Abstract
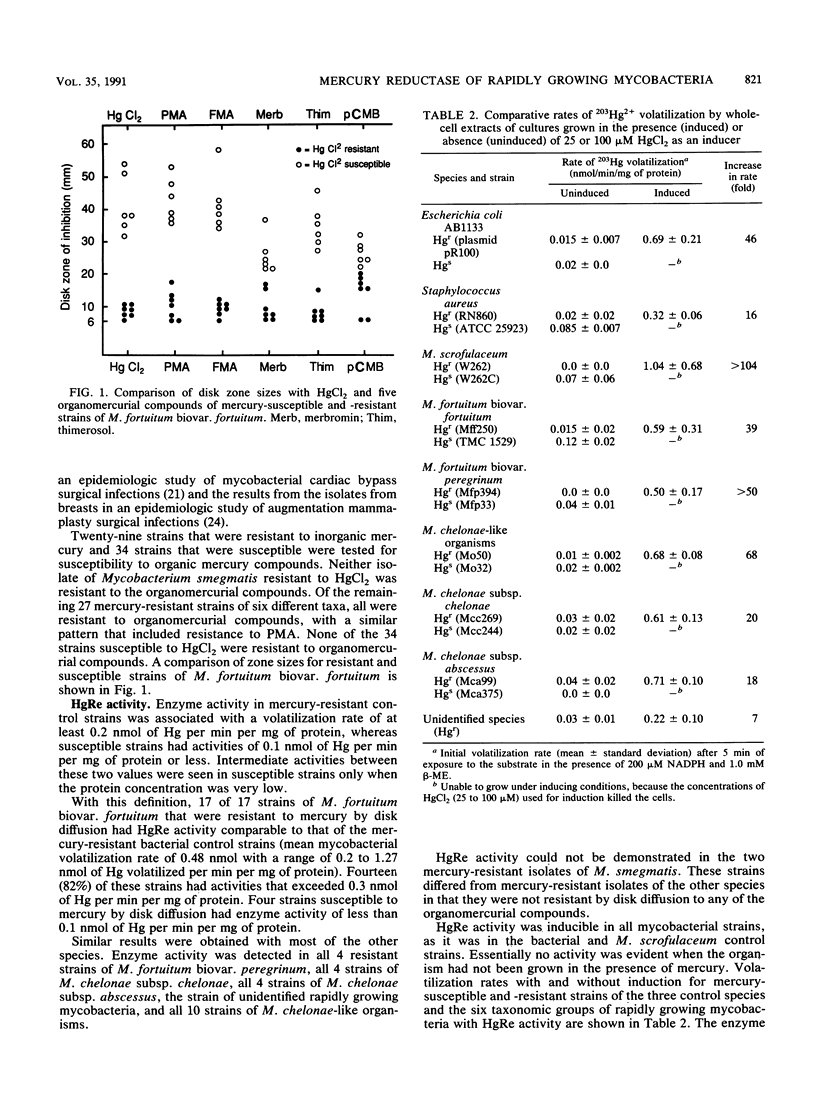
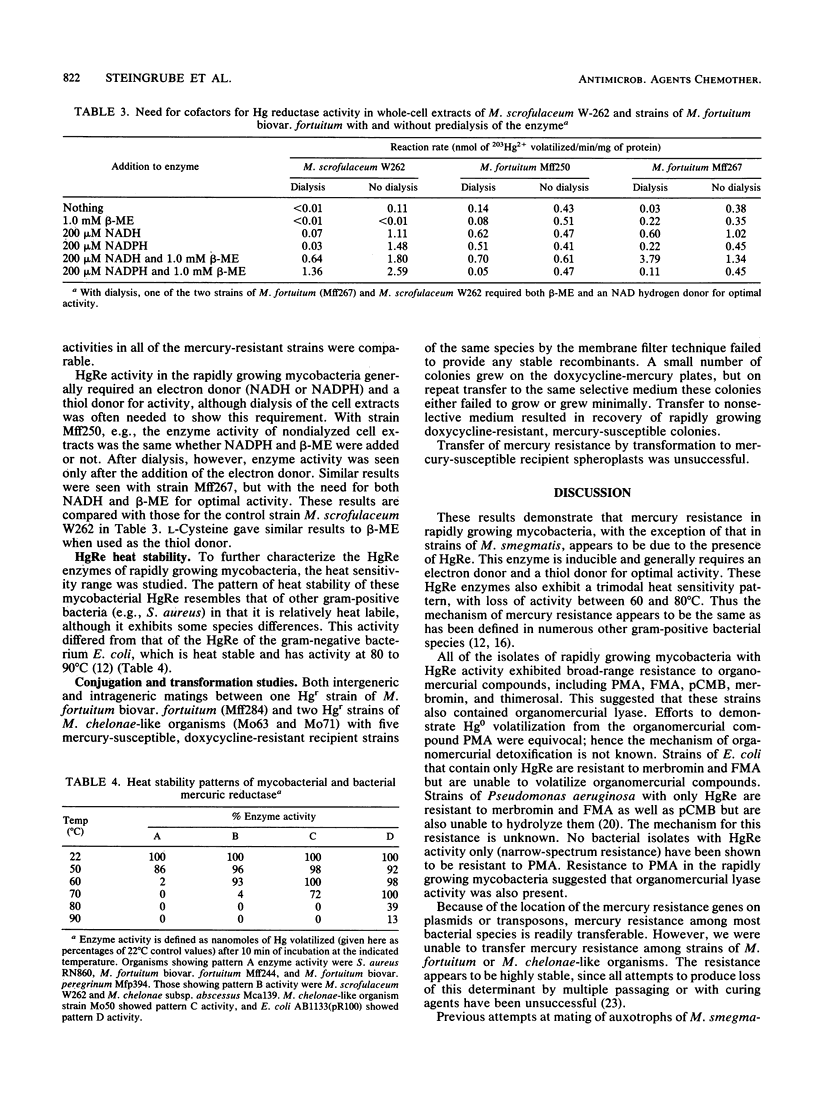
Resistance to mercury was evaluated in 356 rapidly growing mycobacteria belonging to eight taxonomic groups. Resistance to inorganic Hg2+ ranged from 0% among the unnamed third biovariant complex of Mycobacterium fortuitum to 83% among M. chelonae-like organisms. With cell extracts and 203Hg(NO3)2 as the substrate, mercuric reductase (HgRe) activity was demonstrable in six of eight taxonomic groups. HgRe activity was inducible and required NADPH or NADH and a thiol donor for optimai activity. Species with HgRe activity were also resistant to organomercurial compounds, including phenylmercuric acetate. Attempts at intraspecies and intragenus transfer of HgRe activity by conjugation or transformation were unsuccessful. Mercury resistance is common in rapidly growing mycobacteria and appears to function via the same inducible enzyme systems already defined in other bacterial species. This system offers potential as a strain marker for epidemiologic investigations and for studying genetic systems in rapidly growing mycobacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band J. D., Ward J. I., Fraser D. W., Peterson N. J., Silcox V. A., Good R. C., Ostroy P. R., Kennedy J. Peritonitis due to a mycobacterium chelonei-like organism associated with intermittent chronic peritoneal dialysis. J Infect Dis. 1982 Jan;145(1):9–17. doi: 10.1093/infdis/145.1.9. [DOI] [PubMed] [Google Scholar]
- Bhaduri S., Demchick P. H. Simple and rapid method for disruption of bacteria for protein studies. Appl Environ Microbiol. 1983 Oct;46(4):941–943. doi: 10.1128/aem.46.4.941-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. L., Misra T. K., Winnie J. N., Schmidt A., Seiff M., Silver S. The nucleotide sequence of the mercuric resistance operons of plasmid R100 and transposon Tn501: further evidence for mer genes which enhance the activity of the mercuric ion detoxification system. Mol Gen Genet. 1986 Jan;202(1):143–151. doi: 10.1007/BF00330531. [DOI] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, George K. L., Parker B. C., Gruft H. In vitro susceptibility of human and environmental isolates of Mycobacterium avium, M. intracellulare, and M. scrofulaceum to heavy-metal salts and oxyanions. Antimicrob Agents Chemother. 