Abstract
Background and purpose: The sodium channel is a primary target for treating central nervous system disorders such as epilepsy. In this study the anticonvulsant effect of BmK IT2, a sodium channel-specific neurotoxin, was evaluated in different animal models of epilepsy.
Experimental approach: Experiments were performed on freely moving rats made epileptic by administration of either pentylenetetrazole (PTZ) or pilocarpine. BmK IT2 (0.05–0.5 μg in 2 μl) was microinjected into the CA1 area and its effects on PTZ-induced widespread, seizure-like behaviour and cortex epileptiform EEG, as well as on pilocarpine-induced seizure-like behaviour and c-Fos expression were studied.
Key results: Intrahippocampal application of BmK IT2 dose-dependently inhibited PTZ-induced seizure-like behaviour, and reduced the numbers and duration of the high amplitude and frequency discharges (HAFDs) of the epileptiform EEG component induced by PTZ. Similarly, in the pilocarpine-induced status epilepticus (SE) model, BmK IT2 significantly prolonged the latency to onset of the SE, reduced the severity of SE and suppressed hippocampal c-Fos expression during SE.
Conclusions and implications: BmK IT2 showed anticonvulsant activity as it inhibited the widespread seizures induced by PTZ and pilocarpine-induced SE in rats. This activity might be due to the modulation of sodium channels in the hippocampus. Hence, BmK IT2 could be used as a novel tool to explore the molecular and pathological mechanisms of epilepsy with regard to the involvement of sodium channels.
Keywords: BmK IT2, intrahippocampal injection, status epilepticus, epileptic seizures, sodium channels
Introduction
Epilepsy is a common chronic neurological disorder that affects 0.5–1% of the world's population (Sander and Shorvon, 1996). The chronic application of antiepileptic drugs (AEDs) is widely used to treat epileptic seizures (Tunnicliff, 1996). However, many patients suffer from serious side-effects when chronically treated with AEDs, such as chronic toxicity, cognitive impairment, sedation and teratogenesis (Raza et al., 2001). Therefore, new AEDs for the treatment of epilepsy need to be developed (Villetti et al., 2001).
In recent years, a great number of toxins purified from the venoms of arthropods such as scorpions, spiders and wasps have been found to be a rich source of neuroactive substances with a wide range of pharmacological effects (Beleboni et al., 2004). For example, some neurotoxic polypeptides from scorpion or spider venom have been shown to elicit epileptic seizures (Sandoval and Lebrun, 2003) or exert antiepileptic effects (Cairrão et al., 2002). These neurotoxins act on ion channels or receptors in the mammalian nervous system with marked specificity (Carneiro et al., 2003; Purali, 2003). Recent studies on the structures and functions of these different neurotoxins have guided the development of new potential AEDs (McCormick and Meinwald, 1993).
Asian scorpion Buthus martensi Karsch (BmK) belongs to the Buthidae family. Several subtypes of α and β neurotoxins from BmK venom have been demonstrated to act on voltage-gated sodium channels (VGSCs) (Ji et al., 1999; Goudet et al., 2002; Zuo and Ji, 2004). Scorpion α-toxins have been shown to act on site-3 of sodium channels and prolong the inactivation of the channels, whereas scorpion β-toxins were found to be modulators, specifically binding to site-4 of sodium channels in a voltage-dependent manner and altering the activation of the channels (Catterall, 1986). BmK I, an α-like neurotoxin, has been shown to induce strong nociceptive effects and epileptic seizures by prolonging the inactivation of sodium currents (Ji et al, 1996; Zhang et al., 2002; Bai et al., 2003, 2006; Chen et al, 2005). BmK IT2, a β-like neurotoxin containing 61 amino acid residues, was found to be toxic to insects but not mammals (Ji etal., 1994). When injected peripherally BmK IT2 was found to produce significant antinociceptive effects in different models of nociception (Wang et al., 2000; Tan et al., 2001; Zhang et al., 2003). In addition, electrophysiological recordings showed that BmK IT2 alters the activation of sodium channels and inhibits the peak sodium currents on rat dorsal root ganglion neurons, suggesting that the antinociceptive effects of BmK IT2 may be attributed to the modulation of sodium channels (Tan et al., 2001). As distribution of sodium channel subtypes in the central nervous system is similar to that in the peripheral nervous system (Trimmer and Rhodes, 2004), BmK IT2 might act on VGSCs in the central nervous system and exert similar pharmacological effects. In traditional Chinese medicine, BmK venom/body has been used to cure epilepsy, as according to Chinese herbalism ‘Combat poison with poison' (since Song Dynasty). This implies that some components of BmK venom/body may account for its antiepileptic effect. Therefore, in the present study, to elucidate the central pharmacological activities of BmK IT2 and its underlying mechanisms, the following specific questions were addressed: (a) can intrahippocampal administration of BmK IT2 produce anticonvulsant effects in different experimental models of epilepsy? (b) Is BmK IT2 effective at suppressing the widespread behavioural and electrographic seizures induced by PTZ? (c) Is the severity of the status epilepticus (SE) induced by lithum-pilocarpine and the expression of hippocampal c-Fos modified by BmK IT2 treatment?
Methods
Animals
Male Sprague–Dawley rats (250–300 g body weight) from Shanghai Experimental Animal Center, Chinese Academy of Sciences were used. Rats, four in a cage with water and food available ad libitum, were kept in a room with temperature maintained at 22±1 °C and on a 12 h light/dark cycle. All animal procedures were approved by the Committee of Laboratory Animals, Chinese Academy of Sciences. Efforts were made throughout the study to minimize animal suffering.
