg−1 min−1 for 3 min each. At 5 min into the first cycle, a concurrent infusion of clofilium 20 nmol kg−1 min−1 was started. At the end of the cycle, both infusions were switched off and there was a 10 min drug-free interval. The doses of phenylephrine used in the second and third cycles were the same as in the first cycle, whereas the rate of infusion of clofilium was increased to 60 nmol kg−1 min−1 for the second cycle and 200 nmol kg−1 min−1 in the third cycle. This drug infusion protocol has been illustrated in previous publications (Batey and Coker, 2002; Farkas and Coker, 2002). Rabbits were assigned randomly to one of four experimental groups (n=6–7 per group). Two groups received phenylephrine and clofilium but in one of these groups both vagi were sectioned 10 min before commencing the drug administration protocol. In the third group, phenylephrine was replaced by angiotensin II (0.25, 0.5, 0.75 and 1 nmol kg−1 min−1) and the fourth group received saline (0.9% w/v NaCl solution) instead of phenylephrine. Angiotensin II was chosen as an alternative to phenylephrine as a drug that would cause vasoconstriction via a different receptor and because it has been reported to cause less reflex bradycardia than phenylephrine in rabbits (Guo and Abboud, 1984; Kumagai and Reid, 1994). The doses of angiotensin II were selected with the aim of achieving similar increases in arterial blood pressure to those obtained with phenylephrine.
ECG analysis and arrhythmia diagnosis
ECG intervals were measured manually in beats originating from the sino-atrial (SA) node that were not preceded or followed by ectopic beats or conduction block, as described previously (Farkas et al., 2004). QT intervals were measured from the beginning of the Q wave to the end of the T wave or U wave (if present); see Figure 1. QT intervals were corrected for heart rate using a correction factor derived from baseline heart rate and QT intervals from each animal in this study. The correction factor used here, QTc=QT−0.370(RR−250), was based on that originally described by Carlsson et al. (1993) and subsequently modified by others (Batey and Coker, 2002; Farkas and Coker, 2002, 2003). Ventricular premature beats (VPBs), bigeminy, salvos, ventricular tachycardia and ventricular fibrillation were identified according to the definitions in the Lambeth Conventions (Walker et al., 1988). TdP was defined as a polymorphic ventricular tachycardia of four or more beats, where twisting of the QRS complex around the isoelectric baseline was visible in at least one ECG lead (Figure 1). Normally, it was also accompanied by a decline in arterial blood pressure towards zero with little pulsatile activity. Conduction block, either intraventricular such as bundle branch block, or atrio-ventricular block (see Farkas et al. (2004) for examples) was also identified and quantified.
Figure 1.
(a–e) Examples of Lead II ECGs recorded at (a) baseline (−10 min), then (b) 1 min, (c) 5 min, (d) 10 min and (e) 15 min after starting drug administration in a rabbit receiving phenylephrine and clofilium. These examples illustrate drug-induced QT prolongation and slowing of the heart rate. When U waves were present these were included in measurement of the QT interval. In (d, e), clear U waves can be seen and are indicated by arrows. (f) An example of arrhythmias recorded towards the end of the second cycle of drug administration in the same rabbit. At the beginning of the recording there is bigeminy, then a three-beat salvo and an episode of TdP, which spontaneously reverted to sinus rhythm.
Beat-to-beat variability of repolarization and action potential triangulation
To assess beat-to-beat variability of RR and QT intervals, values for 30 consecutive beats of sinus origin were measured manually before the start of the experimental protocol, that is, at baseline and immediately before the first VPB. Poincaré plots were produced by plotting each value against the former value. Short-term variability (STV) was determined as the mean orthogonal distance from the diagonal to the points of the plots and calculated according to the formula described previously (Thomsen et al., 2004) where STV=∑∣Dn+1−Dn∣/[30√2] and D represents RR or QT duration.
Further analysis of variability in repolarization was carried out on the MAP data. The epicardial MAP signal was analysed using Electrophysiology Data Recorder software (Version 2.8; John Dempster, University of Strathclyde, Glasgow, UK). The Po-Ne-Mah data files (recorded at 1000 Hz) were imported into the Electrophysiology Data Recorder application, a low-pass filter of 100 Hz was applied and any baseline drift was subtracted. MAP durations were measured at 30 and 90% repolarization. From MAP duration at 90% repolarization (action potential duration at 90% repolarization (APD90)), Poincaré plots were constructed and STV was calculated as described above at baseline, immediately before the first VPB, before the first episode of TdP (or at an equivalent time point in rabbits that did not have TdP; that is, the middle of the second cycle) and during the third cycle of drug administration. Triangulation of the MAP (APD90–APD30), as defined by Hondeghem et al. (2001), was also calculated at these time points.
Statistics
The incidence of TdP and conduction block was compared using Fisher's exact tests. Within- and among-group comparisons of heart rate, arterial blood pressure, QT intervals and MAP duration were performed using Friedman tests and Kruskal–Wallis tests, respectively, at selected time points. Beat-to-beat variability of QT intervals was compared using unpaired Student's t-tests. One-way ANOVA, with post hoc Dunnett's tests for within-group comparisons with baseline and Tukey–Kramer tests for between-group comparisons, was used to compare STV, triangulation of the MAPs, blood gases, pH and K+. Differences were considered statistically significant when P<0.05.
Drugs
Angiotensin II, clofilium tosylate and phenylephrine (L-phenylephrine HCl) were purchased from Sigma-Aldrich, Poole, UK. Solutions of clofilium and phenylephrine in saline were prepared freshly each day as described previously (Farkas and Coker, 2002). Angiotensin II was dissolved in saline, divided into aliquots and stored at −20 °C until required.
Results
Arrhythmias
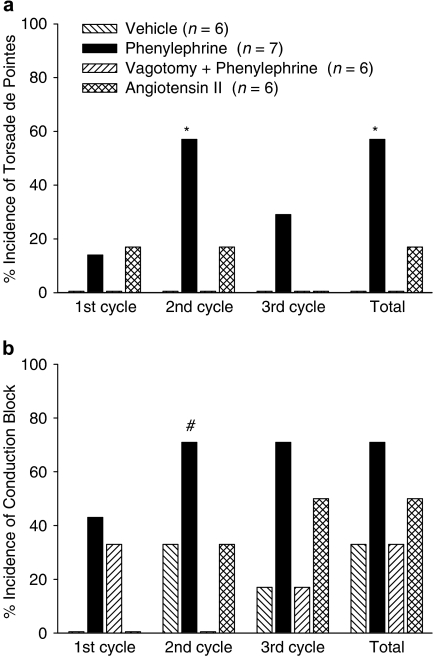
The standard protocol of administration of clofilium and phenylephrine caused TdP in four out of seven rabbits, whereas none of the vagotomized rabbits given clofilium and phenylephrine had TdP (Figure 2a). Omitting phenylephrine and replacing it with saline also prevented the occurrence of TdP. When phenylephrine was replaced by angiotensin II only one out of six rabbits had TdP (Figure 2a). Thus, either vagotomy or omission of phenylephrine prevented the occurrence of TdP.
