Abstract
Background and purpose:
We investigated the effect of rimonabant on inflammation and enhanced platelet reactivity in type 2 diabetic Zucker rats, an experimental model of impaired glucose tolerance and the metabolic syndrome.
Experimental approach:
Rimonabant (10 mg kg−1 by gavage) was fed for 2 weeks to 3-month-old male obese Zucker rats as an impaired glucose tolerance model and for 10 weeks to 6-month-old male obese Zucker rats as a model of the metabolic syndrome. RANTES (Regulated upon Activation, Normal T cell Expressed, and Secreted) and MCP-1 (monocyte chemotactic protein-1) serum levels were determined by ELISA. Leukocyte populations were quantitatively assessed using a veterinary differential blood cell counter. Platelet activation was assessed by flow-cytometry, platelet aggregation, and adhesion of isolated platelets to immobilized fibrinogen.
Key results:
RANTES and MCP-1 serum levels were increased in obese vs lean Zucker rats and significantly reduced by long-term treatment with rimonabant, which slowed weight gain in rats with the metabolic syndrome. Neutrophils and monocytes were significantly increased in young and old obese vs lean Zucker rats and lowered by rimonabant. Platelet-bound fibrinogen was significantly enhanced in obese vs lean Zucker rats of both age, and was reduced by rimonabant. Platelets from obese rats were more sensitive to thrombin-induced aggregation and adhesion to fibrinogen, which were both attenuated by rimonabant therapy.
Conclusions and implications:
We demonstrate positive modulation of circulating neutrophil and monocyte numbers, reduced platelet activation and lower RANTES and MCP-1 levels by rimonabant in Zucker rats. This may potentially contribute to a reduction of cardiovascular risk.
Keywords: diabetes, endocannabinoid blockade, platelets, chemokines, leukocytes
Introduction
Cardiovascular thrombotic events facilitated by pre-existing atherosclerotic lesions account for about two-thirds of deaths in diabetic patients. In addition to macrovascular events, activated platelets contribute to microvascular occlusion, embolization of platelet–platelet or platelet–leukocyte aggregates and amplification of athero- and thrombogenesis (Tschoepe and Menart-Houtermans, 2002). Thus, activated platelets have a major impact on morbidity and mortality as most diabetic patients die from cardiovascular atherothrombotic events (Resnick et al., 2000). This is clinically reflected by the fact that patients with type 2 diabetes without prior cardiovascular events have a risk of myocardial infarction similar to that among non-diabetic patients with prior myocardial infarction (Haffner et al., 1998). Therefore, diabetes is recognized as a coronary heart disease risk equivalent, and even pre-diabetic states such as the metabolic syndrome are associated with increased cardiovascular risk (Ryden et al., 2007). We recently demonstrated that reduction of glucose uptake during impaired glucose tolerance (IGT) reduces platelet activation in Zucker rats (Schäfer et al., 2004c) and this is reflected by a significant reduction in the risk of cardiovascular disease in humans (Chiasson et al., 2003). Activated platelets are the essential step in promoting leukocyte adhesion and determining the progression of atherosclerotic lesion formation (Massberg et al., 2002; Huo et al., 2003). Moreover, platelet activation plays a critical role in initiation of atherosclerosis, as demonstrated in a model of accelerated atherosclerosis in mice, in which inhibition of activated platelets using glycoprotein IIb/IIIa inhibitors prevented the development of atherosclerotic lesions (Massberg et al., 2002).
Overweight and obesity show a much higher prevalence than a decade ago (Ogden et al., 2006). This is linked to an increased rate of obesity-related atherosclerosis-associated diseases such as diabetes, stroke, myocardial infarction and coronary heart disease (Murphy et al., 2006). Pharmacological and non-pharmacological interventions have failed to successfully reduce the disease burden in general, whereas treatment with orlistat, sibutramine or rimonabant only modestly reduced body weight and slightly improved cardiovascular risk factors in overweight patients (Rucker et al., 2007). New strategies for treatment of severe obesity include bariatric surgery, which is associated with long-term weight loss and decreased mortality in selected cases, but is not a preventive option for larger patient populations (Sjostrom et al., 2007). Therefore, new treatment strategies need to be found and implemented. In contrast to previous strategies targeting secondary modification on an end-organ level, a therapeutic approach is required, which would intervene before damage occurs, so ideally prevent the activation of the cascade of deleterious events. Current focus has been on the prevention of excessive calorie intake, primarily by lifestyle modification, but secondarily by pharmacological intervention.
