Abstract
Background and purpose:
Inhibition of squalene synthesis could transform unstable, macrophage/lipid-rich coronary plaques into stable, fibromuscular plaques. We have here treated WHHLMI rabbits, a model for coronary atherosclerosis and myocardial infarction, with a novel squalene synthase inhibitor, lapaquistat acetate (TAK-475).
Experimental approach:
Young male WHHLMI rabbits were fed a diet supplemented with lapaquistat acetate (100 or 200 mg per kg body weight per day) for 32 weeks. Serum lipid levels were monitored every 4 weeks. After the treatment, lipoprotein lipid and coenzyme Q10 levels were assayed, and coronary atherosclerosis and xanthomas were examined histopathologically or immunohistochemically. From histopathological and immunohistochemical sections, the composition of the plaque was analysed quantitatively with computer-assisted image analysis. Xanthoma was evaluated grossly.
Key results:
Lapaquistat acetate decreased plasma cholesterol and triglyceride levels, by lowering lipoproteins containing apoB100. Development of atherosclerosis and xanthomatosis was suppressed. Accumulation of oxidized lipoproteins, macrophages and extracellular lipid was decreased in coronary plaques of treated animals. Treatment with lapaquistat acetate increased collagen concentration and transformed coronary plaques into fibromuscular plaques. Lapaquistat acetate also suppressed the expression of matrix metalloproteinase-1 and plasminogen activator inhibitor-1 in the plaque and increased peripheral coenzyme Q10 levels. Increased coenzyme Q10 levels and decreased very low-density lipoprotein cholesterol levels were correlated with improvement of coronary plaque composition.
Conclusion and implications:
Inhibition of squalene synthase by lapaquistat acetate delayed progression of coronary atherosclerosis and changed coronary atheromatous plaques from unstable, macrophage/lipid accumulation-rich, lesions to stable fibromuscular lesions.
Keywords: coenzyme Q10, coronary atherosclerosis, hypolipidemic effects, lapaquistat acetate, macrophages, matrix metalloproteinase, oxidized LDL, squalene synthase inhibitor, WHHLMI rabbits
Introduction
Cardiac events are the major cause of death in developed countries, and every year, more than 20 million people around the world experience a cardiac event (Naghaviet al., 2003). Many of these people take inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) (Blum et al., 2004), a rate-limiting enzyme for cholesterol biosynthesis. Statin trials have showed suppression of coronary events in about 35% or fewer patients with cardiovascular disease (Sever et al., 2003). To treat the remaining 65% of patients in this group, development of additional treatments is required. In the pathway of cholesterol biosynthesis, squalene synthase is a rate-limiting enzyme that operates distal to 3-hydroxy-3-methylglutaryl Co A reductase. Therefore, inhibitors of squalene synthase (SSI) could provide another option for treating hypercholesterolaemia (Hiyoshi et al., 2000; Amano et al., 2003; Nishimoto et al., 2003) and atherosclerosis (Tavridou et al., 2007). Although the hypolipidemic effects and anti-atherosclerotic effects of SSI were similar to those of statins, the effects of SSI on coronary atherosclerosis and the plaque composition have not been examined.
In the pathway of cholesterol biosynthesis, statins inhibit mevalonate synthesis at the early stage, and SSIs inhibit squalene synthesis from farnesyl pyrophosphate at a later stage. Therefore, statins reduce mevalonate pathway intermediates but SSIs increase those intermediates (Bergstrom et al., 1993; Hiyoshi et al., 2003). Studies in vitro have shown that decreased mevalonate pathway intermediates induced by statins correlate with their anti-atherosclerotic effects (Libby and Aikawa, 2003). Thus, increasing mevalonate pathway intermediates might induce atherogenesis or the destabilization of atheromatous plaques, so it is important to determine the effects of SSIs on atherosclerotic lesions.
To examine this issue, we administered an SSI, lapaquistat acetate (TAK-475) (Miki et al., 2002), to WHHLMI rabbits, a strain prone to coronary atherosclerosis and myocardial infarction (Shiomi et al., 2003). The WHHLMI rabbit is derived from the WHHL rabbit (Watanabe, 1980), an low-density lipoprotein (LDL) receptor-deficient animal model (Goldstein et al., 1983), and showed spontaneous hypercholesterolaemia due to increased plasma LDL, spontaneous coronary atherosclerosis and myocardial infarction. These features including the lipoprotein profile and histopathological findings resemble those found in human hypercholesterolaemia (Shiomi et al., 2004). Therefore, this animal model is useful for examining potential hypolipidemic and anti-atherosclerotic agents. This is the first study designed to determine whether SSI can prevent coronary atherosclerosis and suppress the destabilization of coronary atheromatous plaques.
Methods
Animals
This study was approved by the Committee on Animal Experimentation, Kobe University School of Medicine (permission number P-011209) and was carried out following the Guidelines for Animal Experimentation at Kobe University. Male WHHLMI rabbits, aged 2 months, were divided into control (n=11), low-dose (100 mg kg−1, n=11) and high-dose (200 mg kg−1, n=11) lapaquistat treatment groups. We selected doses of lapaquistat acetate (supplied by Takeda Pharmaceutical Company Limited (Osaka, Japan) based on previous animal studies using statins (Shiomi et al., 1995, 2001, 2005) and TAK-475 (Amano et al., 2003; Nishimoto et al., 2003). Previous statin studies suggested that we had to decrease serum cholesterol levels by about 20% or more to suppress atherosclerotic lesions of WHHL rabbits. Rabbits were given standard rabbit chow (CR3, Japan CLEA Inc., Tokyo, Japan) or chow supplemented with lapaquistat acetate for 32 weeks. Rabbits were housed individually in metal cages in a room maintained at constant temperature (20–24 °C). The room illumination was set at 12 h light and dark cycle.