Figure 2.
(a) Incidence of TdP and (b) incidence of conduction block in anaesthetized rabbits that received clofilium and either saline, angiotensin II or phenylephrine (with or without bilateral vagotomy at −10 min). *P<0.05 compared with vehicle and vagotomy+phenylephrine groups, #P<0.05 compared with vagotomy+phenylephrine group, Fisher's exact test.
When saline was given instead of a vasoconstrictor, the only arrhythmias that were observed were VPBs that occurred in two out of six rabbits. In contrast, VPBs occurred in all of the rabbits given either phenylephrine or angiotensin II. The majority of the rabbits given phenylephrine had complex arrhythmias. Salvos were seen in six out of seven of the intact rabbits and also in four out of six of the vagotomized group. Ventricular tachycardia (monomorphic or polymorphic but without the twisting characteristic of TdP) occurred in four out of seven intact rabbits and three out of six vagotomized rabbits given phenylephrine. In contrast, in the group that received angiotensin II, only 1 rabbit had ventricular tachycardia and 2 had salvos.
In the group given clofilium and phenylephrine, conduction block occurred in all three cycles of drug administration and five out of the seven rabbits had conduction block at some time during the experimental protocol (Figure 2b). Conduction block was also observed in the other three groups. During the second cycle of drug administration, the incidence of conduction block in rabbits receiving clofilium and phenylephrine was significantly greater than that in the vagotomized rabbits, but considering the total incidence of conduction block over the duration of the whole experiment there were no significant differences among the groups (Figure 2b).
Arterial blood pressure and heart rate
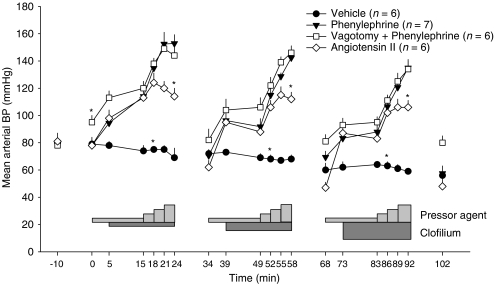
Phenylephrine increased blood pressure in a dose-dependent manner in each cycle of drug administration (Figure 3). When the infusion of phenylephrine was switched off at the end of each cycle, blood pressure returned to baseline values. Bilateral vagotomy caused a modest but significant increase in arterial blood pressure; however, the phenylephrine-induced increases in blood pressure were almost identical to those seen in the rabbits with intact vagi (Figure 3). The increases in blood pressure seen in response to infusion of the lower doses of angiotensin II were similar to those induced by the lower doses of phenylephrine but the pressor action of the highest dose of angiotensin II was less than that of the highest dose of phenylephrine. At the end of infusion of the second dose of pressor agent in each cycle, there were no significant differences in blood pressure between the groups receiving either angiotensin II or phenylephrine.
Figure 3.
Mean arterial blood pressure (BP) in anaesthetized rabbits that received clofilium and either saline, angiotensin II or phenylephrine (with or without bilateral vagotomy at −10 min). The light grey bars indicate when pressor agents were given and the dark grey bars indicate administration of clofilium. Values are mean±s.e.mean. To aid clarity, symbols indicating within-group differences have been omitted. *P<0.05 compared with all other groups, Kruskal–Wallis test.
During the experimental protocol, heart rate did not change significantly in the angiotensin II group. In the vehicle group, heart rate declined slightly but significantly by the end of the first cycle with no further significant changes in subsequent cycles. Marked bradycardia occurred during the first cycle of administration of phenylephrine, which did not reverse when the drug infusions were stopped at the end of this cycle. Further progression of this bradycardia occurred in the second and third cycles. In the vagotomized rabbits, phenylephrine caused similar reductions in heart rate in the first cycle; however, there was some reversal of this bradycardia by the start of the second cycle. As a consequence, during the second and third cycles, the phenylephrine-induced bradycardia was reduced significantly in the vagotomized rabbits compared with the intact rabbits (Figure 4).
Figure 4.
Heart rate in anaesthetized rabbits that received clofilium and either saline, angiotensin II or phenylephrine (with or without bilateral vagotomy at −10 min). Values are mean±s.e.mean. To aid clarity, symbols indicating within-group differences have been omitted. *P<0.05 compared with vehicle and angiotensin II groups, #P<0.05 compared with phenylephrine group, Kruskal–Wallis test.
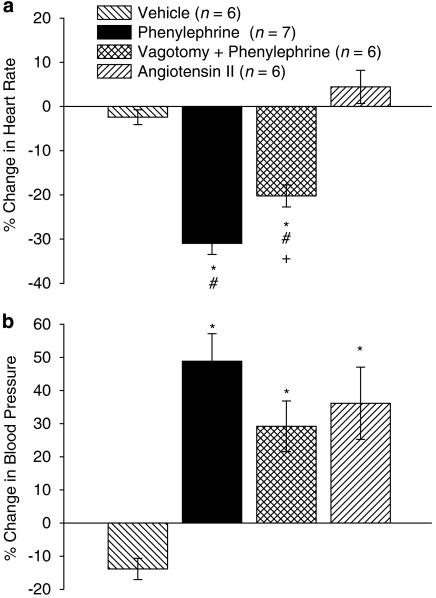
Although vagotomy did not alter basal heart rate, arterial blood pressure was increased. To facilitate comparison of the effects of phenylephrine and angiotensin II, the percentage changes in heart rate and mean arterial blood pressure from the beginning of the drug administration protocol (0 min) to the middle of the second cycle (52 min) have been calculated (Figure 5). Significant changes in heart rate only occurred in rabbits given phenylephrine but the magnitude of the reduction in heart rate was less in the vagotomized rabbits (Figure 5a). In contrast, blood pressure was increased by both phenylephrine and angiotensin II and there were no differences in the extent of the increase in blood pressure among these groups (Figure 5b).
Figure 5.