Recently, the endocannabinoid system has been characterized as a major regulator of food intake and energy homoeostasis in humans and animals. The endocannabinoid system comprises two cannabinoid receptors (CB1 and CB2), the endocannabinoids (anandamide and 2-arachidonyl glycerol, oleoylethanolamide, and several other N-acylethaolamines, arachidonoyl amino acids and monoacylglycerides) and the enzymes involved in their synthesis and degradation as recently reviewed (Lambert and Muccioli, 2007). Blockade of the endocannabinoid system by the selective CB1 receptor antagonist rimonabant resulted in decreased food intake, body weight reduction and an improvement of the metabolic risk profile (Boyd and Fremming, 2005; Henness et al., 2006). This was predominantly attributable to reduced incidence of metabolic syndrome, increased insulin sensitivity and reduced triglyceride levels.
In the present study, we examined the effects of short- and long-term treatment with the CB1 antagonist rimonabant on lipid profiles, circulating leukocytes and platelets as well as on platelet activation in young Zucker rats with IGT as well as in old Zucker rats, an established experimental model of the metabolic syndrome. The study investigates the modulation of cardiometabolic factors by rimonabant in the absence of potentially interfering pharmacological substances such as antiplatelet (for example, acetylsalicylic acid, thienopyridines), lipid-lowering (for example, statins, fibrates) or antidiabetic agents, which would almost always have to be used in patients with the metabolic syndrome.
Methods
Animals
All animal procedures in this investigation conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Male lean and obese Zucker rats were obtained from Harlan-Winkelmann (Borchen, Germany) and housed in temperature-controlled cages (20–22 °C) with a 12-h light-dark cycle and given free access to water and chow. Rimonabant (10 mg kg−1 body weight, solubilized in gum arabic; Sanofi-Aventis, Berlin, Germany) was fed by gavage for 2 weeks to 3-month-old male obese Zucker rats and for 10 weeks to 6-month-old male obese Zucker rats and compared with placebo-treated age-matched lean and obese Zucker rats. The old obese Zucker rat is a model for metabolic syndrome/type 2 diabetes based on IGT caused by the inherited obesity gene mutation (fa/fa), which leads to insulin resistance. Animals of the age group of about 10 weeks are an established model of IGT, and the lean animals (Fa/fa) are the respective healthy controls (Schäfer et al., 2004c). Basal non-fasting blood glucose levels were slightly higher in obese vs lean Zucker rats of both ages (data not shown). The chosen dose was not the lowest dose of rimonabant used in rat studies; however, results from the manufacturer suggested that the dose of 10 mg kg−1 would be more favourable in the Zucker rat with regard to weight gain and lipid modifications (Janiak et al., 2007).
Platelet sampling
Deep general anaesthesia was induced using isoflurane. The abdominal cavity was opened under deep anaesthesia, determined by total absence of reaction to pain under spontaneous respiration and blood was taken by direct puncture of the inferior caval vein into a tube containing 3.8% sodium citrate. A whole blood count was immediately performed from EDTA-anticoagulated blood using the rat-specified profile on a veterinary haematology analyser (Sysmex XT-2000iV; Sysmex, Norderstedt, Germany). Platelet counts were always performed using the platelet-specific fluorescence signal in the RET channel.
Serum levels of the chemokines CCL5 (regulated upon activation, normal T cell expressed and secreted; RANTES) and CCL2 (monocyte chemotactic protein-1; MCP-1) were determined by ELISA (MMR00 RANTES immunoassay and MJE00 JE/MCP-1 immunoassay, both from R&D Systems, Wiesbaden, Germany).
Flow cytometry
For determination of modulation of in vivo platelet activation, whole blood was diluted with phosphate-buffered saline (free of Ca2+ and Mg2+, enriched with D-glucose (5.5 mM) and 0.5% BSA). Platelet surface-expressed P-selectin was detected with a fluorescein isothiocyanate-labelled anti-P-selectin (CD62P) antibody (5108-F100T; BioCytex, Marseille, France). Following incubation with the antibodies, platelets were fixed with methanol-free formaldehyde (1.5%) for 10 min and subsequently analysed as reported before (Schäfer et al., 2006).
Phosphorylation of vasodilator-stimulated phosphoprotein (VASP) was evaluated using fluorescein isothiocyanate-labelled antibodies against phosphorylation of Ser157 as previously described (Schäfer et al., 2003a, 2003b).