Drugs
BmK IT2 was purified from BmK venom by RP-HPLC according to the method described previously by Ji et al. (1994). The purity of the BmK IT2 isolated was checked by mass spectrometry analysis and only that with purity above 99% was used. PTZ, pilocarpine hydrochloride, methylscopolamine bromide and VPA were purchased from Sigma-Aldrich. All drugs were dissolved in sterile isotonic saline for intrahippocampal or intraperitoneal injection.
Surgery
The animals were anaesthetized with sodium pentobarbital (40 mg kg−1 body weight, i.p.) and placed in a stereotaxic frame (Narishige, Tokyo, Japan). The head was shaved and then prepared in sterile fashion with povidone-iodine. A midline incision was made on the scalp to expose the coronal, sagittal and lambdoid sutures. Then, a stainless steel guide cannula for CA1 microinjection was implanted into the right lateral dorsal hippocampus (AP −4.3 mm posterior to bregma, L 2.2 mm lateral to the midline of the skull and V 2.5 mm ventral to the dura surface) according to the Atlas of Paxinos and Watson (1998). Similarly for the EEG recording, the recording electrode was positioned stereotaxically into the frontal cortex (AP −3.5 mm, R 2.0 mm and V 1.5 mm) contralateral to the guide cannula, whereas the reference electrode was placed on the cerebellum (AP −10.0 mm). The guide cannula and the electrodes were fixed and the wound was closed with dental cement. The rats were allowed to recover for at least 3–4 days. At the end of the experiments, the animals were killed and the brains were removed for cresyl violet staining according to the method described previously (Bai et al., 2006). Only animals with cannulae positioned accurately were used for data collection.
Seizure behaviour observation procedures
For PTZ-induced seizures, the rats were placed in a 40 × 30 × 50 cm transparent glass box and allowed to adapt to their environment for at least 1 h before the assays. Rats were then anaesthetized by ether inhalation, and BmK IT2 (dissolved in 2 μl saline) or saline was injected into the hippocampus CA1 through the guide cannula by using a metal syringe; 10 min later, PTZ (85 mg kg−1, i.p.) was administered to induce seizures. After PTZ injection, the rats were placed back in the box and their behaviour was continuously observed for a period of 2 h. Six groups, each containing 6–15 rats, were used to assess the effects of BmK IT2 on PTZ-induced seizures. The six groups of animals were randomly assigned to the following treatments:
Group 1 received saline plus PTZ (n=15) as a negative control;
Group 2 received VPA plus PTZ (n=6) as a positive control;
Group 3–5 received different doses of BmK IT2 (0.05, 0.1 and 0.5 μg in 2 μl saline) plus PTZ (n=12, 10, and 11, respectively);
Group 6 received BmK IT2 (1 μg in 2 μl saline, n=6) to evaluate the effect of BmK IT2 on naive rats.
During experiments, the administration of the drugs and the behaviour observations were ‘double blinded' to eliminate the possibility of human error. Racine's five-point scale (Racine, 1972), modified by Fathollahi et al. (1997), was used for the classification of seizure-like behaviour: Stage 0, no response; Stage 1, ear and facial twitching; Stage 2, myoclonic jerks without upright position; Stage 3, myoclonic jerks, upright position with bilateral forelimb clonus; Stage 4, clonic-tonic seizure; Stage 5, generalized clonic-tonic seizures, loss of postural control. The assessment of the anticonvulsant activity of BmK IT2 was based on the latency, seizure duration, numbers and severity of the seizures during the first 2 h period after PTZ injection. The latency was defined as the average length of time between PTZ administration and the onset of the first seizure above stage 2. A seizure episode was defined as the course from the beginning of the seizure onset to the recovery from the seizure. For the quantification of duration and numbers, seizure-like behaviour was grouped into four categories according to Racine's five-point scale. The minimal interval between two countable seizures was set for 5 s throughout the quantification of all seizure numbers. The duration and number of seizures were calculated as the sums of these multiple seizures for each animal, respectively. The seizure severity was based on a maximal seizure score graded from 0 to 5, as previously described (Bai et al., 2006).
For the lithium–pilocarpine-induced SE experiments, all naive rats received lithium chloride injection (3 mmol kg−1, i.p). On the following day, methylscopolamine bromide (1 mg kg−1, s.c.) was administered 30 min before the pilocarpine to reduce the peripheral effects of pilocarpine. For intrahippocampal administration, eight rats were administered saline as a control; three groups containing six, six and seven rats were microinjected (2 μl) with BmK IT2 at the doses of 0.05, 0.1 and 0.5 μg, respectively. SE was induced by pilocarpine hydrochloride (25 mg kg−1, i.p.) 10 min after the administration of saline or BmK IT2. The rats were then placed back in the box and their behaviour was continuously observed for a period of 2 h. After an initial period of immobility, the onset of SE was characterized by repetitive clonic activity of the trunk and limbs, occurring following repeated rearing with forelimb clonus and falling over for 30 min. The latency was defined as the average length of time between pilocarpine administration and the onset of SE. The seizure severity and classification were evaluated according to Racine's classification as previously indicated (Racine, 1972).