Percentage change in (a) heart rate and (b) arterial blood pressure from time 0 to 52 min (mid second cycle) in anaesthetized rabbits that received clofilium and either saline, angiotensin II or phenylephrine (with or without bilateral vagotomy at −10 min). Values are mean±s.e.mean. *P<0.05 compared with vehicle group, #P<0.05 compared with angiotensin II group, +P<0.05 compared with phenylephrine group, Kruskal–Wallis test.
QT intervals and action potential duration
QT intervals were increased substantially by phenylephrine and clofilium in the first cycle of drug administration, whereas in the presence of either saline or angiotensin II, clofilium only increased QT intervals slightly. QT intervals were significantly higher in the rabbits given phenylephrine than in the saline or angiotensin II groups (Figure 6a). As noted above for heart rate, there was some reversal of the increase in QT intervals by the start of the second cycle in the vagotomized rabbits but not in the intact rabbits (Figure 6a). In the second cycle of drug administration, the QT interval was greater in the phenylephrine group than in the vagotomy+phenylephrine group (Figure 6a). A similar pattern of changes in rate-corrected QT (QTc) intervals was observed (Figure 6b).
Figure 6.
(a) QT intervals and (b) rate-corrected QT (QTc) intervals in anaesthetized rabbits that received clofilium and either saline, angiotensin II or phenylephrine (with or without bilateral vagotomy at −10 min). Values are mean±s.e.mean. At some time points, n is less than the stated values because arrhythmias prevented measurement of ECG intervals. To aid clarity, symbols indicating within-group differences have been omitted. *P<0.05 compared with vehicle and angiotensin II groups, #P<0.05 compared with phenylephrine group, Kruskal–Wallis test.
The profile of changes in epicardial MAP durations in each group (Figure 7) was similar to those for QT and QTc intervals. Once again the increase in MAP duration that occurred in the first cycle in the intact rabbits given phenylephrine was sustained during the second and third cycles, whereas there appeared to be some reversal of this effect by the beginning of the second cycle in the vagotomized rabbits. However, in contrast to the QT and QTc data, the apparent differences in MAP duration between the phenylephrine group and the vagotomy+phenylephrine group in the second and third cycles did not reach statistical significance (Figure 7).
Figure 7.
Epicardial monophasic action potential (MAP) duration measured at 100% repolarization using the Po-Ne-Mah software in anaesthetized rabbits that received clofilium and either saline, angiotensin II or phenylephrine (with or without bilateral vagotomy at −10 min). Values are mean±s.e.mean. At some time points, n is less than the stated values because arrhythmias prevented measurement of action potential duration. To aid clarity, symbols indicating within-group differences have been omitted. *P<0.05 compared with vehicle and angiotensin II groups, Kruskal–Wallis test.
Beat-to-beat variability and action potential triangulation
In the group given phenylephrine and clofilium, beat-to-beat variability of repolarization was investigated by calculating STV of RR and QT intervals. In the rabbits that had TdP (n=4), the STV of QT intervals was 4.1±1.4 ms at baseline (0 min) and 3.8±0.3 ms immediately before the first VPB. At equivalent time points in the rabbits that did not have TdP (n=3), the values were 3.6±1.5 and 3.7±0.3 ms, respectively. Similar analysis of STV of RR intervals also showed no significant differences: 0.9±0.3 and 1.4±0.4 ms at baseline and before the first VPB in rabbits with TdP; 1.9±0.4 and 1.2±0.3 ms, respectively in rabbits that did not have TdP. There were also no changes in STV of RR intervals measured before the first episode of TdP (or at an equivalent time point in those that did not have TdP): 1.5±0.3 and 2.0±1.0 ms, respectively.
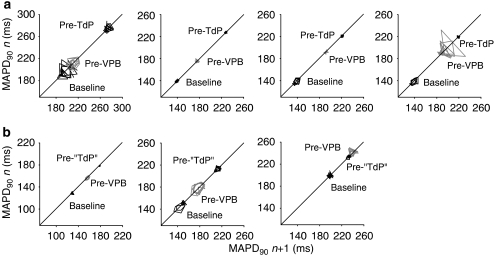
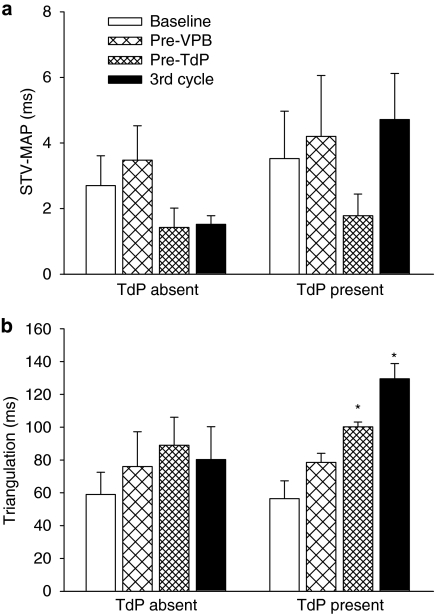
Beat-to-beat variability of repolarization was also assessed from measurements of epicardial MAP duration measured at 90% repolarization (MAPD90) (Figure 8). Poincaré plots of epicardial MAPD90 at baseline, immediately before the first VPB and before the first episode of TdP in rabbits with and without TdP are shown in Figures 8a and b. No differences in STV were found between rabbits that had TdP and those that did not at the time points illustrated in the Poincaré plots or during the third cycle (Figure 9a). There were also no significant changes in STV of epicardial MAPD90 during the progression of the experimental protocol (Figure 9a).
Figure 8.
Poincaré plots of monophasic action potential duration measured at 90% repolarization (MAPD90) at baseline, immediately before the first ventricular premature beat (VPB) and (a) before the first episode of TdP (pre-TdP) or (b) at similar time points in rabbits that did not have TdP (pre-‘TdP') in each rabbit that received clofilium and phenylephrine.
Figure 9.
(a) Short-term variability of monophasic action potential duration measured at 90% repolarization (STV-MAP) and (b) triangulation (APD90−APD30) at various time points in rabbits that had TdP (TdP present, n=4) and those that did not (TdP absent, n=3) in the group given clofilium and phenylephrine. Values are mean±s.e.mean. *P<0.05 compared with baseline, one-way ANOVA.
Assessment of triangulation of the action potential at similar time points indicated no significant changes with time in the rabbits that did not have TdP. However, in the rabbits that did have TdP, triangulation increased with time and was significantly greater before the first episode of TdP with a further increase in the third cycle of drug administration. At the latter time point, triangulation tended to be greater in the rabbits that had TdP compared with those that did not, but this apparent difference did not reach statistical significance (P=0.058, Figure 9b).