For determination of in vitro platelet reactivity, citrated whole blood was centrifuged at 180 g for 10 min to obtain platelet-rich plasma, which was diluted with phosphate-buffered saline to obtain a final platelet concentration of 250 000 μL−1. Hence, platelet-rich plasma samples were stimulated with ADP (5, 10, 15 and 20 μM) for 10 min, and P-selectin expression was assessed as detailed above.
Platelet aggregation
Aggregation in platelet-rich plasma was induced by thrombin using a commercial platelet-aggregation profiler (PAP-8; BioData, Hilden, Germany).
Flow chamber adhesion
Platelet adhesion under arterial flow conditions (shear of 1000 s−1) was assessed using a parallel-plate perfusion chamber (FCS2; Bioptechs Inc., Butler, PA, USA), which was mounted on the stage of an inverted microscope (TE 2000-S; Nikon, Düsseldorf, Germany), using a protocol similar to experiments with human platelets (Schulz et al., 2007). Images were recorded from 5–8 different microscopic fields (× 20 objectives) using a digital photo camera (DS-2M; Nikon).
Statistics
For clarity, values are shown as means±s.e.mean in figures. However, the statistical comparisons were made using non-parametric tests throughout. Group comparisons were made using the Kruskal–Wallis test. In case of significance, Student's t-test was applied after testing for equality of variances by Levene's test. To correct for multiple testing, Bonferroni's method was used. Otherwise, a P-value <0.05 was considered statistically significant.
Reagents
Unless stated otherwise, all chemicals were obtained from Sigma (Deisenhofen, Germany) in the highest purity available.
Results
Metabolic parameters
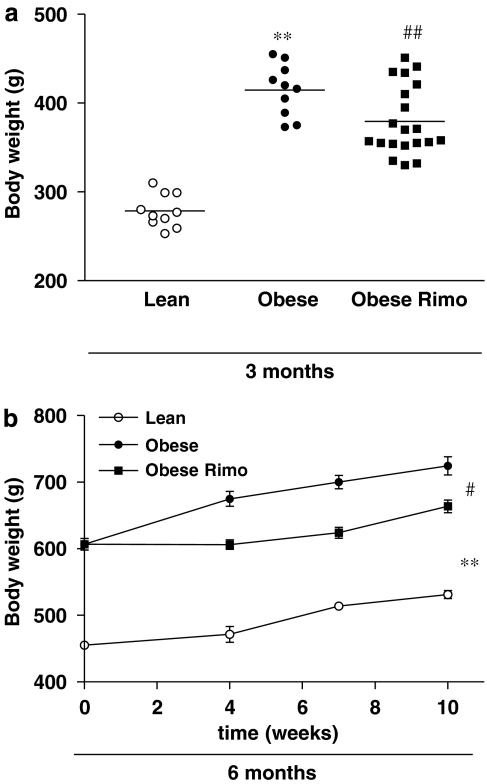
Young and old obese Zucker rats had significantly higher body weights compared with age-matched lean Zucker rats. Two weeks of treatment with rimonabant reduced body weight of young obese Zucker rats (Figure 1a) and 10 weeks of treatment significantly attenuated weight gain in older obese Zucker rats (Figure 1b).
Figure 1.
Body weight in 3-month-old obese Zucker rats after 2 weeks of treatment with or without rimonabant (Rimo, a) compared with lean Zucker rats of the same ages, and weight gain in 6-month-old lean and obese Zucker rats after 10 weeks of treatment with or without rimonabant (Rimo, b). n=10–20, **P<0.01 vs lean; #P<0.05, ##P<0.01 vs placebo.
Circulating platelets and leukocyte subpopulations
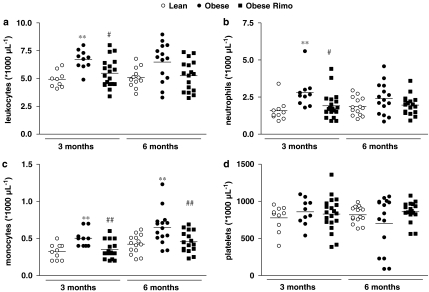
Total leukocyte counts (Figure 2a) as well as circulating neutrophils (Figure 2b) were significantly higher in young obese vs lean Zucker rats and decreased following treatment with the CB1 antagonist, whereas there was only a nonsignificant trend in the older group. Monocyte numbers were increased in obese rats of both age groups and significantly reduced by rimonabant (Figure 2c). The number of circulating platelets did not differ significantly between the treatment groups (Figure 2d).
