Abstract
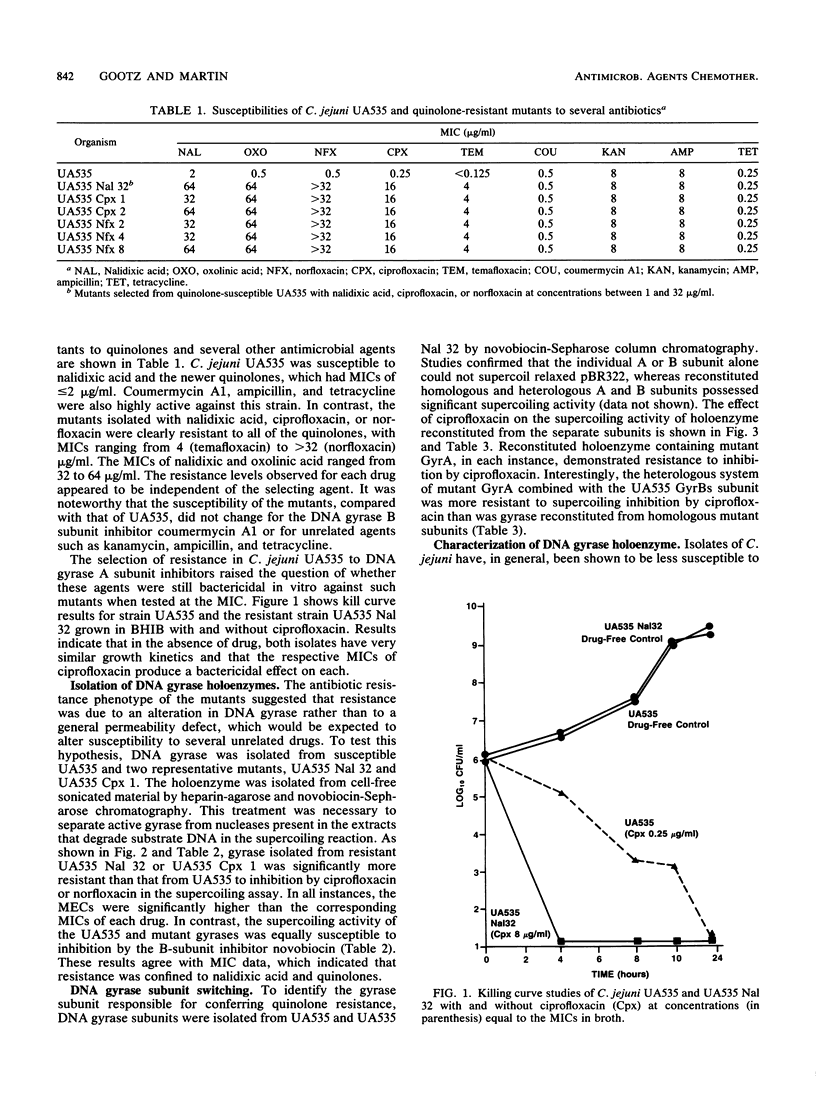
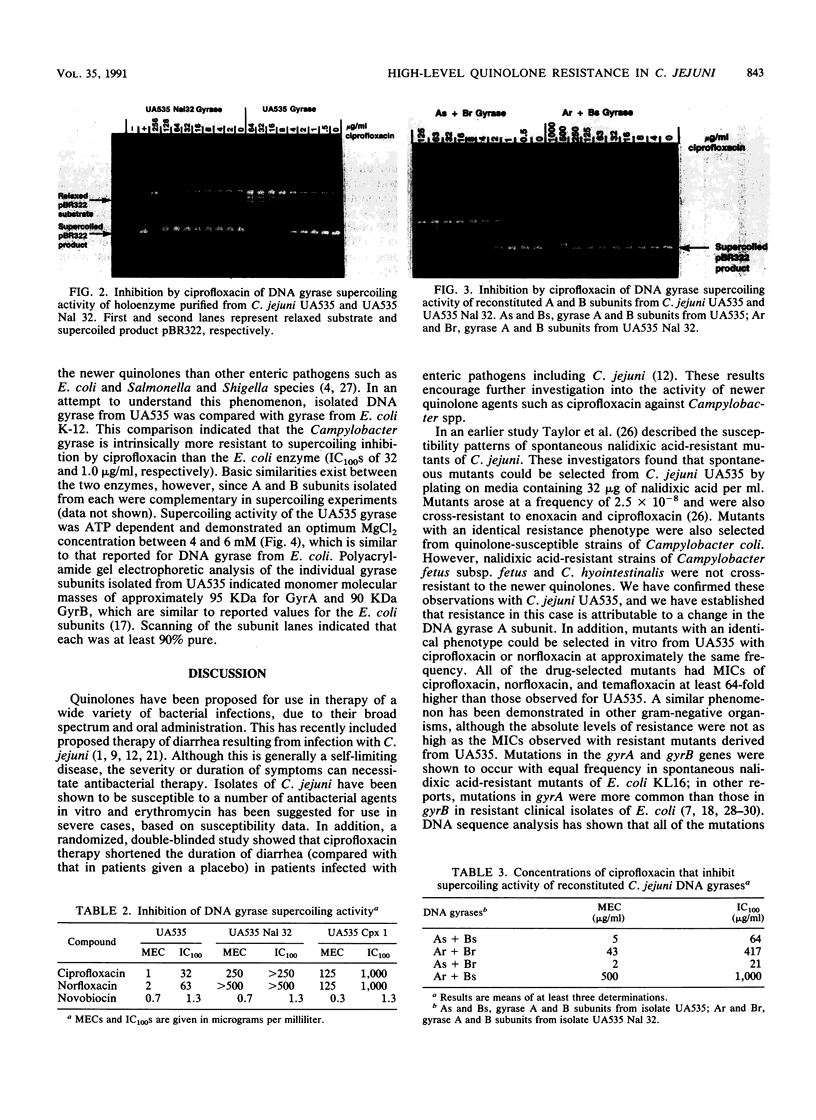
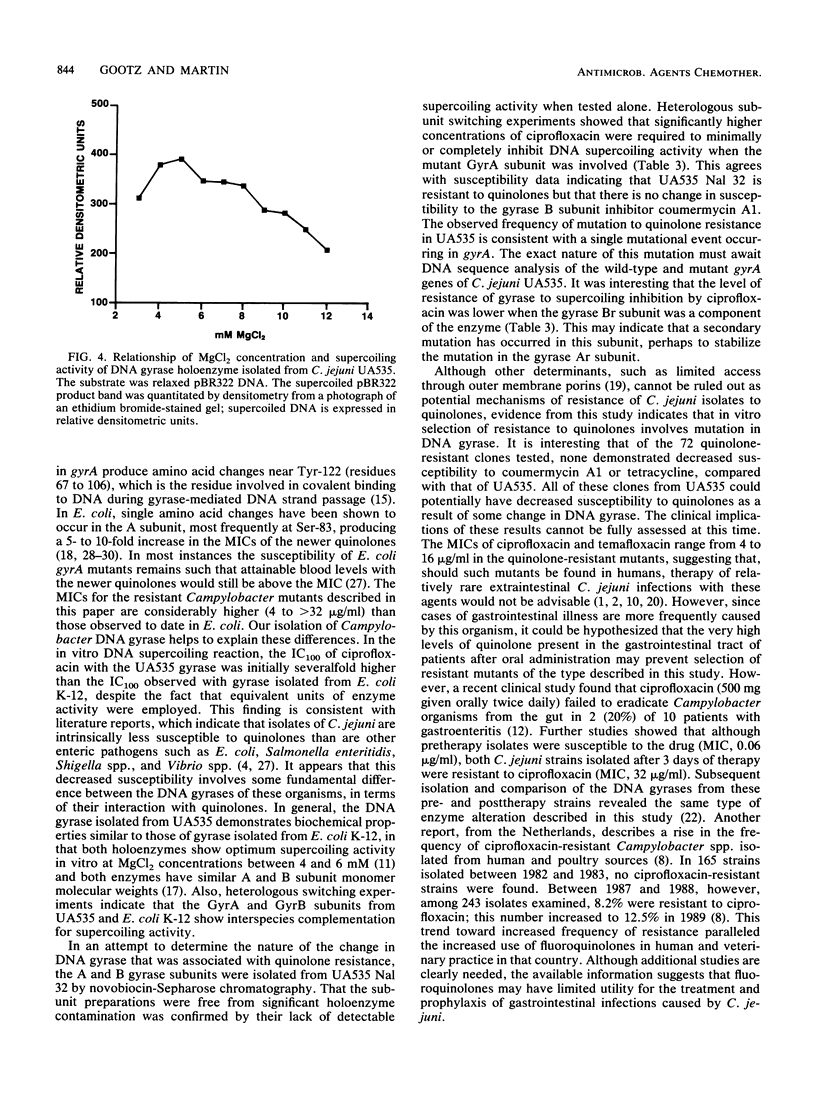
High-level resistance to quinolones has previously been shown to occur in Campylobacter spp. both in vitro and in patients treated with quinolones. We have selected isolates that are resistant to quinolones by plating cells from a susceptible C. jejuni strain, UA535, on medium containing nalidixic acid at 32 micrograms/ml. Fluctuation analysis indicated that resistance occurred by mutation at a frequency of 5 x 10(-8) per cell plated. Unlike what is observed with other gram-negative organisms, the nalidixic acid-resistant mutants demonstrated high-level cross-resistance (MIC, greater than or equal to 4 micrograms/ml) to newer quinolones, including ciprofloxacin, norfloxacin, and temafloxacin, yet remained susceptible to coumermycin A1 and several other unrelated antibiotics. Mutants with an identical resistance phenotype could also be selected from UA535 with ciprofloxacin and norfloxacin at a similar frequency. To study the mechanism of quinolone resistance, DNA gyrases were purified from C. jejuni UA535 and two resistant mutants by heparin-agarose and novobiocin-Sepharose chromatography. After the respective enzyme concentrations were adjusted to equivalent units of activity in the DNA supercoiling reaction, the DNA gyrases from the resistant mutants were found to be 100-fold less susceptible than the wild-type enzyme to inhibition by quinolones. Subunit switching experiments with purified A and B subunits from the wild type and one of the quinolone-resistant mutants indicated that an alteration in the A subunit was responsible for resistance. These results show that a single-step mutation can occur in vitro in the gene encoding DNA gyrase in C. jejuni, producing clinically relevant levels of resistance to the newer quinolones.