Abstract
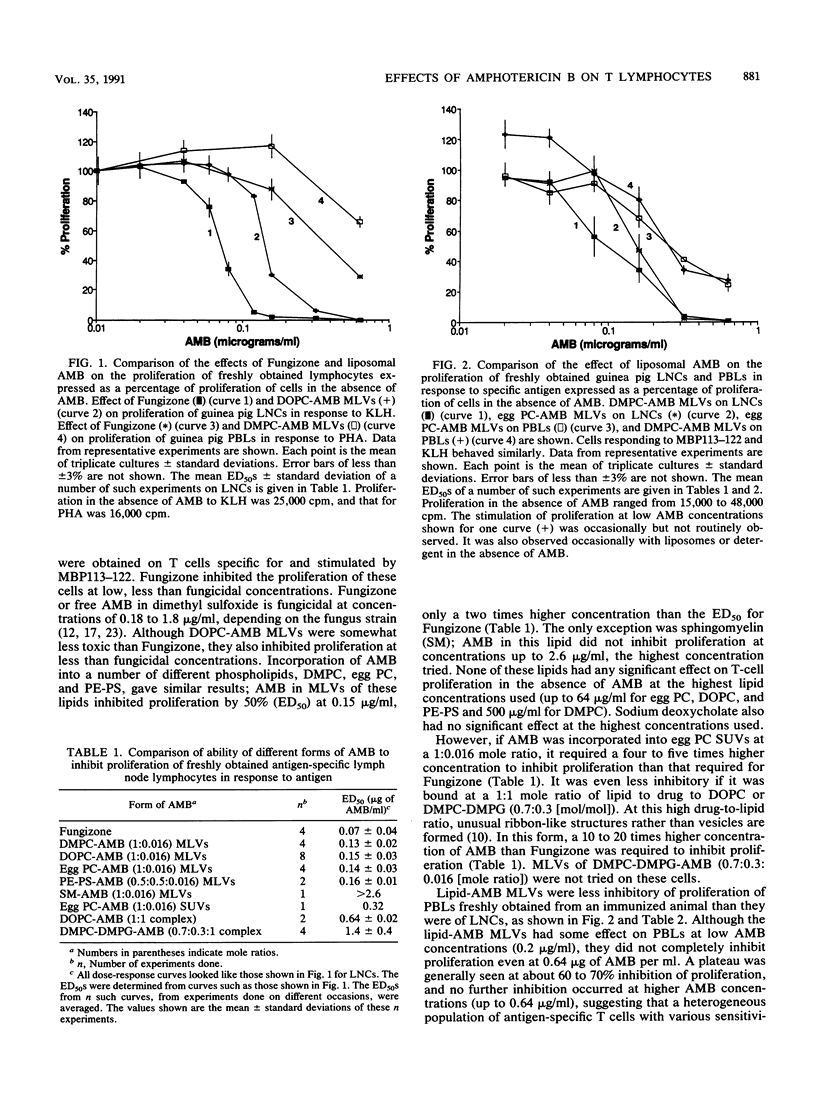
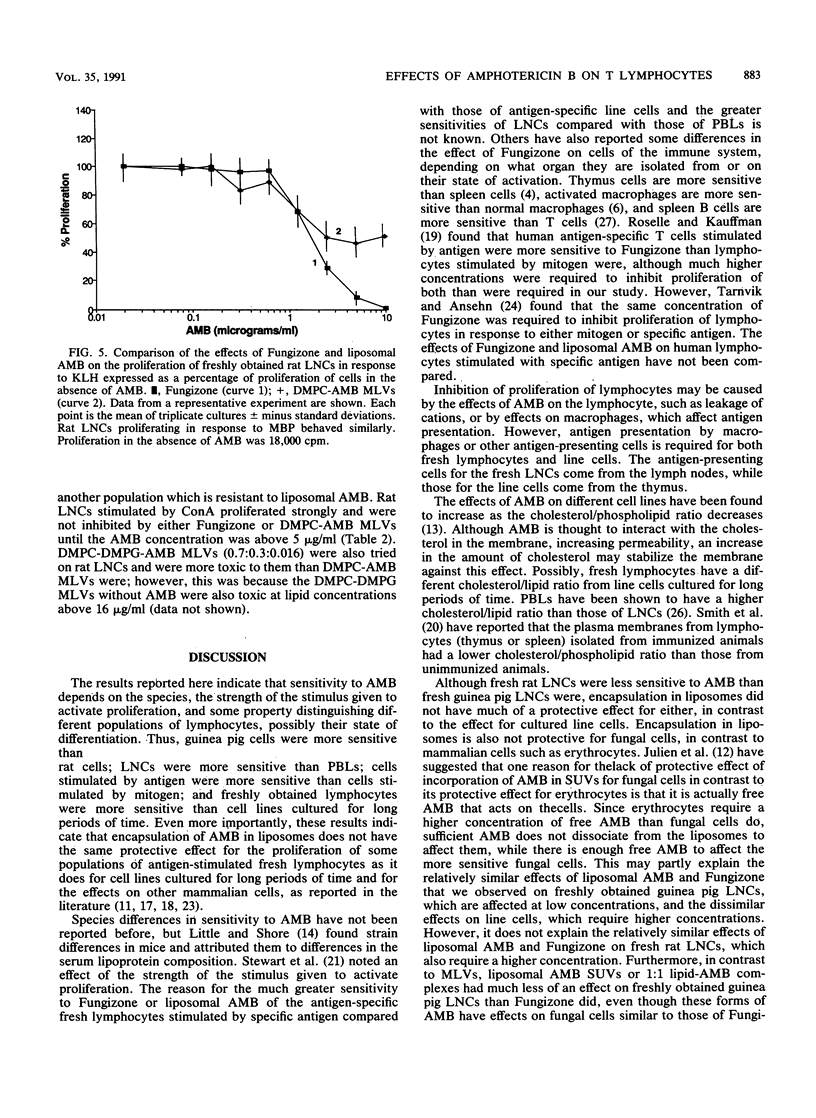
The effects of free amphotericin B (as Fungizone) and amphotericin B (AMB) incorporated into liposomes on the proliferation of lymphocytes were determined. Freshly obtained guinea pig and rat antigen-specific lymphocytes were compared with rat T-lymphocyte cell lines cultured for a long period of time. Incorporation of AMB into multilayered vesicles significantly reduced its effect relative to that of Fungizone on cultured T-cell lines, as reported by others for mammalian cells. In contrast, the effects on freshly obtained antigen-specific lymphocytes were different. Fungizone inhibited proliferation of antigen-specific lymph node cells freshly obtained from immunized guinea pigs at fungicidal concentrations, and incorporation into multilayered lipid vesicles did not have much of a protective effect. Higher concentrations of Fungizone were required to inhibit proliferation of fresh rat lymph node cells, but incorporation into multilayered lipid vesicles still did not have much of a protective effect. Some T lymphocytes in the peripheral circulation of guinea pigs and in the lymph nodes of rats were more resistant to liposomal AMB than another more sensitive T-lymphocyte population was. Proliferation of lymphocytes in response to mitogens was inhibited less than that in response to specific antigen was. Thus, sensitivity to AMB depended on the species, the strength of the stimulus used to activate the lymphocytes, and on some other property of the lymphocytes, possibly their state of differentiation. Regardless of the reason for the difference in effects on freshly obtained lymph node lymphocytes and cultured line cells, the former may be more relevant to effects in vivo and should be considered in a complete evaluation of the in vivo toxicity of these forms of the drug. Incorporation into sonicated unilamellar vesicles had more of a protective effect, while equimolar drug-lipid complexes had even more of a protective effect. These forms of AMB might have less of an immunosuppressive potential than multilayered vesicles containing low amounts of AMB do.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Sarkar A. K., Bachhawat B. K. Design of liposomes to improve delivery of amphotericin-B in the treatment of aspergillosis. 1989 Nov 23-Dec 19Mol Cell Biochem. 91(1-2):85–90. doi: 10.1007/BF00228082. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol. 1982 Jul;129(1):303–308. [PubMed] [Google Scholar]