Biochemical analysis
Serum total cholesterol and triglyceride levels were measured enzymatically every 4 weeks during the treatment and the area under the concentration curve (AUC) was calculated. Plasma lipoproteins were fractionated by ultracentrifugation (very low-density lipoprotein, VLDL, d<1.006 g mL−1; LDL, 1.006 g mL−1<d<1.063 g mL−1, high-density lipoprotein, HDL, d>1.063 g mL−1). Coenzyme Q10 (CoQ10) was extracted according to the method of Hiroshima and Shino (1993) and analysed with liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS). Plasma concentrations of lapaquistat acetate and active metabolites (Nishimoto et al., 2003) were determined with LC/MS/MS using plasma obtained 20 h after lapaquistat acetate administration.
Preparation of histological sections
At the end of the treatment, rabbits were anaesthetized by intravenous injection of ketamine hydrochloride (11.5 mg kg−1) plus xylazine hydrochloride salt (3.5 mg kg−1) and perfused with cold saline solution. After perfusion, the aorta was excised, and the coronary arteries were perfused with 10% buffered formalin solution or periodate–lysine–paraformaldehyde fixative. The aorta was immediately cut open longitudinally and photographed macroscopically with a digital camera to measure the surface areas of gross lesions. Thereafter, the aorta was cut beneath the origin of the second and eighth intercostal arteries, and the origin of the celiac artery and inferior mesenteric artery. Each segment was immersion-fixed with the fixatives described above, and embedded in paraffin. Sections, 4 μm thick, were cut serially from each segment and stained with elastic van Gieson stain. As the left circumflex artery is a major coronary artery in rabbits and atherosclerotic lesions are prevalent in this artery in WHHLMI rabbits (Shiomi et al., 1994; Ito et al., 2004), coronary atherosclerosis was evaluated using this tissue. Twelve coronary sections were prepared at 500 μm intervals. Coronary sections of each segment were stained with elastic van Gieson stain, Azan–Mallory stain and picrosirius red stain. In the sections showing the greatest cross-sectional narrowing (CSN, lesion area/area circumscribed by internal elastic lamina × 100) of each rabbit, immunohistochemical staining was carried out. Serial coronary sections were stained for macrophages (RAM-11; Dako A/S, Glastrup, Denmark), smooth muscle cell (1A4; Dako A/S), matrix metalloproteinase (MMP)-1 (F-67; Daiichi Fine Chemical Co. Ltd., Toyama, Japan), plasminogen activator inhibitor (PAI)-1 (American Diagnostica Inc., Stamford, CT, USA), oxidized lipoprotein (FOH1a/DLH3, generously donated by Itabe et al., 1994) and proliferating cell nuclei (Ki-67; Dako A/S). Staining of sections incubated with mouse IgG2a (for MMP-1) and IgM (for DLH3) instead of specific antibodies was negligible (data not shown). Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling staining was carried out by using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore Corp., Bellerica, MA, USA).
Evaluation of atheromatous plaques and xanthomas at the digital joints
All parameters for atherosclerotic lesions were measured by computer-assisted colour image analysis (Image-Pro Plus, version 4.5, Media Cybernetics Inc., Silver Spring, MD, USA). Aortic atherosclerosis was evaluated using the percent surface lesion area on the whole aorta (surface area of lesion/surface area of the whole intima) and average intimal thickness (intimal area/internal elastic lamina length) (Shiomi et al., 1990). The degree of coronary atheroma was evaluated as the distribution of all sections from 12 proximal segments of the coronary artery as graded every 10 or 25% CSN (Shiomi et al., 2001). The frequency of vulnerable plaques was evaluated by two independent pathologists from the cross-sections showing the greatest CSN of coronary arteries, using the classification of ‘vulnerable' plaques proposed by Naghavi et al. (2003). Arterial sections were graded as follows: one point for ‘+/−' and two points for ‘+' for each classification category of vulnerable plaque and summed for each artery. Rupture-prone plaques (lipid core including macrophages covered with thin fibrous cap) were graded on the basis of percent area of macrophage and lipid accumulation in the plaque, and areas between 10 and 25% were graded as ‘+/−' and plaques with greater than 25% lipid core were graded as ‘+'; Erosion-prone plaques were those showing denudation of endothelial cells without accompanying adherent platelets graded as ‘+/−', and those showing endothelial cell denudation with adherent platelets were graded as ‘+'. Plaques with calcium accumulation showing area of calcified nodules between 5 and 10% were graded ‘+/−' and plaques with calcified nodules showing greater than 10% in the plaque area were graded ‘+'. The scores of two independent observers were summed. Xanthomas were evaluated at the digital joints of forelegs and hind legs following the method reported previously (Shiomi et al., 1990).
Quantitative analyses of composition of coronary atheromatous plaques
Lesional components were analysed for sections showing the greatest CSN in each coronary artery. Lesion area and area of each lesional component were measured with Image-Pro Plus and the percent area of each lesional component was calculated (Shiomi et al., 1994, 1995, 2001, 2005). To evaluate plaque morphological changes, we calculated the plaque vulnerability index (degree of macrophage/lipid content against fibromuscular component in the plaque) by dividing the sum of the areas for macrophages and extracellular lipid deposits by the sum of the areas for smooth muscle cell and collagen fibres (Suzuki et al., 2003; Shiomi et al., 2001, 2005) based on the concept of Davies (1996).