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black R. E., Levine M. M., Clements M. L., Hughes T. P., Blaser M. J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988 Mar;157(3):472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Perez G. P., Smith P. F., Patton C., Tenover F. C., Lastovica A. J., Wang W. I. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: host factors and strain characteristics. J Infect Dis. 1986 Mar;153(3):552–559. doi: 10.1093/infdis/153.3.552. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- Bryan J. P., Waters C., Sheffield J., Krieg R. E., Perine P. L., Wagner K. In vitro activities of tosufloxacin, temafloxacin, and A-56620 against pathogens of diarrhea. Antimicrob Agents Chemother. 1990 Feb;34(2):368–370. doi: 10.1128/aac.34.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. The pathobiology of Campylobacter infections in humans. Annu Rev Med. 1989;40:269–285. doi: 10.1146/annurev.me.40.020189.001413. [DOI] [PubMed] [Google Scholar]
- Cullen M. E., Wyke A. W., Kuroda R., Fisher L. M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989 Jun;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endtz H. P., Mouton R. P., van der Reyden T., Ruijs G. J., Biever M., van Klingeren B. Fluoroquinolone resistance in Campylobacter spp isolated from human stools and poultry products. Lancet. 1990 Mar 31;335(8692):787–787. doi: 10.1016/0140-6736(90)90897-e. [DOI] [PubMed] [Google Scholar]