EEG recordings
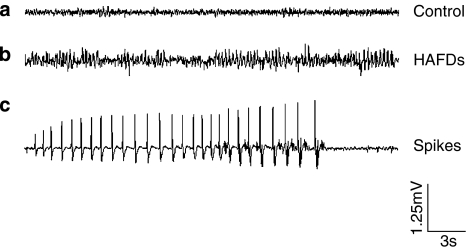
The electrographic activity was recorded by bioamplifier (Model SMUP-E Bioelectric Signals Processing System, Shanghai Medical College of Fudan University, China), with a range of 100 mV and a pass band of 1.6–100 Hz. Analogue data were sampled at 1000 Hz. After a 30 min period of baseline recording, BmK IT2 or saline was injected into the hippocampus, as described above, 10 min later, PTZ (85 mg kg−1, i.p.) was administered to induce seizures and the EEG was continuously recorded for 2 h. The rats were divided into four groups: one group was injected with 2 μl saline plus PTZ (n=6) as control, and the other three groups were treated with different doses of BmK IT2 (0.05, 0.1 and 0.5 μg) plus PTZ (n=6, 6, and 6, respectively). Each EEG file was analysed manually by scanning through the EEG recording on the computer screen. An electrographic spontaneous seizure was defined as a high-frequency (>5 Hz), high-amplitude (>2 × baseline) discharge that lasted for at least 5 s. The latency to the first onset of electrographic epileptiform discharges was recorded. The severity of an electrographic seizure was assessed by counting the number and duration of HAFDs, which are typically associated with seizure-like behaviour. The definition for a HAFD was a discharge that lasted for at least 5 s, frequency of at least 8 Hz, and had an amplitude that was at least two times that of the baseline EEG. The spikes were also analysed. An epileptic spike was defined as a spike with a duration <100 ms or spike-and-wave duration <200 ms (Pitkänen et al., 2005). Spikes were counted with a MFlab200 v3.02 programme (Shanghai Medical College of Fudan University, China).
Fos immunohistochemistry
After the induction of SE and the behavioural observations had been carried out the rats were killed, 2 h after the administration of saline or BmK IT2, for the evaluation of hippocampal c-Fos expression. The animals were deeply anaesthetized by administration of sodium pentobarbital (60 mg kg−1 body weight, i.p.) and perfused intracardially with 200 ml sterile saline followed by 400 ml fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and postfixed in the same fixative for 12 h, and then cryoprotected in 0.1 M phosphate buffer containing 20% sucrose until the tissue sank to the bottom of the container. Frozen serial coronal sections (30 μm thickness) were cut in a cryostat and mounted on gelatin-coated slices.
The sections were immunostained for the c-Fos protein by the avidin–biotin-peroxidase method as previously described (Bai et al., 2003, 2006). Briefly, the sections were rinsed in 0.01 M PBS (pH 7.4), and then incubated with a polyclonal antibody raised in a rabbit against a peptide mapped at the amino terminus of human c-Fos P62 (1:2000, diluted in 0.01 M PBS containing 1% BSA, 0.03% sodium azide, 0.3% Triton X-100, Sc-52, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 48 h at 4 °C. Then, the sections were incubated with biotinylated goat anti-rabbit IgG (1:200, diluted in 0.01 M PBS, Vector, Burlingame, CA, USA) and avidin–biotin-peroxidase complex (1:200, diluted in 0.01 M PBS, Vector, USA) at room temperature for 2 h. The reactive product was visualized by the nickel-diaminobenzidine glucose-oxidase method.
Counting and analysis of the hippocampal c-Fos expression
The spatial levels of c-Fos expression in rat hippocampus (AP −3.80 to −4.30 mm) were evaluated by cell counting. For each animal, the numbers of labelled FLI neurons from 9 to 11 sections in the hippocampal pyramidal cells of CA1, CA2, CA3 and the granular cells of the DG were counted and averaged (n=6–8). The counting did not take into account staining intensity. All data were collected blind to drug treatment of each group.
Statistical analysis
All data are presented as mean±s.e.mean. The data were analysed by use of one-way ANOVA. Tukey's test was employed to compare the differences between all the groups. P<0.05 was considered to be significant.
Results
Effects of BmK IT2 on seizure-like behaviour induced by PTZ
BmK IT2 (1 μg in 2 μl) alone microinjected into CA1 area did not induce any seizure behaviour in all six rats tested. PTZ (85 mg kg−1, i.p.) induced reoccurring onsets of isolated myoclonic jerks followed by the outburst of generalized clonic-tonic seizures in all rats in the saline microinjected control group. PTZ-induced seizures led to a high mortality rate of rats in this group (53.3%, n=15). BmK IT2 (0.05, 0.1, 0.5 μg) microinjected into the CA1 area dose-dependently protected the PTZ-injected rats from death; the number dead being reduced to 33.3, 0 and 0%, respectively. BmK IT2 also significantly reduced all the seizure parameters measured in the PTZ-treated rats (Table 1). Owing to the high mortality rate, the statistic analysis excluded those rats that died within the 2 h recording period, thus the anticonvulsant effect of BmK IT2 might be underestimated in this study. For those rats that survived, the latency of the PTZ-induced seizure activity was prolonged dose-dependently by pretreatment with BmK IT2, and this effect reached significance with the 0.5 μg dose group. In addition, the mid- and high-doses of BmK IT2 significantly reduced the number and the duration of the stage 3–5 seizure-like behaviours in the PTZ-treated rats, but had a limited effect on the stage 1–2 seizure-like behaviour (Table 1). Furthermore, we validated this epilepsy model and compared the anticonvulsant activity of BmK IT2 with VPA. As a positive control, VPA (200 mg kg−1, i.p) pretreatment effectively blocked the tonic–clonic convulsions evoked by PTZ. From the results, 0.1 and 0.5 μg, BmK IT2 appeared to exhibit a similar anticonvulsant effect on seizures to that of VPA.
Table 1.