Statistical analysis
Values were expressed as means±s.e.mean. Statistical analyses were carried out with a two-tailed non-parametric Shirley–Williams test or two-tailed parametric Williams test (comparison with the control group) for serum lipid levels or atherosclerotic lesions, and with the Cochran–Mantel–Haenszel test for frequency. These statistical tests are suitable for data derived from dose-dependent study (Williams, 1986). Correlation analysis was carried out with Pearson's correlation analysis. Significance was set at P<0.05.
Results
Plasma drug concentration and changes in levels of biochemical parameters
One rabbit of each control and high-dose group were excluded from the experiments because of the formation of hair balls in the stomach. There were no significant differences in body weight increase among each group and none of the rabbits showed abnormal findings related to treatment. At the end of treatment, lapaquistat acetate was not detected in the plasma obtained 20 h after administration but the levels of the active metabolite (Nishimoto et al., 2003) were 49.6±13.9 nM in the low-dose group and 129±31.8 nM in the high-dose group.
At the start of the examination, there were no differences in serum total cholesterol and triglyceride levels among the groups (data not shown). As shown in Table 1, compared with the control group, AUC of serum total cholesterol decreased dose-dependently by 17.8% (P=0.006) in the low-dose group and by 30.3% (P<0.001) in the high-dose group; AUC of triglyceride also decreased by 28.0% (P=0.024) and 36.8% (P<0.001), respectively. Reductions in serum lipid levels were due to decreases in lipoproteins containing apoB100 (VLDL+LDL). Compared with the control group, the liver CoQ10 levels increased 1.6-fold in the low-dose group and 1.9-fold in the high-dose group; those in plasma increased 1.3-fold in the low- and high-dose groups; and those in soleus muscle increased 1.4-fold in the low- and high-dose groups.
Table 1.
Effects of lapaquistat acetate on lipid levels and CoQ10 concentrations
|
Dose of lapaquistat acetate (mg per kg body weight) |
|||
|---|---|---|---|
| 0 (n=10) | 100 (n=11) | 200 (n=10) | |
| Total cholesterol (mM) | |||
| VLDL | 1.32±0.13 | 0.94±0.09 (P=0.007) | 0.64±0.05 (P<0.001) |
| LDL | 19.6±0.9 | 18.5±1.4 (NS) | 16.8±0.8 (NS) |
| HDL | 0.32±0.02 | 0.60±0.27 (NS) | 0.39±0.04 (NS) |
| AUC of serum cholesterol (mM × week) | |||
| 1031±44 | 847±52 (P=0.006) | 718±31 (P<0.001) | |
| Triglyceride (mM) | |||
| VLDL | 0.34±0.05 | 0.37±0.07 (NS) | 0.33±0.03 (NS) |
| LDL | 1.95±0.12 | 1.60±0.14 (P=0.050) | 1.44±0.10 (P=0.008) |
| HDL | 0.16±0.01 | 0.22±0.02 (P=0.008) | 0.21±0.01 (P=0.013) |
| AUC of serum triglyceride (mM× week) | |||
| 84.3±7.4 | 60.7±4.0 (P=0.003) | 53.3±2.9 (P<0.001) | |
| CoQ10 | |||
| Liver (μg g−1) | 68.4±2.3 | 110±6 (P<0.001) | 130±5 (P<0.001) |
| Plasma (μg mL−1) | 1.09±0.07 | 1.37±0.11 (P=0.040) | 1.39±0.10 (P=0.037) |
| Soleus (μg g−1) | 23.4±1.1 | 32.3±1.7 (P<0.001) | 31.6±1.2 (P<0.001) |
Abbreviations: AUC, area under the concentration curve; CoQ10, coenzyme Q10; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS, not significant; VLDL, very low-density lipoprotein.
At the end of the treatment, lipoprotein was fractionated and CoQ10 was assayed. Values were expressed as means±s.e.mean. Statistical analyses were carried out by two-tailed non-parametric Shirley–Williams test or two-tailed parametric Williams test (compared with the control group).
Prevention of atherosclerosis and xanthoma
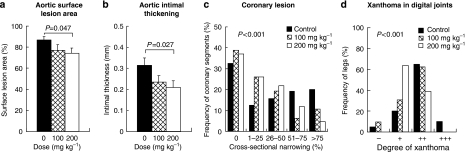
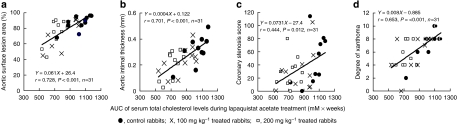
Development of aortic atherosclerosis decreased dose-dependently. The surface lesion area (Figure 1a) and intimal thickness (Figure 1b) of the high-dose group were 15.1% (P=0.047) and 34.2% (P=0.027) lower than those of the control group. Based on analysis of the distribution of coronary CSN (Figure 1c), development of coronary atherosclerosis was suppressed (P<0.001) dose-dependently. The frequency of coronary sections showing greater than 75% CSN was 4.2% in the high-dose group. This was 79% lower than that of the control group (20%). Development of xanthoma (Figure 1d) was suppressed dose-dependently (P<0.001). The frequency of xanthoma showing grades ‘+' and ‘++' was 37.5% in the high-dose group, which was 50% of the control frequency. In addition, correlation analyses showed that AUC of serum total cholesterol levels significantly related to the degree of aortic atherosclerosis, coronary stenosis and xanthoma (Figure 2).