Blood gases, pH and K+
There were no differences in PO2, PCO2, pH or K+ among the drug treatment groups at baseline and no changes in PO2 and PCO2 during the course of the experiments (Table 1). In both groups that were given phenylephrine (intact and vagotomized rabbits) pH decreased as the experiments progressed, whereas increases in K+ were only significant in the vagotomized group (Table 1). The K+ concentrations measured in blood taken from an ear vein before the induction of general anaesthesia were 4.21±0.30, 4.60±0.32, 4.61±0.13 and 4.54±0.22 mM in the vehicle, phenylephrine, phenylephrine+vagotomy and angiotensin II groups, respectively. Induction of general anaesthesia with sodium pentobarbital reduced K+ to a similar extent in all groups (see Table 1 for post-anaesthesia values).
Table 1.
Body temperature and arterial blood gases, pH and K+ measured at baseline and 5 min after finishing the drug infusions in the first, second and third cycles
| Temperature (°C) | PO2 (mm Hg) | PCO2 (mm Hg) | pH (units) | K+ (mM) | |
|---|---|---|---|---|---|
| Vehicle (n=6) | |||||
| Baseline | 38.5±0.2 | 91±2 | 38±2 | 7.42±0.02 | 2.24±0.11 |
| First cycle | 38.6±0.1 | 91±2 | 37±1 | 7.42±0.01 | 2.34±0.17 |
| Second cycle | 38.6±0.1 | 94±2 | 36±1 | 7.42±0.01 | 2.34±0.11 |
| Third cycle | 38.7±0.2 | 100±5 | 35±2 | 7.40±0.01 | 2.59±0.12 |
| Phenylephrine (n=7) | |||||
| Baseline | 39.1±0.3 | 98±4 | 34±3 | 7.41±0.01 | 2.17±0.27 |
| First cycle | 38.9±0.3 | 97±6 | 33±3 | 7.35±0.02 | 2.61±0.36 |
| Second cycle | 38.8±0.3 | 98±5 | 33±2 | 7.34±0.02 | 2.34±0.25 |
| Third cycle | 38.7±0.2 | 103±6 | 31±3 | 7.29±0.03a | 2.47±0.28 |
| Phenylephrine+Vagotomy (n=6) | |||||
| Baseline | 38.9±0.4 | 99±3 | 39±1 | 7.42±0.01 | 2.35±0.07 |
| First cycle | 38.8±0.2 | 101±3 | 41±2 | 7.36±0.02 | 2.87±0.09 |
| Second cycle | 38.4±0.2 | 104±2 | 39±1 | 7.31±0.02a | 3.05±0.18a |
| Third cycle | 38.0±0.1 | 107±5 | 35±2 | 7.30±0.02a | 3.24±0.30a |
| Angiotensin II (n=6) | |||||
| Baseline | 38.6±0.3 | 88±6 | 37±1 | 7.43±0.02 | 2.31±0.09 |
| First cycle | 38.6±0.1 | 88±4 | 35±1 | 7.45±0.01 | 2.42±0.17 |
| Second cycle | 38.9±0.1 | 93±5 | 35±1 | 7.42±0.02 | 2.44±0.16 |
| Third cycle | 38.6±0.2 | 96±3 | 34±1 | 7.40±0.02 | 2.31±0.15 |
P<0.05 compared with baseline, one-way ANOVA.
Values are mean±s.e.mean.
Discussion
The results presented here demonstrate, for the first time, that bilateral vagotomy prevented drug-induced TdP in an in vivo model. Vagally mediated reductions in heart rate were necessary for the development of TdP, whereas increases in arterial blood pressure were not essential. In addition, beat-to-beat instability of repolarization assessed by measurement of STV of QT intervals or monophasic APD90 could not predict TdP, and action potential triangulation was similar in rabbits that had TdP and those that did not.
Importance of vagally mediated bradycardia
It has been known for some time that bradycardia increases the torsadogenicity of drugs that block IKr because these drugs block K+ channels in a reverse-use-dependent manner (Hondeghem and Snyders, 1990; Tande et al., 1990). Rabbits given phenylephrine and clofilium exhibited marked bradycardia, which developed during the first cycle of drug administration and progressed during the second and third cycles. In contrast, in the group receiving saline and clofilium there were only slight reductions in heart rate by the end of the first cycle, and just a tendency for heart rate to decline further with time. This is perhaps a little surprising as the IKr blocker E-4031, in rabbit single SA nodal cells prolonged cycle length as a consequence of reducing the spontaneous diastolic depolarization rate (Verheijck et al., 1995). The lack of a major bradycardic effect of clofilium alone suggests that phenylephrine was mainly responsible for the reduced heart rate observed in the rabbits given phenylephrine and clofilium. This raises the question of how phenylephrine reduced heart rate in these experiments.
Bilateral vagotomy reduced, but did not abolish, the bradycardia induced by phenylephrine indicating that although some of the bradycardia was mediated via a reflex increase in vagal activity, there was also another mechanism. The non-vagal component of the phenylephrine-induced bradycardia could be due to direct stimulation of α1-adrenoceptors on cardiac pacemaker cells (Dukes and Vaughan Williams, 1984) and subsequent blockade of K+ channels (Fedida et al., 1990; Thomas et al., 2004). It has also been suggested that α1-adrenoceptor agonists may block K+ channels directly (Parker et al., 1999). These mechanisms could account for the residual bradycardia seen in the vagotomized rabbits but may only occur with high concentrations of phenylephrine.
A more likely explanation for the non-vagal component of the phenylephrine-induced bradycardia is that it was a consequence of reduced sympathetic activity. It has been shown that increasing arterial blood pressure with phenylephrine caused a baroreflex-mediated inhibition of sympathetic nerve activity in conscious rabbits (Weinstock and Rosin, 1984; Dorward et al., 1985; Undesser et al., 1985) and anaesthetized rabbits (Thames and Ballon, 1984). Such a reduction in sympathetic activity would reduce the release of endogenous noradrenaline and adrenaline, thus reducing stimulation of β1-adrenoceptors on SA nodal cells and therefore lowering heart rate.