- Blanke T. J., Little J. R., Shirley S. F., Lynch R. G. Augmentation of murine immune responses by amphotericin B. Cell Immunol. 1977 Sep;33(1):180–190. doi: 10.1016/0008-8749(77)90145-9. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Goundalkar A., Doganoglu F., Samji N., Kurantsin-Mills J., Koshy K. M. Antigen-targeted liposome-encapsulated methotrexate specifically kills lymphocytes sensitized to the nonapeptide of myelin basic protein. J Neuroimmunol. 1987 Dec;17(1):35–48. doi: 10.1016/0165-5728(87)90029-4. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Hibbs J. B., Jr Modulation of macrophage tumoricidal capability by polyene antibiotics: support for membrane lipid as a regulatory determinant of macrophage function. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4349–4353. doi: 10.1073/pnas.75.9.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S., Moscarello M. A. Effect of bovine basic protein charge microheterogeneity on protein-induced aggregation of unilamellar vesicles containing a mixture of acidic and neutral phospholipids. Biochemistry. 1985 Apr 9;24(8):1909–1914. doi: 10.1021/bi00329a016. [DOI] [PubMed] [Google Scholar]
- Coune A. Liposomes as drug delivery system in the treatment of infectious diseases. Potential applications and clinical experience. Infection. 1988 May-Jun;16(3):141–147. doi: 10.1007/BF01644088. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Janoff A. S., Boni L. T., Popescu M. C., Minchey S. R., Cullis P. R., Madden T. D., Taraschi T., Gruner S. M., Shyamsunder E., Tate M. W. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Grant C. W., Barber K. R., Kalp M. A. Mechanism of the selective toxicity of amphotericin B incorporated into liposomes. Mol Pharmacol. 1987 Jan;31(1):1–11. [PubMed] [Google Scholar]
- Jullien S., Brajtburg J., Bolard J. Affinity of amphotericin B for phosphatidylcholine vesicles as a determinant of the in vitro cellular toxicity of liposomal preparations. Biochim Biophys Acta. 1990 Jan 15;1021(1):39–45. doi: 10.1016/0005-2736(90)90381-w. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Schlessinger D. Sensitivity to amphotericin B and the cholesterol: phospholipid molar ratios of 3T3, L, BHK and HeLa cells. Biochem Pharmacol. 1977 Apr 15;26(8):705–710. doi: 10.1016/0006-2952(77)90212-x. [DOI] [PubMed] [Google Scholar]