Figure 1.
Effects of lapaquistat acetate on aortic ((a) percent surface lesion area in the whole aorta; (b) average intimal thickening), and coronary atherosclerosis (c) and xanthoma at the digital joints (d) of WHHLMI rabbits. Data for each group were derived from 10 rabbits each in control and high-dose groups, and 11 rabbits in the low-dose group. Statistical analyses were carried out by two-tailed non-parametric Shirley–Williams test or two-tailed parametric Williams test for average and by Cochran–Mantel–Haenszel test for frequency.
Figure 2.
Correlation between AUC of serum total cholesterol levels during treatments and aortic surface lesion area (a), average intimal thickening of aortic atherosclerosis (b), coronary stenosis score of proximal 12 segments (c) and degree of xanthoma (d). Evaluation of atherosclerosis was described in the Methods section. Degree of xanthoma was evaluated as sum of points at four legs (one point, slight lesions (+); two points, mild lesions (++); and three points, severe lesions (+++)). AUC, area under concentration curve.
Changes in composition of coronary atheromatous plaques
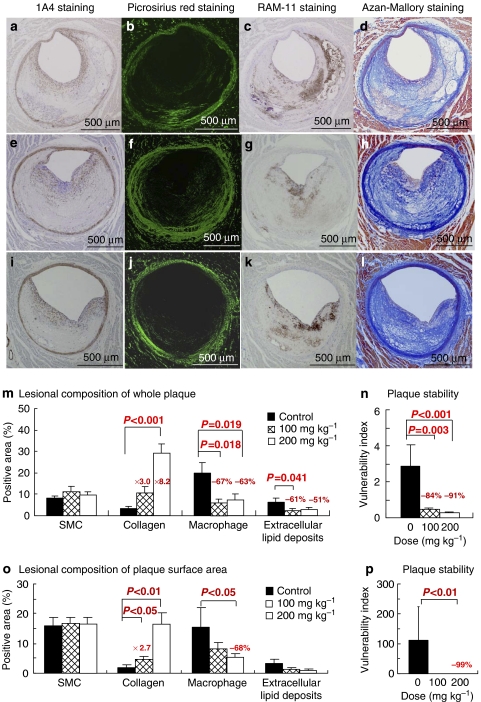
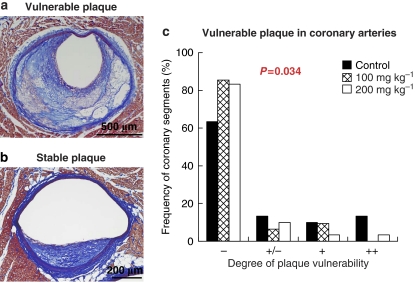
Atherosclerotic lesions did not develop in one rabbit in the control group, and one rabbit in the low-dose group had a lesion in the left circumflex artery that was too small to analyse. Therefore, we examined coronary plaques in 9 rabbits in the control group and 10 rabbits each in the low- and high-dose groups. Figure 3 shows coronary sections stained histopathologically and immunohistochemically (Figures 3a–l) and the results of quantitative analysis of the lesional components of whole plaque (Figures 3m and n) and plaque surface area that was in the region of 100 μm distance from the plaque surface (Figures 3o and p). In coronary whole plaques (Figure 3m), although there were no differences in the smooth muscle cell concentration among groups, the collagen concentration was increased dose-dependently after treatment with lapaquistat (28.9±4.6% in the high-dose group versus 3.5±0.7% in the control group, P<0.001). Compared with the control group, the macrophage concentration was decreased by 67% (P=0.018) in the low-dose group and 63% (P=0.019) in the high-dose group, and the concentration of extracellular lipid deposits decreased by 60% (P=0.041) and 51% (P=0.061), respectively. The vulnerability index decreased by 83.6% in the low-dose group (P=0.003) and by 90.5% in the high-dose group (P<0.001) (Figure 3n). The effects of lapaquistat acetate on the plaque surface area (Figures 3o and p) were similar to the effects on whole plaque. We also analysed coronary plaques by histopathological observations based on the definitions of Naghavi et al. (2003). Figure 4a indicates a representative ‘vulnerable' plaque showing a partially thinned fibrous cap with the accumulation of large numbers of macrophages and lipid clusters. In contrast, a representative ‘stable' plaque was fibromuscular (Figure 4b). The frequency of coronary segments corresponding to vulnerable plaques in humans (as proposed by Naghavi et al., 2003) was decreased dose-dependently (Figure 4c). ‘Vulnerable' coronary sections showing more than one point comprised 60% in the control group, 20% in the low-dose group and 10% in the high-dose group.
Figure 3.
Coronary atheromatous plaques of WHHLMI rabbits stained with a monoclonal antibody 1A4 for smooth muscle cell actin (a, e and i), picrosirius red polarization through 540 nm filter for collagen fibres (b, f and j), a monoclonal antibody RAM-11 for macrophages (c, g and k) Azan–Mallory staining (d, h and l), the composition of whole plaque (m), plaque surface area (n) and plaque vulnerability index (o and p). The upper panel photomicrographs (a–d) are coronary sections of a rabbit in the control group; the middle panel (e–h) is coronary sections of a rabbits in the low-dose group; and in the lower panel (i–l) are coronary sections of a rabbit in the high-dose group. The vulnerability index was calculated by dividing the area of macrophages and extracellular lipid deposits by the area of fibromuscular components (Shiomi et al., 2001). Data for each group were derived from 9 rabbits in the control group and 10 rabbits in each treated group. Statistical analyses were carried out by two-tailed non-parametric Shirley–Williams test.