It is interesting to note that although the pressor effect of phenylephrine reversed within the 10 min drug-free intervals, the bradycardia did not reverse much in the intact rabbits but did reverse substantially in the vagotomized group. This may suggest that there was persistent vagal activation in the intact rabbits given phenylephrine. Studies on the sympathetic system indicated that nerve activity was suppressed for a longer period than the duration of preceding pressor responses induced by phenylephrine (Undesser et al., 1985). Thus, it is possible that reflex vagal activation could also continue after removal of the pressor stimulus. Persistent vagal activation may also explain the differences in QTc intervals between the intact and vagotomized groups given phenylephrine and clofilium. Part of the greater prolongation of the QT interval and epicardial MAP duration in the intact rabbits could be due to the sustained bradycardia but a similar pattern in the QTc data suggests that these changes were not solely dependent on heart rate. In rabbit hearts, ACh prolonged MAP duration independently of heart rate (D'Alonzo et al., 1999), suggesting that vagally released ACh could contribute to the progressive increases in QTc intervals seen in the intact rabbits given phenylephrine and clofilium.
Although vagotomy attenuated the phenylephrine-induced bradycardia, the prevention of TdP by vagotomy may not be due to the reduced bradycardia per se. Dividing the rabbits in the phenylephrine and clofilium groups into those with and without TdP revealed no difference in the extent of bradycardia: 32±4 and 30±2%, respectively by the middle of the second cycle. The reduced bradycardic response to phenylephrine in the vagotomized rabbits does confirm that in the intact rabbits ACh was being released from vagal nerve endings. This ACh could have actions on other parts of the heart as well as the SA node. Vagal innervation of the atria and nodal cells is much denser than in the ventricles (Kawano et al., 2003) but ventricular cells do respond to ACh (Zang et al., 2005) and display regional variations in responsiveness (Yang et al., 1996; Zang et al., 2005), possibly as a result of variations in the expression of the channels carrying the ACh-activated K+ current (IKAch) (Yang et al., 1996). Regional variations in IKr have also been found in the rabbit heart with IKr being greater in the apex than in basal regions and E-4031 causing more prolongation of action potentials in the apex than the base of the heart (Cheng et al., 1999). If the regional distribution of IKr and IKAch is opposite in the rabbit heart, that is, more IKAch at the base than the apex, then ACh-induced shortening of APD in the base of the heart would add to spatial dispersion of repolarization. This argument is supported by observations of greater parasympathetic innervation of the base of the ventricles compared with the apex (Kawano et al., 2003). In addition, in rabbit isolated perfused hearts, bilateral vagal stimulation increased dispersion of repolarization and reversed the normal repolarization sequence such that the base of the heart repolarized before the apex (Mantravadi et al., 2007). Removal of these effects by vagotomy could reduce this spatial dispersion of repolarization, which may explain why vagotomy prevented TdP. There are also transmural differences in the density of IKAch (Yang et al., 1996) and IKr (Bryant et al., 1998), and transmural dispersion of repolarization has been shown to be important in the development of TdP induced by IKr blockers in rabbit hearts (Milberg et al., 2004). Thus, alteration of spatial dispersion of repolarization by vagotomy may involve transmural differences as well as those between the base and apex of the heart.
Angiotensin II increased arterial blood pressure but did not alter heart rate significantly. The lack of change in heart rate in response to the pressor effect of angiotensin II is probably due to any reflex-mediated bradycardia being offset by tachycardia. In isolated right atria, angiotensin II had only a modest direct positive chronotropic action mediated via AT1 receptors (Nakashima et al., 1982; Li et al., 1996). In vivo, however, stimulation of AT1 receptors on sympathetic nerve terminals and in the adrenal medulla enhanced the release of noradrenaline and adrenaline (Dendorfer et al., 1998) which, via activation of β1-adrenoceptors on SA nodal cells, will increase heart rate.
Changes in arterial blood pressure
Stepwise increases in the rate of infusion of phenylephrine produced corresponding increases in arterial blood pressure during each cycle of drug administration. The doses of angiotensin II were selected with the aim of achieving similar increases in arterial blood pressure to those seen after administration of phenylephrine. As can be seen in Figure 3, almost identical increases in blood pressure were achieved with the two lower doses of phenylephrine and angiotensin II; however, there was some attenuation of the pressor response to the two higher doses of angiotensin II. This latter effect could either be due to angiotensin II having already achieved its maximum response or because the direct pressor effect of angiotensin II was being offset by an indirect vasodilator action. It is possible that, in a setting where blood pressure was already elevated, the β2-adrenoceptor-mediated vasodilator effect of adrenaline, released into the circulation by the action of angiotensin II on the adrenal medulla (Dendorfer et al., 1998), was revealed.
It is unlikely that the reduced pressor response seen with the highest doses of angiotensin II was responsible for the low incidence of TdP in that group. The mean changes in blood pressure in the angiotensin II group by the midpoint of the second cycle, when TdP occurred most frequently in the phenylephrine and clofilium groups, were not different from those in the phenylephrine group (see Figure 5).
Another possibility is that intraventricular stretch, resulting from α1-adrenoceptor-mediated vasoconstriction and increased peripheral resistance, could trigger arrhythmias such as TdP. Mechanical stretch can induce complex changes in cardiac muscle including action potential prolongation and early after-depolarizations (Janse et al., 2003). In rabbit isolated perfused hearts, however, it has been shown that stretch does not precipitate TdP (Farkas et al., 2006). Interestingly, it was also found that an α1-adrenoceptor agonist did not enhance dofetilide-induced TdP in these isolated hearts leading to the conclusion that it was stimulation of extracardiac α1-adrenoceptors that was important for the development of TdP in vivo (Farkas et al., 2006).
Prediction of TdP
In the rabbits receiving phenylephrine and clofilium, TdP could not be predicted from measurements of STV of QT intervals or monophasic APD90. Although the power of these comparisons is low in the present study, the results are in agreement with those of another larger study in the same model where phenylephrine was given with either the IKr blocker E-4031 alone, or E-4031 in combination with the slowly activating delayed rectifier potassium current (IKs) blocker, HMR1556, or the INa enhancer, ATX-II (Michael et al., 2007). In contrast, in other models, either action potential STV (Thomsen et al., 2004) or ‘instability' (Hondeghem et al., 2001), which is measured in a similar but not identical manner, do predict TdP. Both of these models involve atrio-ventricular block, either acute in rabbit isolated perfused hearts (Hondeghem et al., 2001) or chronic in dogs (Thomsen et al., 2004). The reduced ventricular rate in these models may be important in revealing the predictive power of these measurements of beat-to-beat variability of repolarization but, at least in the chronic atrio-ventricular blocked dog model, it is the remodelling process rather than bradycardia that leads to increases in STV (Thomsen et al., 2007). In a recent publication, however, increased STV of QT intervals was associated with TdP induced by IK blockers in chloralose-anaesthetized rabbits (Lengyel et al., 2007). Interestingly, phenylephrine was not used in the experiments of Lengyel et al. (2007). A more recent publication found opposite results in phenylephrine-stimulated rabbits anaesthetized with either chloralose or pentobarbital, as STV of QT intervals and several other indices of variability of repolarization did not predict TdP (Vincze et al., 2008). These results suggest that the presence or absence of phenylephrine may influence the predictive power of STV of repolarization parameters rather than the use of any particular anaesthetic.