- Little J. R., Shore V. Modulation by lipoproteins of amphotericin B-induced immunostimulation. Cell Immunol. 1985 Jun;93(1):212–221. doi: 10.1016/0008-8749(85)90401-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Fainstein V., Hopfer R., Mehta K., Sullivan M. P., Keating M., Rosenblum M. G., Mehta R., Luna M., Hersh E. M. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. J Infect Dis. 1985 Apr;151(4):704–710. doi: 10.1093/infdis/151.4.704. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Mehta R. T., Mehta K., Lopez-Berestein G., Juliano R. L. Effect of liposomal amphotericin B on murine macrophages and lymphocytes. Infect Immun. 1985 Feb;47(2):429–433. doi: 10.1128/iai.47.2.429-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R., Lopez-Berestein G., Hopfer R., Mills K., Juliano R. L. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim Biophys Acta. 1984 Mar 14;770(2):230–234. doi: 10.1016/0005-2736(84)90135-4. [DOI] [PubMed] [Google Scholar]
- Roselle G. A., Kauffman C. A. Amphotericin B and 5-fluorocytosine: in vitro effects on lymphocyte function. Antimicrob Agents Chemother. 1978 Sep;14(3):398–402. doi: 10.1128/aac.14.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. I., Ladoulis C. T., Misra D. N., Gill T. J., Bazin H. Lymphocyte plasma membranes. III. Composition of lymphocyte plasma membranes from normal and immunized rats. Biochim Biophys Acta. 1975 Apr 8;382(4):506–525. doi: 10.1016/0005-2736(75)90218-7. [DOI] [PubMed] [Google Scholar]
- Stewart S. J., Spagnuolo P. J., Ellner J. J. Generation of suppressor T lymphocytes and monocytes by amphotericin B. J Immunol. 1981 Jul;127(1):135–139. [PubMed] [Google Scholar]
- Supapidhayakul S. R., Kizlaitis L. R., Andersen B. R. Stimulation of human and canine neutrophil metabolism by amphotericin B. Antimicrob Agents Chemother. 1981 Feb;19(2):284–289. doi: 10.1128/aac.19.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F. C., Jr, Milholland D., Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob Agents Chemother. 1987 Mar;31(3):421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C., Barza M., Fiore C., Szoka F. Efficacy of liposome-intercalated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother. 1984 Aug;26(2):170–173. doi: 10.1128/aac.26.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tärnvik A., Ansehn S. Effect of amphotericin B and clotrimazole on lymphocyte stimulation. Antimicrob Agents Chemother. 1974 Nov;6(5):529–533. doi: 10.1128/aac.6.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls E. V., Kay J. E. Inhibition of proliferation of a murine myeloma cell line and mitogen-stimulated B lymphocytes by the antibiotic amphotericin B (Fungizone). Immunology. 1982 Sep;47(1):115–121. [PMC free article] [PubMed] [Google Scholar]