Figure 2.
Quantitative assessment of circulating total leukocytes (a), neutrophils (b), monocytes (c) and platelets (d) using the automatized veterinary differential blood counter Sysmex XT-2000iV. n=10–20, **P<0.01 vs lean, #P<0.05, ##P<0.01 vs placebo.
Serum levels of chemokines and lipoproteins
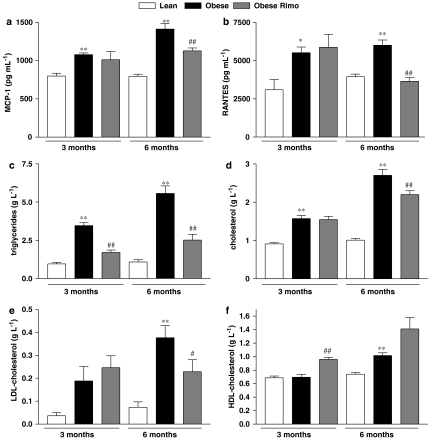
Serum levels of the pro-atherosclerotic chemokine MCP-1 (Figure 3a), the release of which is facilitated by activated platelets (Gawaz et al., 1998, 2005; Massberg et al., 2003), and of RANTES (Figure 3b), which is released by platelets and triggers monocyte recruitment to the vascular wall (von Hundelshausen et al., 2001; Schober et al., 2002; Huo et al., 2003), were significantly increased in obese vs lean Zucker rats of both ages and decreased following chronic 10-week treatment with rimonabant in older obese Zucker rats.
Figure 3.
Serum levels of the pro-atherosclerotic chemokines MCP-1 (a) and RANTES (b) were detected by ELISA. Lipid profiles were performed and triglyceride levels (c) as well as total cholesterol (d), LDL cholesterol (e) and HDL cholesterol (f) were determined. n=5, *P<0.05, **P<0.01 vs lean, #P<0.05, ##P<0.01 vs placebo. HDL cholesterol, high-density lipoprotein cholesterol; LDL cholesterol, low-density lipoprotein cholesterol; MCP-1, monocyte chemotactic protein-1; RANTES, regulated upon activation, normal T cell expressed and secreted.
When analysing the lipid profile in Zucker rats, we found increased levels of triglycerides (Figure 3c), total cholesterol (Figure 3d), and low-density lipoprotein cholesterol (LDL-C) (Figure 3e) in obese vs lean Zucker rats. Triglycerides were already decreased after 2 weeks of CB1 antagonism in young obese Zucker rats, whereas 10 weeks treatment reduced triglycerides as well as total cholesterol and LDL-C. In addition, treatment of both age-groups of obese Zucker rats with rimonabant resulted in raised levels of high-density lipoprotein cholesterol, which were statistically significant in the younger group of Zucker rats (Figure 3f).
Markers of in vivo platelet function
To determine the integrity/activity of the endogenous platelet-inhibiting pathway, we assessed the basal phosphorylation state of platelet VASP in whole blood, which was immediately fixed in formaldehyde after collection. Basal platelet VASP phosphorylation was significantly reduced in obese vs lean Zucker rats of both age groups and improved significantly in obese Zucker rats of both ages when treated with rimonabant (Table 1). In parallel, the extent of in vivo platelet activation was measured by analysis of platelet-bound fibrinogen reflecting glycoprotein IIb/IIIa activation in unstimulated whole blood. Platelet-bound fibrinogen was significantly increased in 6-month-old obese vs lean Zucker rats, and treatment with the CB1 antagonist significantly reduced fibrinogen binding in old obese Zucker rats (Table 1).
Table 1.
