Abstract
Background and purpose:
Body core temperature (Tc) changes affect the QT interval, but correction for this has not been systematically investigated. It may be important to correct QT intervals for drug-induced changes in Tc.
Experimental approach:
Anaesthetized beagle dogs were artificially cooled (34.2 °C) or warmed (42.1 °C). The relationship between corrected QT intervals (QTcV; QT interval corrected according to the Van de Water formula) and Tc was analysed. This relationship was also examined in conscious dogs where Tc was increased by exercise.
Key results:
When QTcV intervals were plotted against changes in Tc, linear correlations were observed in all individual dogs. The slopes did not significantly differ between cooling (−14.85±2.08) or heating (−13.12±3.46) protocols. We propose a correction formula to compensate for the influence of Tc changes and standardize the QTcV duration to 37.5 °C: QTcVcT (QTcV corrected for changes in core temperature)=QTcV–14 (37.5 – Tc). Furthermore, cooled dogs were re-warmed (from 34.2 to 40.0 °C) and marked QTcV shortening (−29%) was induced. After Tc correction, using the above formula, this decrease was abolished. In these re-warmed dogs, we observed significant increases in T-wave amplitude and in serum [K+] levels. No arrhythmias or increase in pro-arrhythmic biomarkers were observed. In exercising dogs, the above formula completely compensated QTcV for the temperature increase.
Conclusions and implications:
This study shows the importance of correcting QTcV intervals for changes in Tc, to avoid misleading interpretations of apparent QTcV interval changes. We recommend that all ICH S7A, conscious animal safety studies should routinely measure core body temperature and correct QTcV appropriately, if body temperature and heart rate changes are observed.
Keywords: hyperthermia, hypothermia, QT interval, dog, correction formula, hyperkalaemia, hypokalaemia, drug-induced temperature changes
Introduction
The QT interval of the ECG is an indirect measurement of the time taken for the ventricles to depolarize and repolarize. Assessment of the QT interval is of clinical importance because prolongation of repolarization is often associated with conditions such as electrical instability and sudden cardiac death (Algra et al., 1991), but can also be indicative of the pro-arrhythmic activity for new chemical entities (De Clerck et al., 2002). As the QT interval is dependent on the length of the cardiac cycle, this interval has to be corrected for changes in heart rate (HR) (Fridericia, 1920; Van De Water et al., 1989). In addition to HR, changes in core temperature (Tc) can also influence the ventricular repolarization time (Alhaddad et al., 2000). However, Tc is rarely measured in Good Laboratory Practice safety studies, even though most radio-telemetry devices used in such studies have a temperature measurement function, and a method for correcting this phenomenon is currently lacking. Tc is normally maintained within narrow well-defined limits, but under certain circumstances, it can be increased, for example, by pharmacological agents (White and Simpson, 1984; Nimmo et al., 1993; serotonin syndrome, see www.fda.gov/cder/drug/advisory/SSRI_SS200607.htm), by fever or by malignant hyperthermia, and can be decreased, for example, during surgery, postoperative recovery and illness. If the QTc interval is used to diagnose prolongation of repolarization during hypo- or hyperthermic situations within drug safety evaluations, then the sole use of a standard QT correction formula may be inadequate and thus misleading. Over-correction or under-correction may lead to artificial results, when body temperature is changed. Although the underlying mechanisms of QT interval changes associated with hypo- and hyperthermia are not fully understood, it is known that serum potassium concentrations can increase during hyperthermia in different species (Spurr and Barlow, 1959; Sprung et al., 1991), and during malignant hyperthermia in humans (Wappler et al., 2000). However, what role these electrolyte alterations have on cardiac repolarization during temperature change is also unknown, as action potential durations are also increased in in vitro studies—using tissue from guinea-pigs (Lathrop et al., 1998), rabbits (unpublished data) and pigs (Roscher et al., 2001), where electrolyte levels are controlled through perfusion of physiological salt solutions. The aim of the present study was to explore the changes of QT interval (QTcV interval; QT interval corrected according to the Van de Water formula), under controlled hypothermic and hyperthermic conditions in anaesthetized dogs, and to propose a mathematical formula to correct the QTcV interval for changes in body temperature. The formula was tested in the same group of dogs, to mimic clinical situations, such as re-warming (analogous to procedures for postoperative or cooled patients) and re-cooling (procedures for heat stroke or fever). Furthermore, we have challenged this formula during exercise tests in conscious telemetered beagle dogs, as exercise also increases body temperature (Febbraio et al., 1996) and none of the existing formulae correct the QT interval properly during exercise (Aytemir et al., 1999; Benatar and Decraene, 2001; Newbold et al., 2007). The applicability of the formula in dogs and humans under different circumstances was further investigated by a review of published data.