- Ericsson C. D., Johnson P. C., Dupont H. L., Morgan D. R., Bitsura J. A., de la Cabada F. J. Ciprofloxacin or trimethoprim-sulfamethoxazole as initial therapy for travelers' diarrhea. A placebo-controlled, randomized trial. Ann Intern Med. 1987 Feb;106(2):216–220. doi: 10.7326/0003-4819-106-2-216. [DOI] [PubMed] [Google Scholar]
- Fleming L. W., Phillips G., Stewart W. K., Scott A. C. Oral ciprofloxacin in the treatment of peritonitis in patients on continuous ambulatory peritoneal dialysis. J Antimicrob Chemother. 1990 Mar;25(3):441–448. doi: 10.1093/jac/25.3.441. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. J., Trenholme G. M., Kaplan R. L., Segreti J., Hines D., Petrak R., Nelson J. A., Mayer K. W., Landau W., Parkhurst G. W. Empiric antimicrobial therapy of domestically acquired acute diarrhea in urban adults. Arch Intern Med. 1990 Mar;150(3):541–546. [PubMed] [Google Scholar]
- Goossens H., De Mol P., Coignau H., Levy J., Grados O., Ghysels G., Innocent H., Butzler J. P. Comparative in vitro activities of aztreonam, ciprofloxacin, norfloxacin, ofloxacin, HR 810 (a new cephalosporin), RU28965 (a new macrolide), and other agents against enteropathogens. Antimicrob Agents Chemother. 1985 Mar;27(3):388–392. doi: 10.1128/aac.27.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T., Retsema J., Girard A., Hamanaka E., Anderson M., Sokolowski S. In vitro activity of CP-65,207, a new penem antimicrobial agent, in comparison with those of other agents. Antimicrob Agents Chemother. 1989 Aug;33(8):1160–1166. doi: 10.1128/aac.33.8.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987 Apr 15;262(11):5339–5344. [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M., O'Dea M. H., Gellert M. Cloning and simplified purification of Escherichia coli DNA gyrase A and B proteins. J Biol Chem. 1984 Jul 25;259(14):9199–9201. [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Nakamura M., Kojima T., Yoshida H. gyrA and gyrB mutations in quinolone-resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):254–255. doi: 10.1128/aac.33.