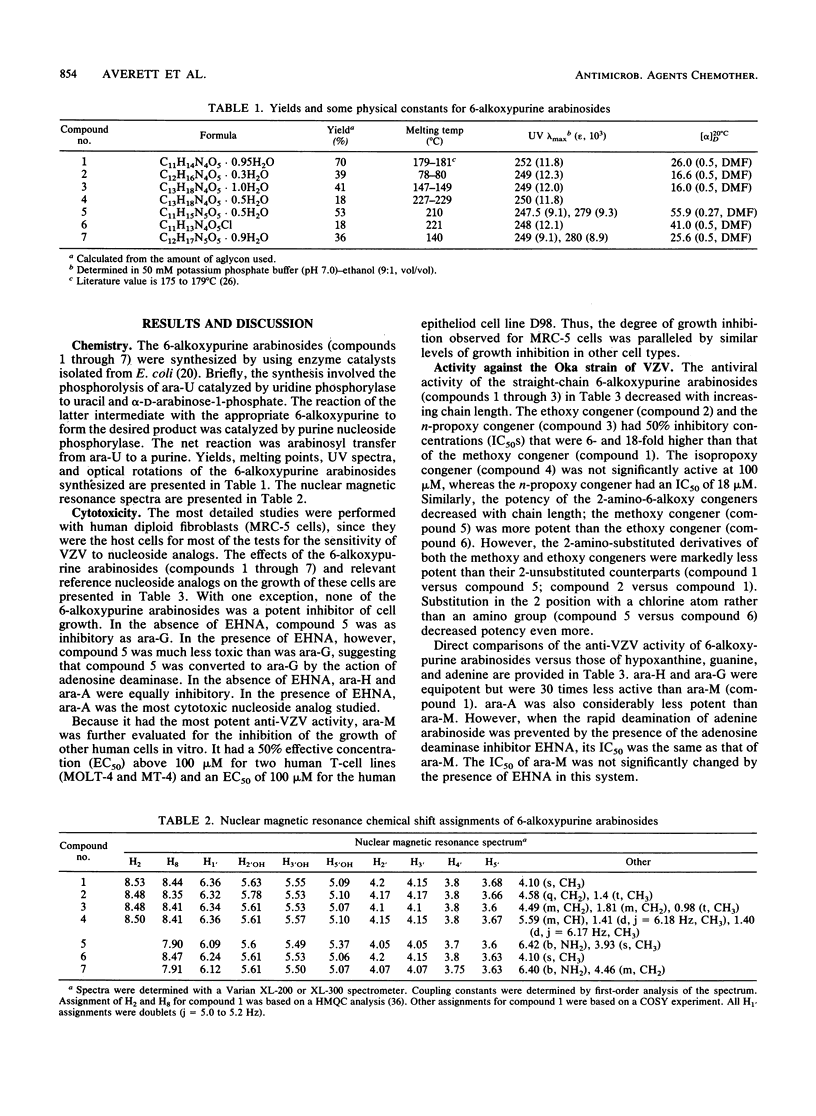
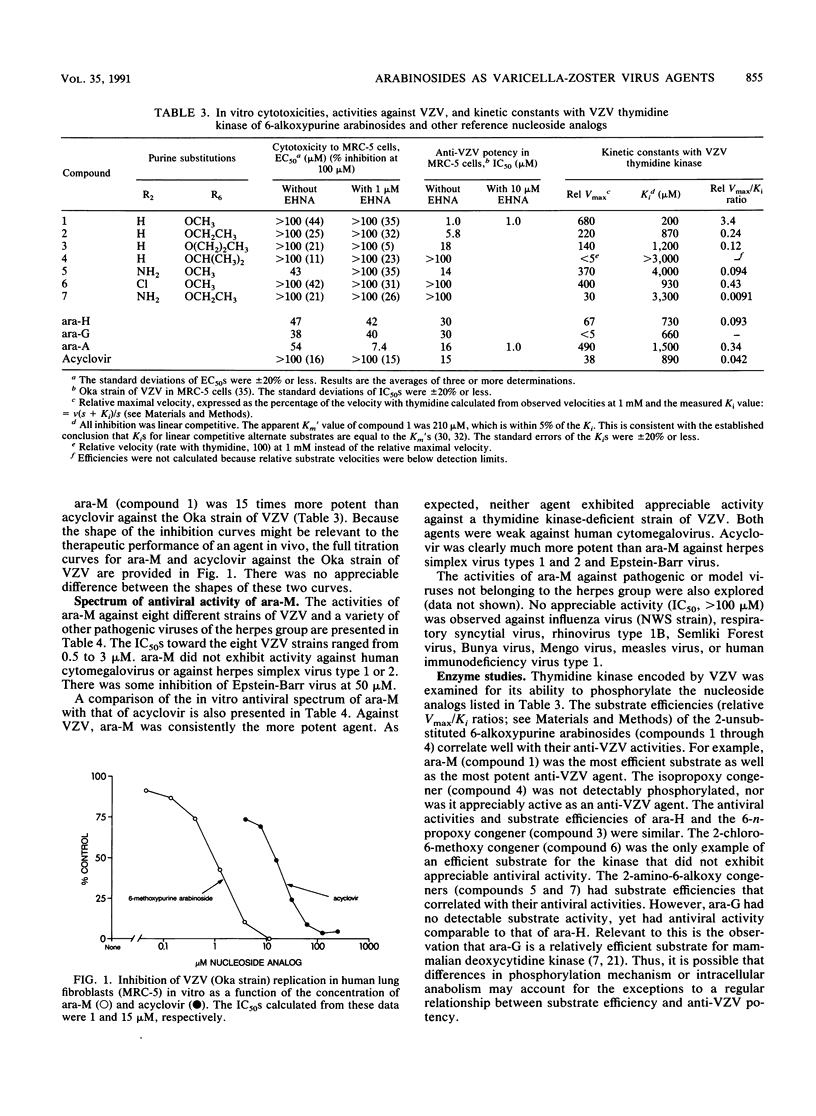
Abstract
Seven 6-alkoxypurine arabinosides were synthesized and evaluated for in vitro activity against varicella-zoster virus (VZV). The simplest of the series, 6-methoxypurine arabinoside (ara-M), was the most potent, with 50% inhibitory concentrations ranging from 0.5 to 3 microM against eight strains of VZV. This activity was selective. The ability of ara-M to inhibit the growth of a variety of human cell lines was at least 30-fold less (50% effective concentration, greater than 100 microM) than its ability to inhibit the virus. Enzyme studies suggested the molecular basis for these results. Of the seven 6-alkoxypurine arabinosides, ara-M was the most efficient substrate for VZV-encoded thymidine kinase as well as the most potent antiviral agent. In contrast, it was not detectably phosphorylated by any of the three major mammalian nucleoside kinases. Upon direct comparison, ara-M was appreciably more potent against VZV than either acyclovir or adenine arabinoside (ara-A). However, in the presence of an adenosine deaminase inhibitor, the arabinosides of adenine and 6-methoxypurine were equipotent but not equally selective; the adenine congener had a much less favorable in vitro chemotherapeutic index. Again, this result correlated with data from enzyme studies in that ara-A, unlike ara-M, was a substrate for two mammalian nucleoside kinases. Unlike acyclovir and ara-A, ara-M had no appreciable activity against other viruses of the herpes group. The potency and selectivity of ara-M as an anti-VZV agent in vitro justify its further study.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averett D. R. Anti-HIV compound assessment by two novel high capacity assays. J Virol Methods. 1989 Mar;23(3):263–276. doi: 10.1016/0166-0934(89)90159-6. [DOI] [PubMed] [Google Scholar]