Figure 4.
(a) Representative unstable (rabbits n=11 of the control group) and (b) stable (rabbit n=8 of the high-dose group) coronary plaques, and (c) the frequency of vulnerable plaques in WHHLMI rabbits treated with lapaquistat acetate. Histopathological sections were stained with Azan–Mallory staining. The frequency of vulnerable plaques was evaluated by two independent pathologists using cross-section showing the greatest cross-sectional narrowing (CSN) of coronary arteries. Based on the classification of vulnerable plaques proposed by Naghavi et al. (2003), arterial sections were graded as follows, one point for ‘+/−' and two points for ‘+' for each classification category of vulnerable plaque and summed for each artery. The scores of two independent observers were summed. Data for each group were derived from 9 rabbits in the control group and 10 rabbits in each treated group. Statistical analyses were carried out by Cochran–Mantel–Haenszel test for distribution.
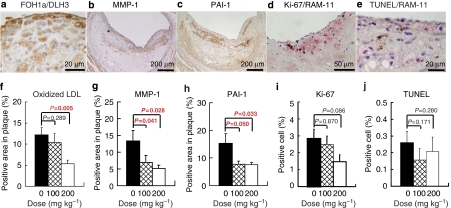
Accumulation of oxidized VLDL/LDL, expression of MMP-1 and PAI-1, and proliferation or apoptosis of arterial cells
Most of oxidized lipoprotein-positive areas (Figure 5a), MMP-1-positive cells (Figure 5b), PAI-1-positive areas (Figure 5c), Ki-67-positive cells (proliferating cells, Figure 5d) and TUNEL-positive cells (Figure 5e) were observed in the plaque surface area. Dose-dependent decreases were observed in the concentration of oxidized lipoprotein (Figure 5f), MMP-1 (Figure 5g) and PAI-1 (Figure 5g). In double staining for Ki-67 and RAM-11 or for TUNEL and RAM-11, almost all Ki-67-positive cells or TUNEL-positive cells were macrophages, and there were no significant differences in the frequency of positive cells among groups (Figure 5i and j).
Figure 5.
Immunohistochemical staining for oxidized lipoprotein (a), matrix metalloproteinase (MMP)-1 (b); plasminogen activator inhibitor (PAI)-1 (c); proliferating cells nuclei ((d) double staining of Ki-67 and RAM-11); TUNEL-positive cells ((e) double staining of TUNEL and RAM-11); the percent area for oxidized lipoprotein (f), MMP-1 (g) and PAI-1 (h); the percent of positive cells showing proliferation (i) and apoptosis (j) of atheromatous plaque of the left circumflex arteries of WHHLMI rabbits treated with lapaquistat acetate. Data for each group were derived from 9 rabbits in the control group and 10 rabbits in each treated group. Statistical analyses were carried out by two-tailed non-parametric Shirley–Williams test.
Correlation analyses between cholesterol levels or CoQ10 levels and coronary atherosclerosis
In correlation analyses, data from rabbits used in analyses of coronary composition were analysed. Table 2 shows the results of Pearson's correlation analysis. AUC of serum cholesterol levels showed positive correlation with the degree of coronary atherosclerosis and negative correlation with collagen content. VLDL cholesterol showed a positive correlation with the concentration of oxidized lipoproteins, macrophages and extracellular lipid deposits, and negative correlation with collagen fibres. However, LDL cholesterol and HDL cholesterol did not relate to coronary atherosclerosis. Surprisingly, soleus CoQ10 levels showed a negative correlation with not only the degree of coronary atherosclerosis but also percent area of oxidized LDL, macrophages and extracellular lipid deposits in the plaque, and positive correlation with smooth muscle cell concentration, although plasma CoQ10 was only correlated with the accumulation of oxidized lipoproteins in the plaque.
Table 2.
Pearson's correlation coefficients between cholesterol levels or CoQ10 levels and coronary atherosclerosis
| VLDL cholesterol | AUC of serum |
CoQ10 |
||
|---|---|---|---|---|
| total cholesterol | Plasma | Soleus | ||
| Degree of coronary CSN | ||||
| Maximum CSN | 0.186 | 0.468 (P=0.010) | −0.073 | −0.489 (P=0.007) |
| CSN score | 0.253 | 0.418 (P=0.024) | −0.035 | −0.457 (P=0.013) |
| Lesional components of coronary atherosclerosis | ||||
| Oxidized lipoproteins | 0.372 (P=0.047) | 0.173 | −0.534 (P=0.003) | −0.453 (P=0.014) |
| Macrophages | 0.402 (P=0.030) | 0.346 | −0.261 | −0.457 (P=0.013) |
| Extracellular lipids | 0.372 (P=0.047) | 0.346 | −0.202 | −0.519 (P=0.004) |
| SMC | −0.254 | −0.280 | 0.119 | 0.520 (P=0.004) |
| Collagen fibers | −0.467 (P=0.011) | −0.407 (P=0.028) | 0.257 | 0.171 |
| Coronary vulnerability index | 0.450 (P=0.014) | 0.304 | −0.276 | −0.428 (P=0.021) |
Abbreviations: AUC, area under the concentration curve; CoQ10, coenzyme Q10; CSN, cross-sectional narrowing; SMC, smooth muscle cell; VLDL, very low-density lipoprotein.
Fractionation of lipoproteins and assay of CoQ10 were carried out at the end of the treatment.
Data from 9 rabbits in the control group, 10 rabbits in the low- and high-dose groups were analysed.