Action potential triangulation also failed to distinguish rabbits that had TdP from those that did not in this model. Although there was a significant increase in triangulation just before the first episode of TdP, this value was not different from that at the same time point in the rabbits that did not have TdP (see Figure 8b). Similar results have been obtained recently in another larger study in this model (Michael et al., 2007) where triangulation could not predict TdP in α1-adrenoceptor-stimulated anaesthetized rabbits. The lack of predictive ability of either action potential triangulation or STV of repolarization in this particular model suggests that caution should be exercised in using these parameters as surrogate end points.
Risk factors for TdP
As well as bradycardia, female gender and hypokalaemia are well-recognized risk factors for TdP (Haverkamp et al., 2000; Gupta et al., 2007) and the occurrence of conduction disturbances may also play a role in the generation of TdP (Farkas et al., 2004). As estrogen exacerbated TdP in this model (Philp et al., 2007), male rabbits were used to avoid any possible complications from variations in hormone concentrations. Although these studies were not designed to investigate the importance of hypokalaemia, the results do raise some interesting possibilities. In previous studies in this particular model, where phenylephrine has been given with various ion channel modulators, K+ has risen as the experiments progressed (Farkas and Coker, 2002, 2003; Michael et al., 2007). This is likely to be an effect of repeated administration of phenylephrine because α1-agonists increased K+ release in anaesthetized rabbits (Coats, 1985). In the present study, there was more scatter in the K+ values in the group given phenylephrine and clofilium and, although there was a tendency for K+ to increase, this did not reach statistical significance. However, in the vagotomized rabbits given phenylephrine and clofilium, K+ increased significantly as the experiments progressed. Although it is possible that the higher K+ values in the vagotomized rabbits could have contributed to the lack of TdP in this group, this is unlikely as high incidences of TdP have been recorded in groups where K+ has increased to ∼4 mM (Michael et al., 2007). In addition, the K+ concentrations in the vehicle and angiotensin II groups remained low but only one of the rabbits in the angiotensin II group had TdP.
Conduction disturbances may contribute to the development of re-entrant arrhythmias such as TdP (Farkas et al., 2004). In the present study, there was significantly less conduction block in the vagotomized rabbits compared with the intact rabbits during the second cycle of administration of phenylephrine and clofilium, which matched the differences in the incidence of TdP. However, in the third cycle more rabbits across all the groups had conduction block but fewer had TdP. This pattern of increasing incidence of conduction block with time, but the greatest incidence of TdP occurring in the second cycle, has been seen previously with clofilium (Batey and Coker, 2002; Farkas and Coker, 2002), and in another recent study in this model there was no link between conduction block and TdP (Michael et al., 2007). Although the absence of conduction block in the second cycle could explain the lack of TdP in the vagotomized rabbits, other mechanisms are probably more important. It is likely that, as discussed above, vagally mediated bradycardia or changes in spatial dispersion of repolarization, had a major influence on the occurrence of TdP in this model.
Limitations and clinical relevance
It is possible that the finding that vagotomy prevented drug-induced TdP may be limited to this particular model. However, in rabbit spontaneously beating, isolated, perfused hearts ACh potentiated dofetilide-induced prolongation of monophasic APD, and revealed the torsadogenic effect of dofetilide (D'Alonzo et al., 1999). Clinical studies have also shown that ACh precipitated TdP (Aizawa et al., 1996; Chinushi et al., 2001) and that atropine suppressed TdP (Tan et al., 1998; Furushima et al., 1999). These clinical findings suggest that vagal nerve activity may influence TdP in man.
Conclusions
The experiments detailed above have demonstrated clearly the importance of vagal influences for the development of drug-induced TdP in α1-adrenoceptor-stimulated anaesthetized rabbits. Whether it is the magnitude of bradycardia that is critical or some other aspect of the influence of vagal nerve activity on the heart remains to be determined. The extent of action potential triangulation did not separate rabbits with TdP from those without, and TdP could not be predicted from measurements of beat-to-beat instability of repolarization. This suggests that temporal dispersion of repolarization is not important in the generation of TdP in this particular model. However, the possibility that activation of vagally mediated reflexes increased spatial dispersion of repolarization and the occurrence of TdP cannot be excluded.
Acknowledgments
The experimental work was funded by the British Heart Foundation (PG96/100). The Hungarian National Research Fund (OTKA F046776) also contributed to the present study.
Abbreviations
- APD90
action potential duration at 90% repolarization
- IKAch
ACh-activated potassium current
- IKr
rapidly activating delayed rectifier potassium current
- MAP
monophasic action potential
- SA
sino-atrial
- STV
short-term variability
- TdP
torsade de pointes
- VPB
ventricular premature beat
Conflict of interest
The authors state no conflict of interest.