Methods
This investigation was conducted in accordance with ‘the provision of the European Convention' on the protection of vertebrate animals, which are used for experimental and other scientific purposes, and with ‘the Appendices A and B', made at Strasbourg on 18 March 1986 (Belgian Act of 18 October 1991).
Animals
Sixteen adult beagle dogs (eight males and eight females) were used in this study, their body weight averaging 11.9 kg (range: 9.8–13.8 kg) and their age averaging 12 months (range: 10–17 months). All dogs were examined before use and found to be healthy and active. Food (but not water) was withheld for at least 12 h prior to anaesthesia and cardiovascular experimentation.
Anaesthesia and measured parameters
Briefly, under total intravenous anaesthesia induced by 0.075 mg kg−1 lofentanil, 0.0015 mg kg−1 scopolamine (Janssen Pharmaceutica, Johnson & Johnson Pharmaceutical R&D (J&J PRD), Beerse, Belgium) and 1.0 mg kg−1 succinylcholine (Myoplegine; Christiaens NV, Brussels, Belgium) and maintained by infusion of etomidate 1.5 mg kg−1 h−1 (J&J PRD), dogs were ventilated with 30% oxygen in pressurized air to normocapnia (PaCO2 between 30 and 50 mm Hg). The ECG (ECG lead II limb leads; Emka, Paris, France) was continuously monitored (sampling frequency, 1000 Hz) and an open lumen catheter was placed into the femoral artery and positioned close to the heart to obtain blood samples for blood gas analysis (ABL700; Radiometer, Brønshøj, Denmark), to monitor stability of the preparation over the course of the experiment.
Heart rate (b.p.m.) was calculated from the pressure signal and the QT interval (measured from the onset of the QRS to the end of the T wave; ms) was taken from the lead II ECG, from which the T-wave morphology was also examined. Furthermore, the QT intervals were corrected for changes in HR according to the Van de Water formula (QTcV; ms, Van de Water et al., 1989). Body Tc (°C) was measured continuously from within the right ventricle of the heart and all signals and parameters were automatically analysed (Notocord-Hem 3.3; Croissy, France) and represented in an Excel file as median values over 1 min.
Study protocol
Eight dogs (five males and three females) were observed during controlled changes of Tc and were divided into two subgroups to investigate a decrease and an increase in Tc. Four dogs were cooled to 34 °C by a blanket of ice and a cold air fan, and another group of four dogs was warmed to 42 °C using a heating plate in combination with a heating lamp. At the end of the study, the results from each group were pooled, to achieve data over a range of temperatures, from normothermia to hypothermia (from 37.5 to 34 °C), and from normothermia to hyperthermia (from 37.5 to 42 °C). Both groups were compared with a set of eight control dogs (three males and five females), evaluated with a constant Tc of approximately 37.5 °C over the same time period. The cooled dogs were thereafter slowly re-warmed to 40 °C by a heating plate in combination with a heating lamp and the heated dogs were slowly re-cooled to 37 °C by turning off all heating equipment. During the re-warming and re-cooling procedures, special attention was given to T-wave morphology, and arterial blood samples were obtained every 15 min in both groups to determine the plasma concentration of potassium.
Calculation of the correction formula
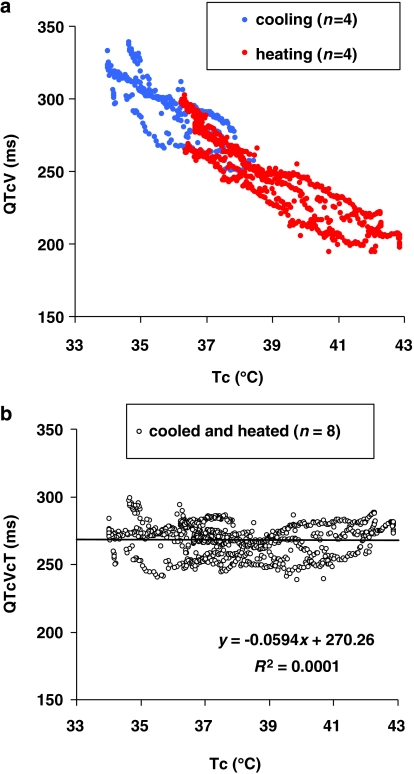
To calculate the temperature correction formula, we plotted the QTcV intervals against Tc (median value of every minute) of all individual cooled (from 37.7 to 34.2 °C) and heated (from 37.3 to 42.1 °C) dogs. As the relationship between the parameters appeared to be linear (Figure 1a) for both groups, and the R2 for a linear relationship was highly significant in all dogs (Table 2), the mean slope of the linear relationship of both parameters in all dogs was used to derive the formula. To validate the formula, we corrected the QTcV intervals for the changes in Tc (=QTcVcT; QTcV corrected for changes in core temperature) to achieve a horizontal line in the QTcVcT/Tc plots in four dogs with a Tc range from 34 to 40 °C. The linear regression line of these plots should have a slope and correlation coefficient (R2) close to zero.