1984 Jan;25(1):137–139. doi: 10.1128/aac.25.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foz A., Roy C., Jurado J., Arteaga E., Ruiz J. M., Moragas A. Mycobacterium chelonei iatrogenic infections. J Clin Microbiol. 1978 Mar;7(3):319–321. doi: 10.1128/jcm.7.3.319-321.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Tuckman M., Bloom B. R. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature. 1987 Jun 11;327(6122):532–535. doi: 10.1038/327532a0. [DOI] [PubMed] [Google Scholar]
- Laddaga R. A., Chu L., Misra T. K., Silver S. Nucleotide sequence and expression of the mercurial-resistance operon from Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5106–5110. doi: 10.1073/pnas.84.15.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner P. S., Falkinham J. O., 3rd Plasmid-encoded mercuric reductase in Mycobacterium scrofulaceum. J Bacteriol. 1984 Feb;157(2):669–672. doi: 10.1128/jb.157.2.669-672.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Suga K., Tokunaga T. Multiple mating types of Mycobacterium smegmatis. Jpn J Microbiol. 1976 Oct;20(5):435–443. doi: 10.1111/j.1348-0421.1976.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I. Frequency of heavy-metal resistance in bacteria from inpatients in Japan. Nature. 1977 Mar 10;266(5598):165–167. doi: 10.1038/266165a0. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I., Kozukue H. Mercury resistance and R plasmids in Escherichia coli isolated from clinical lesions in Japan. Antimicrob Agents Chemother. 1977 Jun;11(6):999–1003. doi: 10.1128/aac.11.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Schottel J. L., Yamada T., Miyakawa Y., Asakawa M., Harville J., Silver S. Mercuric reductase enzymes from Streptomyces species and group B Streptococcus. J Gen Microbiol. 1985 May;131(5):1053–1059. doi: 10.1099/00221287-131-5-1053. [DOI] [PubMed] [Google Scholar]
- Nash D. R., Wallace R. J., Jr, Steingrube V. A., Shurin P. A. Isoelectric focusing of beta-lactamases from sputum and middle ear isolates of Branhamella catarrhalis recovered in the United States. Drugs. 1986;31 (Suppl 3):48–54. doi: 10.2165/00003495-198600313-00012. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Tuovinen O. H. Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: physiological, biochemical, and genetic analyses. Microbiol Rev. 1984 Jun;48(2):95–124. doi: 10.1128/mr.48.2.95-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Taketomo N., Sasaki T. Factors affecting transfer frequency of pAM beta 1 from Streptococcus faecalis to Lactobacillus plantarum. J Bacteriol. 1988 Dec;170(12):5939–5942. doi: 10.1128/jb.170.12.5939-5942.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J. L. The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4341–4349. [PubMed] [Google Scholar]
- Shoemaker S. A., Fisher J. H., Jones W. D., Jr, Scoggin C. H. Restriction fragment analysis of chromosomal DNA defines different strains of Mycobacterium tuberculosis. Am Rev Respir Dis. 1986 Aug;134(2):210–213. doi: 10.1164/arrd.1986.134.2.210. [DOI] [PubMed] [Google Scholar]
- Silcox V. A., Good R. C., Floyd M. M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981 Dec;14(6):686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musser J. M., Hull S. I., Silcox V. A., Steele L. C., Forrester G. D., Labidi A., Selander R. K. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis. 1989 Apr;159(4):708–716. doi: 10.1093/infdis/159.4.708. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Nash D. R., Tsukamura M., Blacklock Z. M., Silcox V. A. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988 Jul;158(1):52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Steele L. C., Forrester G. D., Swenson J. M., Hull S. I. Susceptibilities of Mycobacterium fortuitum biovariant fortuitum and the unnamed third biovariant complex to heavy-metal salts. Antimicrob Agents Chemother. 1984 Oct;26(4):594–596. doi: 10.1128/aac.26.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Steele L. C., Labidi A., Silcox V. A. Heterogeneity among isolates of rapidly growing mycobacteria responsible for infections following augmentation mammaplasty despite case clustering in Texas and other southern coastal states. J Infect Dis. 1989 Aug;160(2):281–288. doi: 10.1093/infdis/160.2.281. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W., Green L., Misra T. K., Silver S. Resistance to mercury and to cadmium in chromosomally resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):663–669. doi: 10.1128/aac.29.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]