Discussion and conclusions
In this study, lapaquistat acetate transformed coronary atheromatous plaques from macrophage/lipid accumulation-rich lesions (unstable lesions) into fibromuscular lesions (stable lesion) in addition to delaying the atherosclerosis and xanthoma. Davies (1996) has characterized ‘dangerous' plaques in humans as those rich in the accumulation of macrophages and extracellular lipids, and poor in fibromuscular components. In this study, coronary plaques were evaluated quantitatively (Figure 3) and qualitatively (Figure 4) based on the classification of human vulnerable atheromatous plaques by Naghavi et al. (2003). Both analyses showed that lapaquistat acetate lowered the accumulation of macrophages and extracellular lipids and increased the fibrous components of coronary whole plaques and the plaque surface area, which corresponded to the fibrous cap region. Therefore, the present findings suggest that coronary plaques were changed beneficially to a more stable form of lesion by lapaquistat treatment.
In this study, lapaquistat acetate decreased serum lipid levels and increased CoQ10 levels in the plasma and tissues (Table 1), and the AUC of serum cholesterol levels during treatment showed significant correlation to the degree of atherosclerosis and xanthoma (Figure 2). The effects of lapaquistat acetate on lipoprotein lipid levels in this study were similar to the previous study using WHHL rabbits (Amano et al., 2003). In addition, VLDL cholesterol showed significant correlation with coronary atherosclerosis (Table 2). In previous studies, cholesterol-rich VLDL related to the coronary atherosclerosis of WHHL rabbits (Shiomi et al., 1992) and stimulated lipid accumulation in macrophages in culture (Kita et al., 1986). Recent clinical studies and in vitro studies showed that remnant lipoproteins including VLDL are highly atherogenic, compared with oxidized LDL (Nakajima et al., 2006). Furthermore, immunoreactive apoB100-containing lipoprotein particles in human atherosclerotic lesions were present in significant amount of VLDL and intermediate-density lipoprotein fraction (Rapp et al., 1994). These studies suggest that VLDL is also atherogenic in humans and WHHL/WHHLMI rabbits and that the reduction of VLDL cholesterol is also important to transform unstable, macrophage/lipid-rich plaques into stable, fibromuscular plaques. In this study, LDL and HDL did not show significant correlation with atherosclerosis. These results may be due to small differences in HDL cholesterol levels among groups, large variances of LDL cholesterol levels in each group and/or small data populations. In addition, LDL cholesterol levels of the treated rabbits may be higher than the threshold of LDL cholesterol level to develop or increase coronary atherosclerosis.
From the analysis of plaque composition, lapaquistat decreased the accumulation of oxidized lipoproteins, macrophages and extracellular lipids, and suppressed expression of MMP-1 while increasing collagen content in the plaques. As in vitro studies have shown that oxidized lipoproteins stimulate transformation of macrophages into foam cells and expression of MMPs in macrophages (Adans et al., 2002; Libby and Aikawa, 2003), one of the reasons for a decrease in macrophages and extracellular lipids and depression of MMP-1 expression in plaques of treated groups was probably due to a reduction of oxidized lipoproteins. As MMP-1 hydrolyzes interstitial collagen (Libby and Aikawa, 2003), suppression of MMP-1 expression in treated plaques probably contributed to the increase in the collagen content. These changes could explain the morphological changes from macrophage/lipid-rich plaques to fibromuscular plaques by lapaquistat acetate.
In addition, lapaquistat acetate increased CoQ10 levels in the plasma and in a peripheral tissue (Table 1), and CoQ10 levels related to a decrease in oxidized lipoproteins accumulation, macrophage content and extracellular lipid accumulation in the coronary plaques (Table 2). Because CoQ10 is an antioxidant (James et al., 2004), increased CoQ10 in peripheral tissues may be important to suppress atherosclerosis. Tavridou and Manolopoulos (2004) and Tavridou et al. (2007) reported that another SSI, EP2306, also showed antioxidative function and inhibitory effects on atherosclerotic lesions developed in cholesterol-fed rabbits. As CoQ10 is located in lipoproteins in the plasma (Mohr et al., 1992) and VLDL receptors are expressed on macrophages in the plaque of WHHL rabbits and humans (Nakazato et al., 1996), plasma CoQ10 may directly affect the atherosclerotic lesions. In our experiments, plasma CoQ10 levels showed a negative correlation with oxidized lipoprotein accumulation in the plaque (Table 2). However, detailed examinations are needed to elucidate fully the effects of CoQ10 on atherogenesis.
As Libby and Aikawa (2003) pointed out, increasing mevalonate pathway intermediates might lead to atherogenesis or destabilization of atheromatous plaques through the activation of the Rho family and proliferation of arterial cells, apoptosis and PAI-1 synthesis. These intermediates were increased in culture by SSIs (Bergstrom et al., 1993; Hiyoshi et al., 2003), and it was therefore important to determine how SSIs affect the composition of atherosclerotic lesions in vivo. In this study, however, lapaquistat acetate did not affect proliferation of arterial cells, decreased oxidative stress and PAI-1 expression (Figures 3 and 5), although synthesis of CoQ10 levels increased (Table 1). Therefore, there was no correlation of increasing isoprenoids with increasing atherogenesis in the present in vivo study. Additional examination will be required to elucidate relation of mevalonate pathway intermediates to atherogenesis.