Activation and reactivity of platelets from 3-month-old lean and obese Zucker rats after 2 weeks as well as from 6-month-old lean and obese Zucker rats after 10 weeks of treatment with rimonabant
| Parameter | Lean, 3 months | Obese, placebo, 3 months | Obese, rimonabant, 3 months | Lean, 6 months | Obese, placebo, 6 months | Obese, rimonabant, 6 months |
|---|---|---|---|---|---|---|
| P-VASP (MFI) | 36.1±1.6 | 25.8±0.9** | 31.6±1.3## | 41.3±3.9 | 23.7±1.9** | 31.8±1.8## |
| Fibrinogen (MFI) | 104.3±5.7 | 123.5±8.2 | 109.2±4.2 | 145.1±14.8 | 207.9±20.3* | 120.6±4.3## |
| CD62P (ADP 20 μM) (MFI) | 91.3±10.7 | 120.9±9.9* | 112.0±7.1 | 190.2±14.4 | 226.9±15.0* | 187.0±7.4# |
| Aggmax (thrombin 0.02 U mL−1 (%)) | 3.9±2.6 | 41.4±2.5* | 16.9±3.6# | 0.0±0.0 | 15.3±4.1 | 4.6±3.2 |
| Firm adhesion (fibrinogen) (n) | 140.4±23.5 | 212.5±40.2 | 114.9±15.4## | 1449±292 | 1068±257 | 618±270# |
Abbreviations: MFI, mean fluorescence intensity; P-VASP, phosphorylation of vasodilator-stimulated phosphoprotein; CD62P, P-selectin; Aggmax, maximum aggregation.
*P<0.05, **P<0.01 vs age-matched lean, #P<0.05, ##P<0.01 vs age-matched obese placebo.
Platelet in vitro stimulation, aggregation and adhesion
ADP (20 μM)-induced P-selectin surface expression was significantly increased in platelets from young and old obese vs lean Zucker rats (Table 1). Chronic treatment of old Zucker rats with rimonabant for 10 weeks significantly reduced ADP-stimulated surface expression of P-selectin (Table 1). Thrombin-induced platelet aggregation at lower concentrations was significantly enhanced in platelet-rich plasma from obese vs lean Zucker rats of both ages, which was attenuated by CB1 receptor antagonism (Table 1).
Washed platelets from obese vs lean Zucker rats of both ages were perfused through a fibrinogen-coated parallel-plate perfusion chamber at arterial shear conditions of 1000 s−1 without any additional pharmacological activation. Although the number of adherent platelets was only modestly increased in suspensions from obese vs lean Zucker rats of both ages, treatment of obese Zucker rats with rimonabant significantly reduced adhesion of unstimulated platelets on isolated fibrinogen under arterial flow conditions (Table 1).
Discussion
In the present study, we examined the effects of blocking CB1 receptors with rimonabant for 2 weeks in young Zucker rats and for 10 weeks in old Zucker rats as an established experimental model of the metabolic syndrome based on IGT. CB1 antagonism by rimonabant positively modulated the lipid profile, reduced circulating neutrophils and monocytes, as well as the pro-atherosclerotic chemokines MCP-1 and RANTES. Platelet activation as well as their susceptibility to agonists or physical stimuli was significantly reduced.
The rimonabant in obesity (RIO)-Europe and -North America trials previously demonstrated weight reduction in obese non-diabetic patients, who all received diet and lifestyle therapy in addition to the study medication. Thereby, an average weight loss of 6.0–6.5% over 2 years was achieved (van Gaal et al., 2005; Pi-Sunyer et al., 2006). In contrast, the animals in this experimental study did not have a restriction in food intake and were at ages of ongoing weight gain. Although rimonabant did not induce weight loss, CB1 antagonism significantly attenuated the weight gain in obese Zucker rats. RIO-Europe (van Gaal et al., 2005), RIO-North America (Pi-Sunyer et al., 2006), RIO-Diabetes (Scheen et al., 2006), as well as the more-specific RIO-Lipids study, which included patients who were dyslipidaemic, non-diabetic and obese (Despres et al., 2005), all demonstrated raised levels of high-density lipoprotein cholesterol and reduction of triglycerides by rimonabant. Both features were confirmed in our study in obese Zucker rats of both age groups. In addition, when rimonabant was used for 10 weeks as the single medication in the older group, we observed a significant reduction in LDL-C as well as total cholesterol levels similar to the LDL-C-lowering effects of rimonabant observed in a murine obesity model (Poirier et al., 2005). Metabolic factors may affect the balance between inflammatory and anti-inflammatory activity controlling the progression of atherosclerosis. They contribute to lipid deposition in the arterial wall and initiate leukocyte recruitment (Hansson, 2005). Levels of circulating leukocytes, which were within the normal limits for rats in all investigated groups, were significantly increased in both the model for IGT as well as the metabolic syndrome compared with their respective lean controls. Treatment with rimonabant significantly reduced the number of circulating leukocytes in total as well as of monocytes more specifically. Similarly, in human diabetes and atherosclerosis, there is low-grade inflammation over decades of atherogenesis whereas individual leukocyte counts remain within ‘normal ranges'. This effect might indicate a potential modulation of low-grade inflammation in these models of developing diabetes. Monocytes circulating in the blood are the precursors of foam cells. The monocyte chemoattractant proteins recruit monocytes to the vascular endothelium, where they enter the subendothelial space, accumulate lipids and differentiate into macrophages and foam cells. MCP-1 is present in these macrophage-rich atherosclerotic plaques and oxidized LDL-C induces the production of MCP-1 in endothelial and smooth muscle cells (Hansson, 2005). MCP-1 causes the expression of inflammatory genes and impairs insulin-dependent glucose uptake. Obese mice with a deletion of CCR2, the main chemokine receptor for MCP-1, show improved insulin resistance, which indicates the important role of MCP-1 in the metabolic syndrome (Weisberg et al., 2006). In this study, rimonabant positively modulated this inflammatory pathway in obese Zucker rats by reducing the substrate LDL-C, by lowering levels of the chemoattractant MCP-1 and by decreasing circulating monocytes as the predominant target cells.