Anticonvulsant effect of BmK IT2 on behavioural seizures induced by PTZ during 2 h
| Treatment | Saline+PTZ | 0.05 μg BmK IT2+PTZ | 0.1 μg BmK IT2+PTZ | 0.5 μg BmK IT2+PTZ | 200 mg kg−1 VPA+PTZ |
|---|---|---|---|---|---|
| Animal number (n) | 15 | 12 | 10 | 11 | 6 |
| Mortality | 8/15 | 4/12 | 0/10 | 0/11 | 0/6 |
| Latency (s) | 75.18±5.30 | 104.93±16.22 | 119.60±27.31 | 231.23±36.00*** | 124.17± 16.75 |
| Seizure duration (s) | |||||
| Stage 1 & 2 seizures | 104.50±25.80 | 66.00±10.62 | 81.50±16.53 | 48.55±5.29* | 54.33±9.83 |
| Stage 3 seizures | 73.17±24.58 | 40.09±7.41 | 5.50±3.02*** | 14.27±4.86** | 18.83±4.93* |
| Stage 4 seizures | 210.00±90.72 | 43.50±14.67*** | 3.50±2.60*** | 1.09±0.60*** | 5.00±2.40*** |
| Stage 5 seizures | 7.00±3.74 | 4.64±2.12 | 0.50±0.34*** | 0*** | 1.50±0.95 |
| Total duration | 358.50±102.48 | 157.00±27.62** | 91.00±16.63*** | 64.09±5.78*** | 79.67±15.38*** |
| Seizure number | |||||
| Stage 1 & 2 seizures | 16.00±6.66 | 13.86±1.89 | 17.60±3.36 | 15.91±2.95 | 9.67±1.63 |
| Stage 3 seizures | 4.00±0.84 | 5.67±0.60 | 0.60±0.27*** | 1.45±0.31*** | 3.17±0.79 |
| Stage 4 seizures | 2.14±0.23 | 3.59±0.27*** | 0.30±0.21*** | 0.27±0.14*** | 0.50±0.22*** |
| Stage 5 seizures | 1.50±0.21 | 1.86±0.18 | 0.10±0.21*** | 0*** | 0.33±0.21*** |
*P<0.05, **P<0.01 and ***P<0.001, compared to group treated with saline (One-way ANOVA, Tukey's test). Rats in the saline group treated with PTZ died within 60 min (n=7), therefore, the number and duration of behavioural seizures for this group was not included in the statistics.
Effects of BmK IT2 on the electrographic seizures induced by PTZ
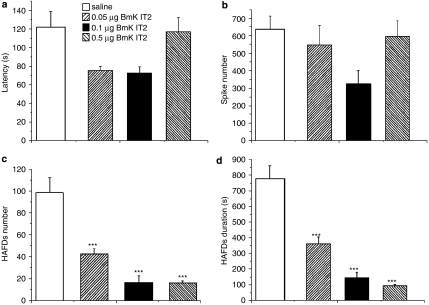
The EEG was recorded through a pre-implanted electrode in the cortex of freely moving rats. Acute epileptiform EEG was triggered and recurred as discrete episodes in all groups after PTZ administration. Representative EEG traces showing isolated and clustered spikes and HAFDs from saline injected rats before and after PTZ treatment are shown in Figure 1. BmK IT2-injected rats showed similar EEG waveform to the saline group, but the frequencies of the spikes and HAFDs were reduced and this was accompanied by decreased seizure-like behaviour. Quantitative analysis of seizure activity demonstrated that there were no marked differences in the latency to first electrographic seizure and isolated spikes between the saline and BmK IT2 groups (Figures 2a and b). Interestingly, administration of BmK IT2 produced a significant reduction in the number and duration of HAFDs in a dose-dependent manner (Figures 2c and d). These results suggested that 0.1–0.5 μg BmK IT2 suppressed epileptiform activity on the electrographic seizures, which are typically associated with seizure-type behaviours.
Figure 1.
Representative normal and epileptiform EEG traces recorded from rat cortex directly above the hippocampus. (a) Normal EEG before PTZ injection; (b) and (c), HAFDs and spikes appeared during seizures after PTZ injection. Note that all EEG recordings were obtained from a rat with saline microinjected into hippocampal CA1 area before and after PTZ injection.
Figure 2.
Effects of BmK IT2 on electrographic seizures induced by PTZ. The EEG results in the saline (n=6) or BmK IT2 (0.05, 0.1, 0.5 μg, n=6 for each dose) groups were counted for 2 h and averaged. (a) Effects of BmK IT2 on the latency to the first EEG seizure induced by PTZ. (b) A quantitative comparison of spikes between the saline and BmK IT2 groups during the first 2 h after PTZ injection. (c)A quantitative comparison of the HAFDs between the saline and BmK IT2 groups during the first 2 h after PTZ injection. (d) A comparison of the duration of the HAFDs between the saline and BmK IT2 groups during the first 2 h after PTZ injection. Data are presented as mean±s.e.mean. *P<0.05 and ***P<0.005 compared with the saline group (One-way ANOVA, Tukey's test).
Effects of BmK IT2 on seizures-type behaviour induced by pilocarpine
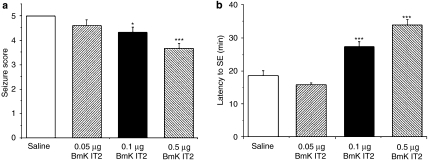
After injection of pilocarpine, the rats displayed alterations in behaviour, including stereotypic scratching, grooming, sniffing, and chewing, resembling stage 1 and 2 seizures. These were followed by seizures above stage 3 that developed approximately 18 min after pilocarpine injection and the seizure activity progressed to SE. BmK IT2 microinjected into the hippocampal CA1 area dose-dependently reduced pilocarpine-induced seizure severity; this effect was significant in the 0.1 and 0.5 μg dose groups (Figure 3a). In addition, one rat in the 0.5 μg BmK IT2 group did not progress to SE. With those rats that progressed to SE after pilocarpine injection, the latency of the SE onset in the control group was 18.61±1.41 min, and 0.1 and 0.5 μg BmK IT2 pretreatment significantly prolonged the latency of the SE onset to 27.36±1.53 and 33.96±1.63 min, respectively compared with that of the saline control group (Figure 3b); the latency in the 0.05 μg BmK IT2 group (15.88±0.53 min) showed a small decrease but was not significantly different from the saline group (Figure 3b).
