Abstract
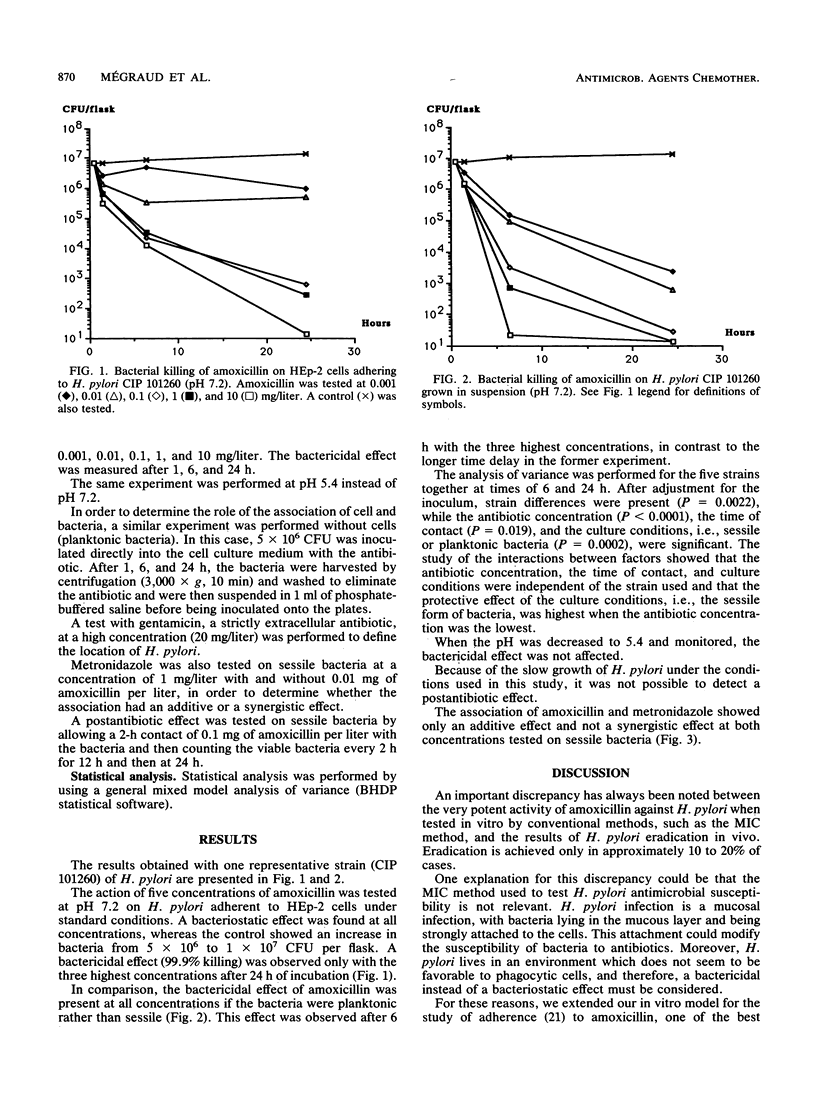
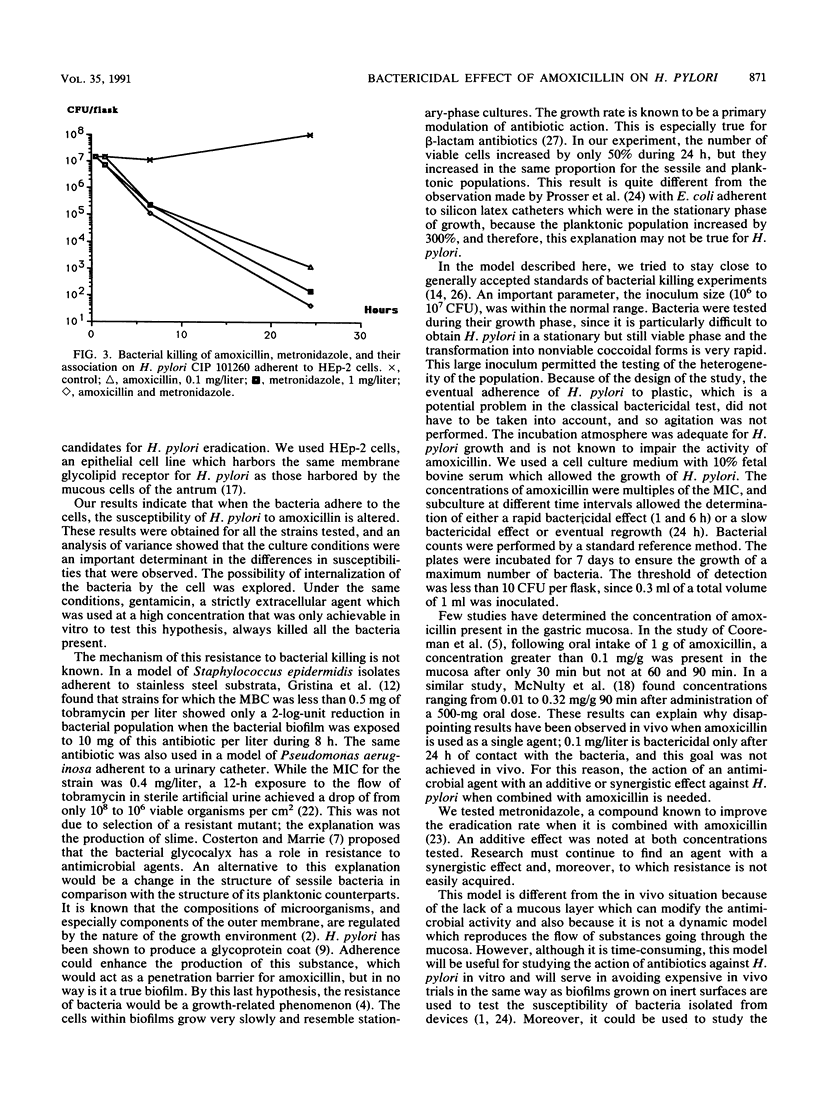
The treatment of Helicobacter pylori with antimicrobial agents has largely been ineffective, and susceptibility results are in disagreement with those obtained by standard in vitro testing. The bactericidal effect of amoxicillin was tested in an in vitro model by using sessile bacteria attached to HEp-2 cells; this bactericidal effect was compared with that against planktonic bacteria. Viable cell counts were performed by standard procedures after 1, 6, and 24 h of contact with the antibiotic at different concentrations. A bactericidal effect (99.9% killing) was observed against sessile bacteria after 24 h with concentrations of only 10, 1, and 0.1 mg/liter, while against planktonic bacteria it was also noted at concentrations of 0.01 and 0.001 mg/liter, and the effect was observed after 6 h with the three highest concentrations. When the results for five strains were studied by analysis of variance at 6 and 24 h, the main variable was the antibiotic concentration, followed by the culture conditions, e.g., planktonic or sessile bacteria, the strain tested, and the time of contact. A decreased pH of 5.4 did not affect the action of amoxicillin. The bactericidal effect of the combination of amoxicillin and metronidazole was additive against sessile H. pylori.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., Costerton J. W. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Sep;34(9):1666–1671. doi: 10.1128/aac.34.9.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar H., Dasgupta M. K., Costerton J. W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990 Nov;34(11):2043–2046. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Allison D. G., Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988 Dec;22(6):777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- Glupczynski Y., Delmee M., Bruck C., Labbe M., Avesani V., Burette A. Susceptibility of clinical isolates of Campylobacter pylori to 24 antimicrobial and anti-ulcer agents. Eur J Epidemiol. 1988 Jun;4(2):154–157. doi: 10.1007/BF00144743. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Blake P., Blincow E. The minimum inhibitory and bactericidal concentrations of antibiotics and anti-ulcer agents against Campylobacter pyloridis. J Antimicrob Chemother. 1986 Mar;17(3):309–314. doi: 10.1093/jac/17.3.309. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Gristina A. G., Hobgood C. D., Webb L. X., Myrvik Q. N. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials. 1987 Nov;8(6):423–426. doi: 10.1016/0142-9612(87)90077-9. [DOI] [PubMed] [Google Scholar]