As inflammation is centrally involved in the development of atherosclerosis (Libby, 2002; Hansson, 2005), we further assessed the pro-atherosclerotic chemokine RANTES in addition to MCP-1. RANTES is secreted by endothelial and smooth muscle cells, activated T cells, macrophages and platelets. RANTES can be delivered by circulating platelets to inflamed endothelium, where it increases leukocyte adhesiveness under flow conditions (Sheikine and Hansson, 2006). The adhesive function of platelets has recently been highlighted as an important recruitment mechanism in atherosclerosis (Massberg et al., 2002). For example, targeted deficiency of P-selectin in platelets reduces atherosclerosis in mice (Huo et al., 2003). Platelets increase monocyte recruitment in atherosclerosis by secreting chemokines such as RANTES (Eriksson, 2004), which then triggers monocyte arrest, and antagonism of RANTES receptors reduces atherosclerotic plaque formation (Veillard et al., 2004). In the present study, chronic block of CB1 receptors with rimonabant reduced serum levels of RANTES in obese Zucker rats and lowered the number of circulating neutrophils. By reducing mediators of pro-inflammatory and pro-atherosclerotic pathways, rimonabant appears to improve the cardiometabolic risk profile.
RANTES is released by activated platelets, which furthermore induce endothelial RANTES and MCP-1 formation (Gawaz et al., 1998, 2005). Activated platelets are nowadays recognized as the essential step in promoting leukocyte adhesion and determining the progression of atherosclerotic lesion formation (Massberg et al., 2002; Huo et al., 2003). Moreover, platelet activation plays a critical role for initiation of atherosclerosis, as demonstrated in a model of accelerated atherosclerosis in mice, in which inhibition of activated platelets using glycoprotein IIb/IIIa inhibitors prevented the development of atherosclerotic lesions (Massberg et al., 2002). Platelet activation by several pro-inflammatory chemokines (Gear et al., 2001; Schäfer et al., 2004b; Weber, 2005) as well as platelet-dependent chemokine-induced leukocyte recruitment has been described (Schulz et al., 2007). In our study, we observed enhanced platelet susceptibility to physical and pharmacological stimuli in obese vs lean Zucker rats, which were attenuated by treatment with rimonabant. Basal platelet VASP phosphorylation regulates and displays tonic endogenous platelet inhibition (Massberg et al., 2004; Schäfer et al., 2004d), a process that is severely affected in diabetes (Schäfer et al., 2004a), and was modified by antagonism of CB1 receptors. Improved VASP phosphorylation resulting in reduced platelet activation and susceptibility has been previously described in experimental diabetes following pharmacological intervention, increasing endogenous nitric oxide (NO)/cGMP signalling as an endogenous platelet inhibitory pathway (Schäfer et al., 2006, 2007). Improved NO/cGMP signalling indicates improved endothelial function, which is markedly disturbed in diabetes and developing atherosclerosis and a very early feature leading to platelet activation, leukocyte adhesion and chemokine release (Schäfer and Bauersachs, 2008). It is therefore tempting to speculate that indirect effects of rimonabant including beneficial effects on leukocytes via CB2 receptors as well as improvement of NO signalling might most probably have contributed to the observed effects. It was beyond the scope of the present study to fully elucidate the complex mechanisms that might be involved in reducing the cardiometabolic risk profile in developing diabetes by CB1 receptor blockade. It is known that Δ9-tetrahydrocannabinol inhibits several immunological functions of macrophages, which can be inhibited by rimonabant, despite the fact that these cells do not express CB1 receptors (Chuchawankul et al., 2004). Ligands of the CB2 receptors expressed on leukocytes have previously been demonstrated to reduce cytokine secretion from human monocytes (Klegeris et al., 2003). A recent study reported that CB2 receptor stimulation attenuated endothelial-cell activation, reduced monocyte chemoattractant protein synthesis and inhibited monocyte–endothelial cell adhesion (Rajesh et al., 2007). Therefore, it might be tempting to speculate that part of the anti-inflammatory modulation observed by treatment with rimonabant in the present study was mediated by alternative signalling through CB2 receptors in the presence of functional CB1 receptor blockade.