Figure 3.
Effects of BmK IT2 on behavioural seizures induced by pilocarpine. (a) The seizure scores of the saline (n=8) and BmK IT2 (n=6 for each dose)-treated groups. (b) The latency to SE onset after the injection of saline or BmK IT2. Data are presented as mean±s.e.mean. *P<0.05 and ***P<0.005 compared with the saline group (One-way ANOVA, Tukey's test).
Effects of BmK IT2 on hippocampal c-fos expression induced by pilocarpine
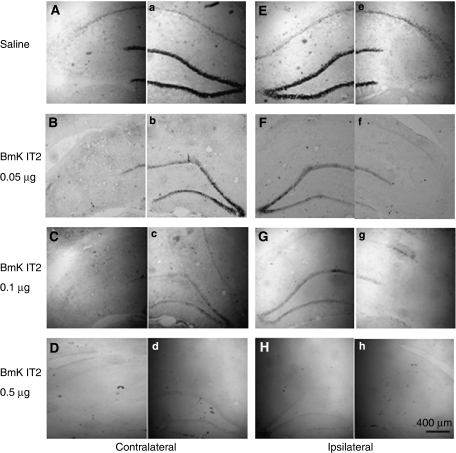
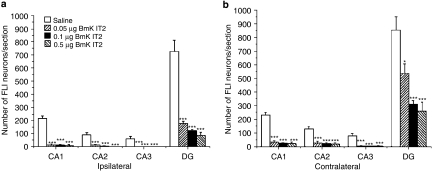
The change in hippocampal c-Fos expression induced by pilocarpine and the effects of BmK IT2 on pilocarpine-induced c-Fos expression were studied from the same animal groups as those used for behavioural observations after SE had been established. Representative sections of the ipsilateral and contralateral hippocampus from the saline and BmK IT2-treated groups are illustrated in Figure 4. In the saline control group, the predominant FLI neurons were detected in the pyramidal cell layer of the hippocampal CA1 and in the granular cell layer of the DG with a few scattered in other layers (Figures 4A and E). Application of different doses of BmK IT2 (0.05, 0.1 and 0.5 μg) induced a dose-dependent suppression of the c-Fos expression in distinct hippocampal regions (Figures 4 and 5). Interestingly, the inhibitory effect of BmK IT2 in the ipsilateral side was stronger than that in the contralateral side. The inhibitory effects of 0.05, 0.1 and 0.5 μg BmK IT2 on pilocarpine-induced c-Fos expression in the ipsilateral FLI neurons were 81.29, 86.82 and 91.15%, respectively whereas those in the contralateral side were 54.78, 72.57 and 76.92%, respectively.
Figure 4.
Representative microphotographs of the suppressive effect of BmK IT2 on hippocampal c-Fos expression induced by pilocarpine. The sections show the c-Fos expression in the ipsilateral and contralateral sides of the hippocampus after the intrahippocampal administration of saline or BmK IT2. Panels (A) and (a), (B) and (b), (C) and (c), (D) and (d), (E) and (e), (F) and (f), (G) and (g), (H) and (h) were taken from the same section, respectively.
Figure 5.
Histograms of the number of labelled FLI neurons in the ipsilateral (a) and contralateral (b) hippocampus in the saline and BmK IT2 groups. The number of labelled FLI neurons in the hippocampus from 9 to 11 sections of each animal was counted and averaged (n=6–8) 2 h after the injection of pilocarpine. Data are shown as mean±s.e.mean. *P<0.05 and ***P<0.005 compared with the saline group (One-way ANOVA, Tukey's test).
Discussion and conclusions
In the present study, BmK IT2 showed anticonvulsant effects on widespread seizures induced by PTZ, as assessed by monitoring behaviour and EEG recordings. In addition, BmK IT2 also decreased the severity of SE and suppressed c-Fos expression during SE induced by lithium–pilocarpine.
Effects of BmK IT2 on behavioural and electrographic seizures induced by PTZ
PTZ, a GABAA receptor antagonist, is well established as a chemical convulsant that can generate clonic or tonic–clonic seizures in animals and man (Fisher, 1989; Panagopoulos et al., 1998). The rodent PTZ model has been widely used for antiepileptic drug discovery and studying the mechanism of seizures (Barkai et al., 1994; Psarropoulou et al., 1994; Loscher, 2002). In the present study, BmK IT2 was found to be effective at inhibiting the widespread seizures induced by PTZ in a dose-dependent manner. In addition, the HAFDs accompanying the hypokinetic events, myclonic jerks or clonic seizures, evoked by PTZ administration, were also markedly reduced by BmK IT2 pretreatment, indicating that BmK IT2 decreases the hyperexcitability evoked by PTZ in rat brain. The appearance of behavioural seizures did not quite coincide with the onset of electrographic seizures. These different latencies are probably due to variations in the site of onset of the seizure in the brain. Studies by Browning (1987) have suggested that seizures originate from two primary brain regions, that is, the forebrain and the brainstem. PTZ, depending on the dose administered, can produce both forebrain and brainstem seizures, and then indirectly induce the occurrence of epileptiform activity in the frontal cortex. This suggests that additional regions of the rat brain are involved in the occurrence and propagation of seizures.