In addition, the hypocholesterolemic effect and anti-atherosclerotic effects of lapaquistat acetate were equivalent to the previous statin studies using WHHL rabbits (Shiomi et al., 1995, 2001, 2005; Fukumoto et al., 2001; Suzuki et al., 2003). This suggests that the reduction of serum cholesterol levels is important in suppression of atherosclerosis. Effects of lapaquistat acetate that were additional to those of the statins include a decrease in serum triglyceride levels and an increase in CoQ10 levels (Table 1). In addition, although one of the important side effects of statins in humans is myopathy, SSI suppressed statin-induced myotoxicity in guinea pig (Nishimoto et al., 2007). These observations strongly suggest that SSI could provide an additional choice or combination treatment in patients with hypercholesterolaemia, metabolic syndrome and coronary heart diseases.
In conclusion, this study provides evidence that inhibition of squalene synthase by lapaquistat acetate resulted in the beneficial alteration of macrophage/lipid-rich plaques (unstable plaques) to fibromuscular plaques (stable plaques). These compositional changes in coronary plaques were probably due to a reduction of oxidized lipoprotein accumulation in the plaque through decreasing serum lipid levels and increasing CoQ10 levels.
Acknowledgments
We thank Dr H Itabe (Showa University School of Pharmaceutical Science, Tokyo, Japan) for providing an antioxidized lipoprotein antibody (FOH1a/DLH3), T Tamura (Kobe University School of Medicine, Kobe, Japan) for technical assistance and R Tozawa (Takeda Pharmaceutical Company Limited, Osaka, Japan) for useful discussion. We thank Takeda Pharmaceutical Company for a Research Grant.
Abbreviations
- AUC
area under the concentration curve
- CoQ10
coenzyme Q10
- CSN
cross-sectional narrowing
- HDL
high-density lipoprotein
- LCX
left circumflex artery
- LDL
low-density lipoprotein
- MMP
matrix metalloproteinase
- PAI
plasminogen activator inhibitor
- SSI
squalene synthase inhibitors
- VLDL
very low-density lipoprotein
Conflict of interest
Y Amano and T Nishimoto are employees of Takeda Pharmaceutical Co. Ltd, suppliers of lapaquistat. The remaining authors state no conflict of interest.
References
- Adans JA, Economou AP, Martinson JM, Jr, Zhou M, Wahl LM. Oxidized low-density and high-density lipoproteins regulate the production of matrix metalloproteinase-1 and -9 by activated monocytes. J Leukoc Biol. 2002;71:1012–1018. [PubMed] [Google Scholar]
- Amano Y, Nishimoto T, Tozawa R, Ishikawa E, Imura Y, Sugiyama Y. Lipid-lowering effects of TAK-475, a squalene synthase inhibitor, in animal models of familial hypercholesterolemia. Eur J Pharmacol. 2003;466:155–161. doi: 10.1016/s0014-2999(03)01549-8. [DOI] [PubMed] [Google Scholar]
- Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bostedor RG, et al. Zaragozic acids: a family of fungal metabolites that are picomolar competitive inhibitors of squalene synthase. Proc Natl Acad Sci USA. 1993;90:80–84. doi: 10.1073/pnas.90.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Simsolo C, Hasin Y. 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins), atherosclerosis and coronary syndromes. Atherosclerosis. 2004;175:1–5. doi: 10.1016/j.atherosclerosis.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Davies MJ. Stability and instability: two faces of coronary atherosclerosis. Circulation. 1996;94:2013–2020. doi: 10.1161/01.cir.94.8.2013. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Libby P, Rabkin E, Hill CC, Enomoto M, Hirouchi Y, et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103:993–999. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Kita T, Brown MS. Defective lipoprotein receptors and atherosclerosis: lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med. 1983;309:288–296. doi: 10.1056/NEJM198308043090507. [DOI] [PubMed] [Google Scholar]
- Hiroshima O, Shino M.Determination of ubiquinones in biological material by high performance liquid chromatography Nippon Rinsho 199351952–958.(in Japanese) [PubMed] [Google Scholar]
- Hiyoshi H, Yanagimachi M, Ito M, Ohtsuka I, Yoshida I, Saeki T, et al. Effect of ER-27856, a novel squalene synthase inhibitor, on plasma cholesterol in rhesus monkeys: comparison with 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors. J Lipid Res. 2000;41:1136–1144. [PubMed] [Google Scholar]
- Hiyoshi H, Yanagimachi M, Ito M, Yasuda N, Okada T, Ikuta H, et al. Squalene synthase inhibitors suppress triglyceride biosynthesis through the farnesol pathway in rat hepatocytes. J Lipid Res. 2003;44:128–135. doi: 10.1194/jlr.m200316-jlr200. [DOI] [PubMed] [Google Scholar]
- Itabe H, Takeshima E, Iwasaki H, Kimura J, Yoshida Y, Imanaka T, et al. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J Biol Chem. 1994;269:15274–15279. [PubMed] [Google Scholar]
- Ito T, Yamada S, Shiomi M. Progression of coronary atherosclerosis relates to the onset of myocardial infarction in an animal model of spontaneous myocardial infarction (WHHLMI rabbits) Exp Anim. 2004;53:339–346. doi: 10.1538/expanim.53.339. [DOI] [PubMed] [Google Scholar]