References
- Aizawa Y, Washizuka T, Igarashi Y, Kitazawa H, Chinushi M, Abe A, et al. Acetylcholine-induced prolongation of the QT interval in idiopathic long QT syndrome. Am J Cardiol. 1996;77:879–882. doi: 10.1016/s0002-9149(97)89189-8. [DOI] [PubMed] [Google Scholar]
- Batey AJ, Coker SJ. Proarrhythmic potential of halofantrine, terfenadine and clofilium in a modified in vivo model of torsade de pointes. Br J Pharmacol. 2002;135:1003–1012. doi: 10.1038/sj.bjp.0704550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L, Antzelevitch C, Vos MA. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol Sci. 2003;24:619–625. doi: 10.1016/j.tips.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Bril A, Gout B, Bonhomme M, Landais L, Faivre JF, Linee P, et al. Combined potassium and calcium channel blocking activities as a basis for antiarrhythmic efficacy with low proarrhythmic risk: experimental profile of BRL-32872. J Pharmacol Exp Ther. 1996;276:637–646. [PubMed] [Google Scholar]
- Brooks RR, Drexler AP, Maynard AE, Al-Khalidi H, Kostreva DR. Proarrhythmia of azimilide and other class III antiarrhythmic agents in the adrenergically stimulated rabbit. Proc Soc Exp Biol Med. 2000;223:183–189. doi: 10.1046/j.1525-1373.2000.22325.x. [DOI] [PubMed] [Google Scholar]
- Bryant SM, Wan X, Shipsey SJ, Hart G. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovasc Res. 1998;40:322–331. doi: 10.1016/s0008-6363(98)00133-3. [DOI] [PubMed] [Google Scholar]
- Buchanan LV, Kabell G, Brunden MN, Gibson JK. Comparative assessment of ibutilide, D-sotalol, clofilium, E-4031, and UK-68,798 in a rabbit model of proarrhythmia. J Cardiovasc Pharmacol. 1993;22:540–549. [PubMed] [Google Scholar]
- Carlsson L. In vitro and in vivo models for testing arrhythmogenesis in drugs. J Intern Med. 2006;259:70–80. doi: 10.1111/j.1365-2796.2005.01590.x. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Abrahamsson C, Andersson B, Duker G, Schiller-Linhardt G. Proarrhythmic effects of the class III agent almokalant: importance of infusion rate, QT dispersion, and early afterdepolarisations. Cardiovasc Res. 1993;27:2186–2193. doi: 10.1093/cvr/27.12.2186. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Almgren O, Duker G. QTU-prolongation and torsades de pointes induced by putative class III antiarrhythmic agents in the rabbit: etiology and interventions. J Cardiovasc Pharmacol. 1990;16:276–285. doi: 10.1097/00005344-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Cheng J, Kamiya K, Liu W, Tsuji Y, Toyama J, Kodama I. Heterogeneous distribution of the two components of delayed rectifier K+ current: a potential mechanism of the proarrhythmic effects of methanesulfonanilide class III agents. Cardiovasc Res. 1999;43:135–147. doi: 10.1016/s0008-6363(99)00061-9. [DOI] [PubMed] [Google Scholar]
- Chinushi M, Nakagawa I, Hori T, Yamashita F, Washizuka T, Aizawa Y. QT interval prolongation and torsades de pointes unmasked by intracoronary acetylcholine administration. Pacing Clin Electrophysiol. 2001;24:1561–1562. doi: 10.1046/j.1460-9592.2001.01561.x. [DOI] [PubMed] [Google Scholar]
- Coats RA. The effects of adrenoceptor agonists and antagonists on plasma potassium concentration in anaesthetized guinea-pigs, rabbits and rats. Br J Pharmacol. 1985;86:827–836. doi: 10.1111/j.1476-5381.1985.tb11104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alonzo AJ, Zhu JL, Darbenzio RB. Effects of class III antiarrhythmic agents in an in vitro rabbit model of spontaneous torsades de pointe. Eur J Pharmacol. 1999;369:57–64. doi: 10.1016/s0014-2999(99)00057-6. [DOI] [PubMed] [Google Scholar]
- Dendorfer A, Raasch W, Tempel K, Dominiak P. Interactions between the renin–angiotensin system (RAS) and the sympathetic system. Basic Res Cardiol. 1998;93 Suppl 2:24–29. doi: 10.1007/s003950050202. [DOI] [PubMed] [Google Scholar]
- Dorward P, Riedel W, Burke S, Gipps J, Korner P. The renal sympathetic baroreflex in the rabbit. Arterial and cardiac baroreceptor influences, resetting, and effect of anesthesia. Circ Res. 1985;57:618–633. doi: 10.1161/01.res.57.4.618. [DOI] [PubMed] [Google Scholar]
- Dukes ID, Vaughan Williams EM. Effects of selective α1-, α2-, β1- and β2-adrenoceptor stimulation on potentials and contractions in the rabbit heart. J Physiol. 1984;355:523–546. doi: 10.1113/jphysiol.1984.sp015436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas A, Batey AJ, Coker SJ. How to measure electrocardiographic QT interval in the anaesthetized rabbit. J Pharmacol Toxicol Methods. 2004;50:175–185. doi: 10.1016/j.vascn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Farkas A, Coker SJ. Limited induction of torsade de pointes by terikalant and erythromycin in an in vivo model. Eur J Pharmacol. 2002;449:143–153. doi: 10.1016/s0014-2999(02)01992-1. [DOI] [PubMed] [Google Scholar]
- Farkas A, Coker SJ. Prevention of clofilium-induced torsade de pointes by prostaglandin E2 does not involve ATP-dependent K+ channels. Eur J Pharmacol. 2003;472:189–196. doi: 10.1016/s0014-2999(03)01910-1. [DOI] [PubMed] [Google Scholar]
- Farkas A, Lepran I, Papp JG. Comparison of the antiarrhythmic and the proarrhythmic effect of almokalant in anaesthetised rabbits. Eur J Pharmacol. 1998;346:245–253. doi: 10.1016/s0014-2999(98)00067-3. [DOI] [PubMed] [Google Scholar]
- Farkas A, Lepran I, Papp JG. Proarrhythmic effects of intravenous quinidine, amiodarone, D-sotalol, and almokalant in the anesthetized rabbit model of torsade de pointes. J Cardiovasc Pharmacol. 2002;39:287–297. doi: 10.1097/00005344-200202000-00016. [DOI] [PubMed] [Google Scholar]
- Farkas AS, Acsai K, Toth A, Dezsi L, Orosz S, Forster T, et al. Importance of extracardiac α1-adrenoceptor stimulation in assisting dofetilide to induce torsade de pointes in rabbit hearts. Eur J Pharmacol. 2006;537:118–125. doi: 10.1016/j.ejphar.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Fedida D, Shimoni Y, Giles WR. α-Adrenergic modulation of the transient outward current in rabbit atrial myocytes. J Physiol. 1990;423:257–277. doi: 10.1113/jphysiol.1990.sp018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furushima H, Niwano S, Chinushi M, Yamaura M, Taneda K, Washizuka T, et al. Effect of atropine on QT prolongation and torsade de pointes induced by intracoronary acetylcholine in the long QT syndrome. Am J Cardiol. 1999;83:714–718. doi: 10.1016/s0002-9149(98)00976-x. [DOI] [PubMed] [Google Scholar]
- Guo GB, Abboud FM. Angiotensin II attenuates baroreflex control of heart rate and sympathetic activity. Am J Physiol. 1984;246:H80–H89. doi: 10.1152/ajpheart.1984.246.1.H80. [DOI] [PubMed] [Google Scholar]
- Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:891–899. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res. 2000;47:219–233. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- Hondeghem L, Snyders D. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM. Thorough QT/QTc not so thorough: removes torsadogenic predictors from the T-wave, incriminates safe drugs, and misses profibrillatory drugs. J Cardiovasc Electrophysiol. 2006;17:337–340. doi: 10.1111/j.1540-8167.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- Janse MJ, Coronel R, Wilms-Schopman FJ, de Groot JR. Mechanical effects on arrhythmogenesis: from pipette to patient. Prog Biophys Mol Biol. 2003;82:187–195. doi: 10.1016/s0079-6107(03)00015-4. [DOI] [PubMed] [Google Scholar]
- Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Reid IA. Angiotensin II exerts differential actions on renal nerve activity and heart rate. Hypertension. 1994;24:451–456. doi: 10.1161/01.hyp.24.4.451. [DOI] [PubMed] [Google Scholar]
- Lawrence CL, Pollard CE, Hammond TG, Valentin JP. Nonclinical proarrhythmia models: predicting torsades de pointes. J Pharmacol Toxicol Methods. 2005;52:46–59. doi: 10.1016/j.vascn.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Lengyel C, Varro A, Tabori K, Papp JG, Baczko I. Combined pharmacological block of IKr and IKs increases short-term QT interval variability and provokes torsades de pointes. Br J Pharmacol. 2007;151:941–951. doi: 10.1038/sj.bjp.0707297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang J, Pfaffendorf M, van Zwieten PA. Direct positive chronotropic effects of angiotensin II and angiotensin III in pithed rats and in rat isolated atria. Br J Pharmacol. 1996;118:1653–1658. doi: 10.1111/j.1476-5381.1996.tb15588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HR, Remeysen P, De Clerck F. Nonselective IKr-blockers do not induce torsades de pointes in the anesthetized rabbit during alpha1-adrenoceptor stimulation. J Cardiovasc Pharmacol. 2000;36:728–736. doi: 10.1097/00005344-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Mantravadi R, Gabris B, Liu T, Choi B-R, de Groat WC, Ng GA, et al. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res. 2007;100:e72–e80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G, Dempster J, Kane KA, Coker SJ. Potentiation of E-4031-induced torsade de pointes by HMR1556 or ATX-II is not predicted by action potential short-term variability or triangulation. Br J Pharmacol. 2007;152:1215–1227. doi: 10.1038/sj.bjp.0707513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberg PMD, Ramtin SMS, Monnig GMD, Osada N, Wasmer KMD, Breithardt GMDFF, et al. Comparison of the in vitro electrophysiologic and proarrhythmic effects of amiodarone and sotalol in a rabbit model of acute atrioventricular block. J Cardiovasc Pharmacol. 2004;44:278–286. doi: 10.1097/01.fjc.0000129581.81508.78. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Angus JA, Johnston CI. Chronotropic effects of angiotensin I, angiotensin II, bradykinin and vasopressin in guinea pig atria. Eur J Pharmacol. 1982;81:479–485. doi: 10.1016/0014-2999(82)90113-3. [DOI] [PubMed] [Google Scholar]
- Parker C, Li Q, Fedida D. Non-specific action of methoxamine on Ito, and the cloned channels hKv 1.5 and Kv 4.2. Br J Pharmacol. 1999;126:595–606. doi: 10.1038/sj.bjp.0702335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp KL, Hart G, Coker SJ. A gender-independent proarrhythmic action of 17β-estradiol in anaesthetized rabbits. Eur J Pharmacol. 2007;575:113–121. doi: 10.1016/j.ejphar.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Shah RR, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005;2:758–772. doi: 10.1016/j.hrthm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Tan HL, Wilde AAM, Peters RJG. Suppression of torsades de pointes by atropine. Heart. 1998;79:99–100. doi: 10.1136/hrt.79.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande PM, Bjornstad H, Yang T, Refsum H. Rate-dependent class III antiarrhythmic action, negative chronotropy, and positive inotropy of a novel Ik blocking drug, UK-68798: potent in guinea pig but no effect in rat myocardium. J Cardiovasc Pharmacol. 1990;16:401–410. doi: 10.1097/00005344-199009000-00008. [DOI] [PubMed] [Google Scholar]
- Thames MD, Ballon BJ. Occlusive summation of carotid and aortic baroreflexes in control of renal nerve activity. Am J Physiol. 1984;246:H851–H857. doi: 10.1152/ajpheart.1984.246.6.H851. [DOI] [PubMed] [Google Scholar]
- Thomas D, Kiehn J, Katus HA, Karle CA. Adrenergic regulation of the rapid component of the cardiac delayed rectifier potassium current, I Kr, and the underlying hERG ion channel. Basic Res Cardiol. 2004;99:279–287. doi: 10.1007/s00395-004-0474-7. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Oros A, Schoenmakers M, van Opstal JM, Maas JN, Beekman JDM, et al. Proarrhythmic electrical remodelling is associated with increased beat-to-beat variability of repolarisation. Cardiovasc Res. 2007;73:521–530. doi: 10.1016/j.cardiores.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Verduyn SC, Stengl M, Beekman JDM, de Pater G, van Opstal J, et al. Increased short-term variability of repolarization predicts D-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110:2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- Undesser KP, Pan JY, Lynn MP, Bishop VS. Baroreflex control of sympathetic nerve activity after elevations of pressure in conscious rabbits. Am J Physiol. 1985;248:H827–H834. doi: 10.1152/ajpheart.1985.248.6.H827. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, van Ginneken ACG, Bourier J, Bouman LN. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circ Res. 1995;76:607–615. doi: 10.1161/01.res.76.4.607. [DOI] [PubMed] [Google Scholar]
- Vincze D, Farkas AS, Rudas L, Makra P, Csik N, Lepran I, et al. Relevance of anaesthesia for dofetilide-induced torsades de pointes in α1-adrenoceptor-stimulated rabbits. Br J Pharmacol. 2008;153:75–89. doi: 10.1038/sj.bjp.0707536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, et al. The Lambeth Conventions—guidelines for the study of arrhythmias in ischemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Rosin AJ. Relative contributions of vagal and cardiac sympathetic nerves to the reflex bradycardia induced by a pressor stimulus in the conscious rabbit: comparison of ‘steady state' and ‘ramp' methods. Clin Exp Pharmacol Physiol. 1984;11:133–141. doi: 10.1111/j.1440-1681.1984.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Yang ZK, Boyett MR, Janvier NC, McMorn SO, Shui Z, Karim F. Regional differences in the negative inotropic effect of acetylcholine within the canine ventricle. J Physiol. 1996;492:789–806. doi: 10.1113/jphysiol.1996.sp021346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang WJ, Chen LN, Yu XJ, Fang P, Lu J, Sun Q. Comparison of effects of acetylcholine on electromechanical characteristics in guinea-pig atrium and ventricle. Exp Physiol. 2005;90:123–130. doi: 10.1113/expphysiol.2004.027888. [DOI] [PubMed] [Google Scholar]