The anticonvulsant effects of an intrahippocampal injection of BmK IT2 suggest that the rat hippocampus is one of the targets that BmK IT2 acts on. This hypothesis is partially supported by results from a surface plasmon resonance assay, which indicated that BmK IT2 binds to rat cerebrocortical synaptosomes and hippocampal synaptosomes (Chai et al., 2006). In addition, BmK IT2 has been demonstrated to inhibit the peak sodium currents of rat DRG neurons (Tan et al., 2001) and those of rNav1.2α expressed in Xenopus oocytes (unpublished observations). Thus, BmK IT2 may decrease the hyperexcitability of the epileptic hippocampus by affecting sodium channels.
We also compared the anticonvulsant effects of BmK IT2 with those of VPA, a commonly used antiepileptic drug. In the present study, VPA similar to previous observations (Piredda et al., 1986; Swinyard et al., 1986), was shown to inhibit PTZ-induced seizures effectively. The predominant antiepileptic action of VPA is probably due to its effect on voltage-gated sodium channels, which would reduce peak sodium currents and inhibit high-frequency firing of neurons (McLean and Macdonald, 1986; Johannessen, 2000; Köhling, 2002). The results from the present study showing that VPA is capable of inhibiting the widespread seizure activity induced by PTZ indicate that VPA is a suitable compound to use as a positive control. A comparison of the antiepileptic properties of VPA with those of BmK IT2 indicated that BmK IT2 has similar or more potent anticonvulsant effects than those of VPA. Hence, BmK IT2 may provide a new direction for developing potential AED for the treatment of epilepsy. The delivery of antiepileptic medication, such as BmK IT2 in the present study, directly into the brain has the advantage of maintaining a long-lasting effect on the focus of the seizures and avoiding problems of systemic toxicity (Costantin et al., 2005); however, it also limits its acceptance clinically. Hence, further studies are needed to explore these results more fully.
Effects of BmK IT2 on pilocarpine-induced SE and c-Fos expression
The lithium–pilocarpine model of epilepsy seems to reproduce some of the developmental, clinical and neurophysiological features of human temporal lobe epilepsy (Cavalheiro, 1996). Compared with the widespread seizures induced by PTZ, convulsions induced by pilocarpine are partial seizures with secondary more general seizures (Lian et al., 2005). In the present study, BmK IT2 did not reduce the occurrence of pilocarpine-induced SE, but did inhibit the general seizures induced by pilocarpine in a dose-dependent manner. This suggests that the most potent effect of BmK IT2 on SE was to slow down or block either synchronization or propagation of focal or general epileptiform activity.
After the induction of an intense limbic and tonic–clonic SE, which lasts for hours, the expression of about 300 genes is triggered in the temporal lobe (Lukasiuk et al., 2004). The gene products presumably participate in the molecular cascades involving the reorganization of neuronal circuits during epileptogenesis. The acute anticonvulsant properties of BmK IT2 were accompanied by a significant suppression of c-Fos expression in the hippocampus. The upregulation of c-Fos expression is regarded as a functional marker mapping the hyperexcitability of neuronal populations in the brain after electrically- or chemically-induced seizures (Daniel and Harold, 1996; Herdegen and Leah, 1998). In the present study, c-Fos expression during the first 2 h after pilocarpine injection was found in the ipsilateral and contralateral hippocampus. This accords with results from previous studies that have shown that the early phase of c-Fos expression in specific brain regions appear transiently after the induction of various types of seizures (Morgan et al., 1987; Yamashita et al., 1991). The c-Fos expression was located predominantly in DG and moderately in CA1 of the hippocampus. The distribution of seizure-activated c-Fos regions in rats indicates neuronal activation that includes increased metabolic activity of the neurons and the firing of action potentials (Daniel and Harold, 1996; Motte et al., 1997; Herdegen and Leah, 1998). Hippocampal pyramidal cells are glutamatergic and excitatory. CA1 pyramidal cells receive excitatory inputs from the CA3 and project onto the adjacent subiculum. The dentate gyrus is considered to act as a gate preventing excessive activity from entering other hippocampal regions. Thus, the activation of dentate granule cells could be a critical event in the development of seizure activity within the temporal lobe (Heinemann et al., 1992). The sprouting mossy fibres and the excitatory interconnections between granule cells have been proposed to contribute to increased synchrony of neuronal firing in many models of temporal lobe epilepsy (Tauck and Nadler, 1985; Sutula et al., 1988; Peng and Houser, 2005). The strong suppression of c-Fos expression induced by BmK IT2 indicates that the cellular hyperactivation during SE was reduced, highly consistent with the decreased severity of behavioural seizures. Furthermore, the higher inhibitory ratio in the ipsilateral than the contralateral hippocampus suggests that the inhibitory effect of BmK IT2 originated from the injection site (CA1) and spread to a specific region. Although our data demonstrate that BmK IT2 has anticonvulsant activity on SE in rodents, which represents the initial precipitating injury, whether BmK IT2 is able to suppress spontaneous recurrent seizures when given after the establishment of chronic temporal lobe epilepsy remains to be assessed.
In conclusion, our study has demonstrated that BmK IT2 is a potent anticonvulsant as it inhibits the seizures induced by PTZ and pilocarpine in freely moving rats. As BmK IT2 has sodium channel modulator properties, the underlying mechanisms of the anticonvulsant effects of BmK IT2 may, in part, be attributed to the modulation of sodium channels in the hippocampus. Thus, BmK IT2 deserves further study to help us explore the molecular and pathological mechanisms of epilepsy with regard to sodium channels and potential AEDs in the therapy of epilepsy.
Acknowledgments
This study was supported by the National Basic Research Program of China (2006CB500801), and in part by a grant from the National Nature Sciences Foundation of China (30270428 and 30370446). We thank Dr Wang Yun for his kind assistance in the article revision.