- James AM, Smith RAJ, Murphy MO. Antioxidant and prooxidant properties of mitochondorial coenzyme Q. Arch Biochem Biophys. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Kita T, Yokode M, Watanabe Y, Narumiya S, Kawai C. Stimulation of cholesteryl ester synthesis in mouse peritoneal macrophages by cholesterol-rich very low density lipoproteins from the Watanabe heritable hyperlipidemic rabbit, an animal model of familial hypercholesterolemia. J Clin Invest. 1986;77:1460–1465. doi: 10.1172/JCI112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Mechanism of plaque stabilization with statins. Am J Cardiol. 2003;91:4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- Miki T, Kori M, Mabuchi H, Tozawa R, Nishimoto T, Sugiyama Y, et al. Synthesis of novel 4,1-benzoxazepine derivatives as squalene synthase inhibitors and their inhibitions of cholesterol synthesis. J Med Chem. 2002;45:4571–4580. doi: 10.1021/jm020234o. [DOI] [PubMed] [Google Scholar]
- Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992;1126:247–254. doi: 10.1016/0005-2760(92)90237-p. [DOI] [PubMed] [Google Scholar]
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaques to vulnerable patients, a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Nakano T, Tanaka A. The oxidative modification hypothesis of atherosclerosis: the comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin Chim Acta. 2006;367:36–47. doi: 10.1016/j.cca.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Nakazato K, Ishibashi T, Shindo J, Shiomi M, Maruyama Y. Expression of very low density lipoprotein receptor mRNA in rabbit atherosclerotic lesions. Am J Pathol. 1996;149:1831–1838. [PMC free article] [PubMed] [Google Scholar]
- Nishimoto T, Amano Y, Tozawa R, Ishikawa E, Imura Y, Yukimasa H, et al. Lipid-lowering properties of TAK-475, a squalene synthase inhibitor, in vivo and in vitro. Br J Pharmacol. 2003;139:911–918. doi: 10.1038/sj.bjp.0705332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto T, Ishikawa E, Anayama H, Hamajyo H, Nagai H, Hirakata M, et al. Protective effects of a squalene synthase inhibitor, lapaquistat acetate (TAK-475), on statin-induced myotoxicity in guinea pigs. Toxicol Appl Pharmacol. 2007;223:39–45. doi: 10.1016/j.taap.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie-Hardman J, et al. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb. 1994;14:1767–1774. doi: 10.1161/01.atv.14.11.1767. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomized controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Hirouchi Y, Enomoto M. Fibromuscular cap composition is important for the stability of established atherosclerotic plaques in mature WHHL rabbits treated with statins. Atherosclerosis. 2001;157:75–84. doi: 10.1016/s0021-9150(00)00708-5. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Shiraishi M, Watanabe Y. Inheritability of atherosclerosis and the role of lipoproteins as risk factors in the development of atherosclerosis in WHHL rabbits: risk factors related to coronary atherosclerosis are different from those related to aortic atherosclerosis. Atherosclerosis. 1992;96:43–52. doi: 10.1016/0021-9150(92)90036-g. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Tsukada T, Yata T, Ueda M. Cell compositions of coronary and aortic atherosclerotic lesions differ: an immunohistochemical study. Arterioscler Thromb. 1994;14:931–937. doi: 10.1161/01.atv.14.6.931. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Tsukada T, Yata T, Watanabe Y, Tsujita Y, et al. Reduction of serum cholesterol levels alters lesional composition of atherosclerotic plaques: effect of pravastatin sodium on atherosclerosis in mature WHHL rabbits. Arterioscle Thromb Vasc Biol. 1995;5:1938–1944. doi: 10.1161/01.atv.15.11.1938. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Watanabe Y, Tsujita Y, Kuroda M, Arai M, et al. Suppression of established atherosclerosis and xanthomas in mature WHHL rabbits by keeping their serum cholesterol levels extremely low: effect of pravastatin sodium in combination with cholestyramine. Atherosclerosis. 1990;83:69–80. doi: 10.1016/0021-9150(90)90132-3. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit) Arterioscler Thromb Vasc Biol. 2003;23:1239–1244. doi: 10.1161/01.ATV.0000075947.28567.50. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Correlation of vulnerable coronary plaques to sudden cardiac events. Lessons from a myocardial infarction-prone animal model (the WHHLMI rabbit) J Atheroscler Thromb. 2004;11:184–189. doi: 10.5551/jat.11.184. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Yamada S, Ito T. Atheroma stabilizing effects of simvastatin due to depression of macrophages or lipid accumulation in the atheromatous plaques of coronary atherosclerosis-prone WHHL rabbits. Atherosclerosis. 2005;178:287–294. doi: 10.1016/j.atherosclerosis.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kobayashi H, Sato F, Yonemitsu Y, Nakashima Y, Sueishi K. Plaque-stabilizing effect of pitavastatin in Watanabe heritable hyperlipidemic (WHHL) rabbits. J Atheroscler Thromb. 2003;10:109–116. doi: 10.5551/jat.10.109. [DOI] [PubMed] [Google Scholar]
- Tavridou A, Kaklamanis L, Papalois A, Kourounakis AP, Rekka EA, Kourounakis PN, et al. EP2306, a novel squalene synthase inhibitor, reduces atherosclerosis in the cholesterol-fed rabbit. J Pharmacol Exp Ther. 2007;323:794–804. doi: 10.1124/jpet.107.126375. [DOI] [PubMed] [Google Scholar]
- Tavridou A, Manolopoulos VG. Antioxidant properties of two novel 2-biphenylmorpholine compounds (EP2306 and EP2301) in vitro and in vivo. Eur J Pharmacol. 2004;505:213–221. doi: 10.1016/j.ejphar.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit) Atherosclerosis. 1980;36:261–268. doi: 10.1016/0021-9150(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Williams DA. A note on Shirley's nonparametric test for comparing several dose levels with a zero-dose control. Biometrics. 1986;42:183–186. [PubMed] [Google Scholar]