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Huyer G., Huyer M., Worobec E. A. Characterization of the porins of Campylobacter jejuni and Campylobacter coli and implications for antibiotic susceptibility. Antimicrob Agents Chemother. 1989 Mar;33(3):297–303. doi: 10.1128/aac.33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennie R. A., Pearson R. D., Barrett L. J., Lior H., Guerrant R. L. Susceptibility of Campylobacter jejuni to strain-specific bactericidal activity in sera of infected patients. Infect Immun. 1986 Jun;52(3):702–706. doi: 10.1128/iai.52.3.702-706.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler H. E., Diridl G., Stickler K., Wolf D. Clinical efficacy of ciprofloxacin compared with placebo in bacterial diarrhea. Am J Med. 1987 Apr 27;82(4A):329–332. [PubMed] [Google Scholar]
- Segreti J., Nelson J. A., Goodman L. J., Kaplan R. L., Trenholme G. M. In vitro activities of lomefloxacin and temafloxacin against pathogens causing diarrhea. Antimicrob Agents Chemother. 1989 Aug;33(8):1385–1387. doi: 10.1128/aac.33.8.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Courvalin P. Mechanisms of antibiotic resistance in Campylobacter species. Antimicrob Agents Chemother. 1988 Aug;32(8):1107–1112. doi: 10.1128/aac.32.8.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Ng L. K., Lior H. Susceptibility of Campylobacter species to nalidixic acid, enoxacin, and other DNA gyrase inhibitors. Antimicrob Agents Chemother. 1985 Nov;28(5):708–710. doi: 10.1128/aac.28.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]