Abbreviations
- AEDs
antiepileptic drugs
- BmK
Buthus martensi Karsch
- DG
dentate gyrus
- FLI
c-Fos-like immunoreactive
- HAFDs
high amplitude and frequency discharges
- PBS
phosphate-buffered saline
- PTZ
pentylenetetrazole
- SE
status epilepticus
- VGSCs
voltage-gated sodium channels
- VPA
valproic acid sodium salt
Conflict of interest
The authors state no conflict of interest.
References
- Bai ZT, Zhang XY, Ji YH. Fos expression in rat spinal cord induced by peripheral injection of BmK I, an alpha-like scorpion neurotoxin. Toxicol Appl Pharmacol. 2003;192:78–85. doi: 10.1016/s0041-008x(03)00260-6. [DOI] [PubMed] [Google Scholar]
- Bai ZT, Zhao R, Zhang XY, Chen J, Liu T, Ji YH. The epileptic seizures induced by BmK I, a modulator of sodium channels. Exp Neurol. 2006;197:167–176. doi: 10.1016/j.expneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Barkai E, Grossman Y, Gutnick MJ. Long-term changes in neocortical activity after chemical kindling with systemic pentylenetetrazole: an in vitro study. J Neurophysiol. 1994;72:72–83. doi: 10.1152/jn.1994.72.1.72. [DOI] [PubMed] [Google Scholar]
- Beleboni RO, Pizzo AB, Fontana ACK, Carolino ROG, Coutinho-Netto J, Santos WF. Spider and wasps neurotoxins: pharmacological and biochemical aspects. Eur J Pharmacol. 2004;17:1–17. doi: 10.1016/j.ejphar.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Browning RA.Effect of lesions on seizures in experimental animals Epilepsy and the Reticular Formation: The role of the Reticular Core in Convulsive Seizures 1987New York; 137–162.Alan R. Liss [Google Scholar]
- Cairrão MAR, Ribeiro AM, Pizzo AB, Fontana ACK, Beleboni RO, Coutinho-Neto J, et al. Anticonvulsant and GABA uptake inhibition properties of P. bistriata and S. raptoria spider venom fractions. Pharm Biol. 2002;40:472–477. [Google Scholar]
- Carneiro AM, Kushmerick C, Koenen J, Arndt MH, Cordeiro MN, Chavez-Olortegui C, et al. Expression of a functional recombinant Phoneutria nigriventer toxin active on K+ channels. Toxicon. 2003;41:305–313. doi: 10.1016/s0041-0101(02)00292-1. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA. The pilocarpine model of epilepsy. Ita J Neurol Sci. 1996;16:33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- Chai ZF, Bai ZT, Liu T, Pang XY, Ji YH. The binding of BmK IT2 on mammal and insect sodium channels by surface plasmon resonance assay. Pharmacol Res. 2006;54:85–90. doi: 10.1016/j.phrs.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Chen J, Tan ZY, Zhao R, Feng XH, Shi J, Ji YH. The modulation effects of BmK I, an alpha-like scorpion neurotoxin, on voltage-gated Na(+) currents in rat dorsal root ganglion neurons. Neurosci Lett. 2005;390:66–71. doi: 10.1016/j.neulet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Costantin L, Bozzi Y, Richichi C, Viegi A, Antonucci F, Funicello M, et al. Antiepileptic effects of botulinum neurotoxin E. J Neurosci. 2005;25:1943–1951. doi: 10.1523/JNEUROSCI.4402-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel GH, Harold AR. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Fathollahi Y, Motamedi F, Semnanian S, Zardoshti M. Examination of persistent effects of repeated administration of pentylenetetrazol on rat hippocampal CA1, evidence from in vitro study on hippocampal slices. Brain Res. 1997;758:92–98. doi: 10.1016/s0006-8993(97)00164-9. [DOI] [PubMed] [Google Scholar]
- Fisher RS. Animal models of the epilepsies. Brain Res Brain Res Rev. 1989;14:245–278. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- Goudet C, Chi WW, Tytgat J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon. 2002;40:1239–1258. doi: 10.1016/s0041-0101(02)00142-3. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system, control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Ji YH, Hattori H, Hsu K, Terakawa S. Molecular characteristics of four new depressant insect neurotoxins purified from venom of Buthus martensi Karsch by HPLC. Sci China B. 1994;37:954–963. [PubMed] [Google Scholar]
- Ji YH, Li YJ, Zhang JW, Song B, Yamaki T, Mochizuki T, et al. Covalent structures of BmK AS and BmK AS-1, two novel bioactive polypeptides purified from Chinese scorpion Buthus martensi Karsch. Toxicon. 1999;37:519–536. doi: 10.1016/s0041-0101(98)00190-1. [DOI] [PubMed] [Google Scholar]
- Ji YH, Mansuelle P, Terakawa S, Kopeyan C, Yanaihara N, Hsu K, et al. Two neurotoxins (BmK I and BmK II) from the venom of the scorpion Buthus martensi Karsch, purification, amino acid sequences and assessment of specific activity. Toxicon. 1996;34:987–1001. doi: 10.1016/0041-0101(96)00065-7. [DOI] [PubMed] [Google Scholar]
- Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochem Int. 2000;37:103–110. doi: 10.1016/s0197-0186(00)00013-9. [DOI] [PubMed] [Google Scholar]
- Köhling R. Voltage-gated sodium channels in epilepsy. Epilepsia. 2002;43:1278–1295. doi: 10.1046/j.1528-1157.2002.40501.x. [DOI] [PubMed] [Google Scholar]
- Lian XY, Zhang ZZ, Stringer JL. Anticonvulsant activity of ginseng on seizures induced by chemical convulsants. Epilepsia. 2005;46:15–22. doi: 10.1111/j.0013-9580.2005.40904.x. [DOI] [PubMed] [Google Scholar]
- Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs: a comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Lukasiuk K, Kontula L, Pitkänen A. cDNA profiling of epileptogenesis in the rat brain. Eur J Neurosci. 2004;17:271–279. doi: 10.1046/j.1460-9568.2003.02461.x. [DOI] [PubMed] [Google Scholar]
- McCormick KD, Meinwald J. Neurotoxin acylpolymines from spider venoms. J Chem Ecol. 1993;19:2411–2413. doi: 10.1007/BF00979674. [DOI] [PubMed] [Google Scholar]
- McLean MJ, MacDonald RL. Sodium valproate, but not ethosuximide, produces use- and voltage-dependent limitation of high frequency repetitive firing of action potentials of mouse central neurons in cell culture. J Pharmacol Exp Ther. 1986;237:1001–1011. [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Motte J, Fernandes MJS, Marescaux C, Nehlig A. Effects of pentylenetetrazol-induced status epilepticus on c-Fos and HSP72 immunoreactivity in the immature rat brain. Mol Brain Res. 1997;50:79–84. doi: 10.1016/s0169-328x(97)00174-5. [DOI] [PubMed] [Google Scholar]
- Panagopoulos NT, Kazanis E, Sotiriou E, Papanastasiou P, Matsokis NA. Effect of the pentylenetetrazole (PTZ) induced seizures on the gabaergic system in the mouse brain. Eur J Neurosci. 1998;10:48. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 1998Academic Press: Sydney; 2nd edn. [Google Scholar]
- Peng Z, Houser CR. Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piredda S, Yonekawa W, Whittingham TS, Kupferberg HJ. Effects of antiepileptic drugs on pentylenetetrazole-induced epileptiform activity in the in vitro hippocampus. Epilepsia. 1986;27:341–346. doi: 10.1111/j.1528-1157.1986.tb03551.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 2005;63:27–42. doi: 10.1016/j.eplepsyres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Psarropoulou C, Matsokis N, Angelatou F, Kostopoulos G. Pentylenetetrazol-induced seizures decrease gamma-aminobutyric acid-mediated recurrent inhibition and enhance adenosinemediated depression. Epilepsia. 1994;35:12–19. doi: 10.1111/j.1528-1157.1994.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Purali N. Stimulation of GABA release by scorpion venom in an isolated synapse in the crayfish (Astacus leptodactylus) Toxicon. 2003;41:383–389. doi: 10.1016/s0041-0101(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raza M, Shahenn F, Choudhary MI, Sombati S, Rafiq A, Suria A, et al. Anticonvulsant activities of ethanolic extract and aqueous fraction isolated from Delphinium denudatum. J Ethonopharmacol. 2001;78:73–78. doi: 10.1016/s0378-8741(01)00327-0. [DOI] [PubMed] [Google Scholar]
- Sander JWAS, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry. 1996;61:433–443. doi: 10.1136/jnnp.61.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval MR, Lebrun I. TsTx toxin isolated from Tityus serrulatus scorpion venom induces spontaneous recurrent seizures and mossy fiber sprouting. Epilepsia. 2003;44:904–911. doi: 10.1046/j.1528-1157.2003.38001.x. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Swinyard EA, Sofia RD, Kupferberg HJ. Comparative anticonvulsant activity and neurotoxicity of felbamate and four prototype antiepileptic drugs in mice and rats. Epilepsia. 1986;27:27–34. doi: 10.1111/j.1528-1157.1986.tb03497.x. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Xiao H, Mao X, Wang CY, Zhao ZQ, Ji YH. The inhibitory effects of BmK IT2, a scorpion neurotoxin on rat nociceptive flexion reflex and a possible mechanism for modulating voltage-gated Na+ channels. Neuropharmarcol. 2001;40:352–357. doi: 10.1016/s0028-3908(00)00168-4. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G. Basis of the antiseizure action of phenytoin. Gen Phamarcol. 1996;27:1091–1097. doi: 10.1016/s0306-3623(96)00062-6. [DOI] [PubMed] [Google Scholar]
- Villetti G, Bregola G, Bassani F, Bergamaschi M, Rondelli I, Pietra C, et al. Preclinical evaluation of CHF3381 as novel antiepileptic agent. Neuropharmacol. 2001;40:866–878. doi: 10.1016/s0028-3908(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Wang CY, Tan ZY, Chen B, Zhao ZQ, Ji YH. Antihyperalgesia effect of BmK IT2 a depressant insect-selective scorpion toxin in rat by peripheral administration. Brain Res Bull. 2000;51:335–338. doi: 10.1016/s0361-9230(00)00355-5. [DOI] [PubMed] [Google Scholar]
- Yamashita YS, Corsi PS, Gombos G. Immunohistochemistry of c-fos in mouse brain during postanatal development: basal levels and changing response to metrazol and kainite injection. Eur J Neurosci. 1991;3:764–770. doi: 10.1111/j.1460-9568.1991.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Bai ZT, Chai ZF, Zhang JW, Liu Y, Ji YH. Suppressive effects of BmK IT2 on nociceptive behavior and c-Fos expression in spinal cord induced by formalin. J Neurosci Res. 2003;74:167–173. doi: 10.1002/jnr.10723. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhang JW, Chen B, Bai ZT, Shen J, Ji YH. Dynamic determination and possible mechanism of amino acid transmitter release from rat spinal dorsal horn induced by the venom and a neurotoxin (BmK I) of scorpion Buthus martensi Karsch. Brain Res Bull. 2002;58:27–31. doi: 10.1016/s0361-9230(02)00752-9. [DOI] [PubMed] [Google Scholar]
- Zuo XP, Ji YH. Molecular mechanism of scorpion neurotoxins acting on sodium channels. Mol Neurobiol. 2004;30:265–278. doi: 10.1385/MN:30:3:265. [DOI] [PubMed] [Google Scholar]