- Biron K. K., Elion G. B. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980 Sep;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Stanat S. C., Sorrell J. B., Fyfe J. A., Keller P. M., Lambe C. U., Nelson D. J. Metabolic activation of the nucleoside analog 9-[( 2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohman C., Eriksson S. Deoxycytidine kinase from human leukemic spleen: preparation and characteristics of homogeneous enzyme. Biochemistry. 1988 Jun 14;27(12):4258–4265. doi: 10.1021/bi00412a009. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Fyfe J. A., St Clair M. H., Weinhold K., Rideout J. L., Freeman G. A., Lehrman S. N., Bolognesi D. P., Broder S., Mitsuya H. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Fyfe J. A., McKee S. A., Keller P. M. Altered thymidine-thymidylate kinases from strains of herpes simplex virus with modified drug sensitivities to acyclovir and (E)-5-(2-bromovinyl)-2'-deoxyuridine. Mol Pharmacol. 1983 Sep;24(2):316–323. [PubMed] [Google Scholar]
- Kelley J. L., Selway J. W., Schaeffer H. J. Purine acyclic nucleosides. 6-Dimethylamino-9-[(2-phenylalanylamido-1-substituted- ethoxy)methyl]purines as candidate antivirals. J Pharm Sci. 1985 Dec;74(12):1302–1304. doi: 10.1002/jps.2600741211. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Freeman G. A., Shaver S. R., Beacham L. M., 3rd, Hurlbert S., Cohn N. K., Elwell L. P., Selway J. W. 3'-Amino-2',3'-dideoxyribonucleosides of some pyrimidines: synthesis and biological activities. J Med Chem. 1983 Jun;26(6):891–895. doi: 10.1021/jm00360a019. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Koszalka G. W., Tuttle J. V. Purine nucleoside synthesis, an efficient method employing nucleoside phosphorylases. Biochemistry. 1981 Jun 9;20(12):3615–3621. doi: 10.1021/bi00515a048. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Koszalka G. W., Chen I. S., Beacham L. M., 3rd, Rideout J. L., Elion G. B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976 Jul 10;251(13):4055–4061. [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein-Barr virus. J Virol. 1984 Apr;50(1):50–55. doi: 10.1128/jvi.50.1.50-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey T. A., Agarwal L. A., Weksler B. B. A rapid fluorometric DNA assay for the measurement of cell density and proliferation in vitro. In Vitro Cell Dev Biol. 1988 Mar;24(3):247–252. doi: 10.1007/BF02623555. [DOI] [PubMed] [Google Scholar]