Endocannabinoids have multiple roles in regulating functions of CNS. Although antagonists of the endocannabinoid system have been used for smoking cessation, severe psychiatric abnormalities have also been reported as side effects. These include depression, anxiety and suicidal tendency, which recently led the US Food and Drug Administration to refuse approval for rimonabant, whereas the European Medicines Agency recognized psychiatric side effects in ∼10% of rimonabant-treated patients, but still considered the beneficial effects to outweigh the adverse side effects.
In conclusion, the selective CB1 antagonist rimonabant represents a promising therapeutic alternative for the treatment of obesity and associated metabolic disorders. In addition to its weight-reducing properties in obese patients, rimonabant in the Zucker rats positively modulated the lipid profile, reduced circulating neutrophils and monocytes, attenuated platelet activation and the release of pro-atherosclerotic chemokines. Thus, rimonabant may reduce cardiovascular risk by lessening pro-inflammatory and pro-atherosclerotic cascades.
Acknowledgments
We thank Juliane Jaitner, Sarah Weinberger and Ilona Mell for expert technical assistance.
Abbreviations
- CB
cannabinoid receptor
- HDL-C
high-density lipoprotein cholesterol
- IGT
impaired glucose tolerance
- LDL-C
low-density lipoprotein cholesterol
- MCP-1
monocyte chemotactic protein-1
- RANTES
regulated upon activation, normal T cell expressed and secreted
- VASP
vasodilator-stimulated phosphoprotein
Conflict of interest
This study was partly supported by a research grant from Sanofi-Aventis.
References
- Boyd ST, Fremming BA. Rimonabant—a selective CB1 antagonist. Ann Pharmacother. 2005;39:684–690. doi: 10.1345/aph.1E499. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- Chuchawankul S, Shima M, Buckley NE, Hartmann CB, McCoy KL. Role of cannabinoid receptors in inhibiting macrophage costimulatory activity. Int Immunopharmacol. 2004;4:265–278. doi: 10.1016/j.intimp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Eriksson EE. Mechanisms of leukocyte recruitment to atherosclerotic lesions: future prospects. Curr Opin Lipidol. 2004;15:553–558. doi: 10.1097/00041433-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M, Neumann FJ, Dickfeld T, Koch W, Laugwitz KL, Adelsberger H, et al. Activated platelets induce monocyte chemotactic protein-1 secretion and surface expression of intercellular adhesion molecule-1 on endothelial cells. Circulation. 1998;98:1164–1171. doi: 10.1161/01.cir.98.12.1164. [DOI] [PubMed] [Google Scholar]
- Gear AR, Suttitanamongkol S, Viisoreanu D, Polanowska-Grabowska RK, Raha S, Camerini D. Adenosine diphosphate strongly potentiates the ability of the chemokines MDC, TARC, and SDF-1 to stimulate platelet function. Blood. 2001;97:937–945. doi: 10.1182/blood.v97.4.937. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Henness S, Robinson DM, Lyseng-Williamson KA. Rimonabant. Drugs. 2006;66:2109–2119. doi: 10.2165/00003495-200666160-00006. [DOI] [PubMed] [Google Scholar]
- Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007;72:1345–1357. doi: 10.1038/sj.ki.5002540. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DM, Muccioli GG. Endocannabinoids and related N-acylethanolamines in the control of appetite and energy metabolism: emergence of new molecular players. Curr Opin Clin Nutr Metab Care. 2007;10:735–744. doi: 10.1097/MCO.0b013e3282f00061. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Grüner S, Konrad I, Garcia Arguinzonis MI, Eigenthaler M, Hemler K, et al. Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood. 2004;103:136–142. doi: 10.1182/blood-2002-11-3417. [DOI] [PubMed] [Google Scholar]
- Massberg S, Vigt F, Dickfeld T, Brand K, Page S, Gawaz M. Activated platelets trigger an inflammatory response and enhance migration of aortic smooth muscle cells. Thromb Res. 2003;110:187–194. doi: 10.1016/s0049-3848(03)00342-6. [DOI] [PubMed] [Google Scholar]
- Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJV. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew–Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O′Connor SE, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007;293:H2210–H2218. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HE, Harris MI, Brock DB, Harris TB. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2000;23:176–180. doi: 10.2337/diacare.23.2.176. [DOI] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden L, Standl E, Bartnik M, Van den BG, Betteridge J, de Boer MJ, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Alp NJ, Cai S, Lygate CA, Neubauer S, Eigenthaler M, et al. Reduced vascular NO bioavailability in diabetes increases platelet activation in vivo. Arterioscler Thromb Vasc Biol. 2004a;24:1720–1726. doi: 10.1161/01.ATV.0000138072.76902.dd. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Bauersachs J. Endothelial dysfunction, impaired endogenous platelet inhibition and platelet activation in diabetes and atherosclerosis. Curr Vasc Pharmacol. 2008;6:52–60. doi: 10.2174/157016108783331295. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Flierl U, Kobsar A, Eigenthaler M, Ertl G, Bauersachs J. Soluble guanylyl cyclase activation with HMR 1766 attenuates platelet activation in diabetic rats. Arterioscler Thromb Vasc Biol. 2006;26:2813–2818. doi: 10.1161/01.ATV.0000249407.92147.12. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Fraccarollo D, Hildemann S, Christ M, Eigenthaler M, Kobsar A, et al. Inhibition of platelet activation in congestive heart failure by selective aldosterone receptor antagonism and ACE inhibition: role of endothelial function and platelet VASP phosphorylation. Thromb Haemost. 2003a;89:1024–1030. [PubMed] [Google Scholar]
- Schäfer A, Fraccarollo D, Vogt C, Flierl U, Hemberger M, Tas P, et al. Improved endothelial function and reduced platelet activation by chronic HMG-CoA-reductase inhibition with rosuvastatin in rats with streptozotocin-induced diabetes mellitus. Biochem Pharmacol. 2007;73:1367–1375. doi: 10.1016/j.bcp.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Schulz C, Eigenthaler M, Fraccarollo D, Kobsar A, Gawaz M, et al. Novel role of the membrane bound chemokine fractalkine in platelet activation and adhesion. Blood. 2004b;103:407–412. doi: 10.1182/blood-2002-10-3260. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Vollkommer T, Burkhardt M, Bauersachs J, Münzel T, Walter U, et al. Endothelium-dependent and -independent relaxation and VASP serines 157/239 phosphorylation by cyclic nucleotide-elevating vasodilators in rat aorta. Biochem Pharmacol. 2003b;65:397–405. doi: 10.1016/s0006-2952(02)01523-x. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Widder J, Eigenthaler M, Bischoff H, Ertl G, Bauersachs J. Increased platelet activation in young Zucker rats with impaired glucose tolerance is improved by acarbose. Thromb Haemost. 2004c;92:97–103. doi: 10.1160/TH04-02-0118. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Wiesmann F, Neubauer S, Eigenthaler M, Bauersachs J, Channon KM. Rapid regulation of platelet activation in vivo by nitric oxide. Circulation. 2004d;109:1819–1822. doi: 10.1161/01.CIR.0000126837.88743.DD. [DOI] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- Schulz C, Schäfer A, Stolla M, Kerstan S, Lorenz M, von Brühl M-L, et al. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood, a critical role for P-selectin expressed on activated platelets. Circulation. 2007;116:764–773. doi: 10.1161/CIRCULATIONAHA.107.695189. [DOI] [PubMed] [Google Scholar]
- Sheikine YA, Hansson GK. Chemokines as potential therapeutic targets in atherosclerosis. Curr Drug Targets. 2006;7:13–27. doi: 10.2174/138945006775270240. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. New Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- Tschoepe D, Menart-Houtermans B.Diabetes mellitus Platelets 2002Academic Press: San Diego; 435–445.In: Michelson AD (ed) [Google Scholar]
- van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005;96:612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]