- Jeffries L., Buckley D. E., Blower P. R., Plumb J. N. Comparative sensitivities to antimicrobial agents of Campylobacter pylori and the gastric campylobacter like organism from the ferret. J Clin Pathol. 1987 Oct;40(10):1265–1267. doi: 10.1136/jcp.40.10.1265-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Anthony B. F. Importance of bacterial growth phase in determining minimal bactericidal concentrations of penicillin and methicillin. Antimicrob Agents Chemother. 1981 Jun;19(6):1075–1077. doi: 10.1128/aac.19.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Mégraud F., Gerbaud G., Courvalin P. Susceptibility of Campylobacter pyloridis to 20 antimicrobial agents. Antimicrob Agents Chemother. 1986 Sep;30(3):510–511. doi: 10.1128/aac.30.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouliatte H. Traitement des gastrites chroniques associées à Campylobacter pylori. Gastroenterol Clin Biol. 1989;13(1 Pt 1):101B–106B. [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Pellizzari A., Sherman P., Drumm B. Gastric glycerolipid as a receptor for Campylobacter pylori. Lancet. 1989 Jul 29;2(8657):238–241. doi: 10.1016/s0140-6736(89)90428-5. [DOI] [PubMed] [Google Scholar]
- McNulty C. A., Dent J. C., Ford G. A., Wilkinson S. P. Inhibitory antimicrobial concentrations against Campylobacter pylori in gastric mucosa. J Antimicrob Chemother. 1988 Nov;22(5):729–738. doi: 10.1093/jac/22.5.729. [DOI] [PubMed] [Google Scholar]
- Megraud F., Bonnet F., Garnier M., Lamouliatte H. Characterization of "Campylobacter pyloridis" by culture, enzymatic profile, and protein content. J Clin Microbiol. 1985 Dec;22(6):1007–1010. doi: 10.1128/jcm.22.6.1007-1010.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neman-Simha V., Mégraud F. In vitro model for Campylobacter pylori adherence properties. Infect Immun. 1988 Dec;56(12):3329–3333. doi: 10.1128/iai.56.12.3329-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderda G., Vaira D., Holton J., Ainley C., Altare F., Ansaldi N. Amoxycillin plus tinidazole for Campylobacter pylori gastritis in children: assessment by serum IgG antibody, pepsinogen I, and gastrin levels. Lancet. 1989 Apr 1;1(8640):690–692. doi: 10.1016/s0140-6736(89)92206-x. [DOI] [PubMed] [Google Scholar]
- Prosser B. L., Taylor D., Dix B. A., Cleeland R. Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrob Agents Chemother. 1987 Oct;31(10):1502–1506. doi: 10.1128/aac.31.10.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauws E. A., Tytgat G. N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990 May 26;335(8700):1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- Taylor P. C., Schoenknecht F. D., Sherris J. C., Linner E. C. Determination of minimum bactericidal concentrations of oxacillin for Staphylococcus aureus: influence and significance of technical factors. Antimicrob Agents Chemother. 1983 Jan;23(1):142–150. doi: 10.1128/aac.23.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R., Tosch W., Zak O., Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986 May;132(5):1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]