- McLaren C., Ellis M. N., Hunter G. A. A colorimetric assay for the measurement of the sensitivity of herpes simplex viruses to antiviral agents. Antiviral Res. 1983 Nov;3(4):223–234. doi: 10.1016/0166-3542(83)90001-3. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Adamczyk D. L., Miller W. H., Koszalka G. W., Rideout J. L., Beacham L. M., 3rd, Chao E. Y., Haggerty J. J., Krenitsky T. A., Elion G. B. Adenosine kinase from rabbit liver. II. Substrate and inhibitor specificity. J Biol Chem. 1979 Apr 10;254(7):2346–2352. [PubMed] [Google Scholar]
- Rideout J. L., Krenitsky T. A., Koszalka G. W., Cohn N. K., Chao E. Y., Elion G. B., Latter V. S., Williams R. B. Pyrazolo[3,4-d]pyrimidine ribonucleosides as anticoccidials. 2. Synthesis and activity of some nucleosides of 4-(alkylamino)-1H-pyrazolo[3, 4-d]pyrimidines. J Med Chem. 1982 Sep;25(9):1040–1044. doi: 10.1021/jm00351a007. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Schwender C. F. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974 Jan;17(1):6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Spector T., Cleland W. W. Meanings of Ki for conventional and alternate-substrate inhibitors. Biochem Pharmacol. 1981 Jan 1;30(1):1–7. doi: 10.1016/0006-2952(81)90277-x. [DOI] [PubMed] [Google Scholar]
- Spector T., Hajian G. Statistical methods to distinguish competitive, noncompetitive, and uncompetitive enzyme inhibitors. Anal Biochem. 1981 Aug;115(2):403–409. doi: 10.1016/0003-2697(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Spector T., Harrington J. A., Morrison R. W., Jr, Lambe C. U., Nelson D. J., Averett D. R., Biron K., Furman P. A. 2-Acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (A1110U), a potent inactivator of ribonucleotide reductases of herpes simplex and varicella-zoster viruses and a potentiator of acyclovir. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1051–1055. doi: 10.1073/pnas.86.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Spector T., Stonehuerner J. G., Biron K. K., Averett D. R. Ribonucleotide reductase induced by varicella zoster virus. Characterization, and potentiation of acyclovir by its inhibition. Biochem Pharmacol. 1987 Dec 15;36(24):4341–4346. doi: 10.1016/0006-2952(87)90682-4. [DOI] [PubMed] [Google Scholar]