Figure 1.
(a) Plot of core temperature (Tc) against QTcV interval (QTcV) of all individual dogs (n=8); cooled (open circles) and heated (closed circles). Regression analysis showed a good linear correlation over all dogs (slope=−14.06, R2=0.88 and P<0.001). (b) Plot of Tc against QTcVcT interval (QTcVcT) of all individual dogs (n=8), linear correlation with slope=−0.06, R2=0.0001 and P=0.758.
Table 2.
ΔTc, ΔQTcV, slopes and R2 values of all individual dogs; cooled (n=4) and heated (n=4)
| Dog | Gender | ΔTc (°C) | ΔQTcV (ms) | Slope | R2 |
|---|---|---|---|---|---|
| Cooled | |||||
| 1 | Male | −3.2 | +66 | −16.33 | 0.91 |
| 2 | Male | −2.5 | +56 | −16.53 | 0.90 |
| 3 | Male | −3.8 | +49 | −14.47 | 0.89 |
| 4 | Female | −4.2 | +40 | −12.06 | 0.88 |
| Heated | |||||
| 5 | Male | +6.5 | −65 | −9.71 | 0.95 |
| 6 | Female | +4.8 | −34 | −17.11 | 0.96 |
| 7 | Male | +3.8 | −45 | −10.82 | 0.97 |
| 8 | Female | +4.4 | −61 | −14.85 | 0.98 |
Validation of the correction formula
To validate and check the applicability of the correction formula, it was used in the same dogs after re-warming and re-cooling. Furthermore, we checked the formula in freely moving telemetered beagle dogs before and after an exercise test. In this study, 36 female dogs were telemetered (ITS (International Telemetry Systems, Dexter, MI, USA) T27F-11) to measure ECG and Tc, at rest and during a speed-increasing protocol of 40 min (from walking to running against 15 km h−1) on a roller band treadmill. During the study, Tc, HR and QT were monitored and after the study QTcV and QTcVcT were calculated. In addition, we conducted a literature survey to investigate published changes in Tc and QTc in dogs and man. To achieve comparable slopes we corrected the QT interval, where necessary, in the dog studies by the de Van de Water formula, and by the Fridericia formula in human studies.
Statistical analysis
Pooled data were expressed as mean±standard deviation. Intergroup comparisons were made with ANOVA Dunnett's test on repeated measures (WINKS SDA Software, Cedar Hill, USA). Comparisons within a group were made with a paired t-test (Microsoft Excel 2000). Linear regression and correlation analysis were made using Pearson's correlation coefficient (WINKS SDA Software, Cedar Hill, USA). A two-tailed P<0.05 was considered statistically significant.
Results
In this study, we used two groups of dogs, eight dogs that were cooled (n=4) or heated (n=4) and eight dogs, which were kept at basal Tc, as a time control group. There were no notable differences between both treated and control groups in gender (male/female=5/3 versus 3/5, respectively), body weight (11.7±1.4 versus 11.9±1.0 kg, respectively), age (12±2 versus 12±1 months, respectively), basal Tc (37.5±1.3 versus 37.8±1.2 °C, respectively), basal HR (88±33 versus 72±13 b.p.m., respectively), basal QT (240±19 versus 257±22 ms, respectively) and basal QTcV (264±4 versus 261±18 ms, respectively).
In the pooled averaged data (Table 1) from the cooled dogs, the decrease of Tc (P=0.003) within 63±13 min induced a decrease in HR (P=0.014), prolongation of QT interval (P=0.007) and QTcV interval (P=0.002). Table 1 also shows that in pooled averaged data from the heated dogs, the increase of Tc (P=0.004) induced an inconsistent increase in HR (P=0.137), but a clear shortening of QT (P=0.005) and QTcV intervals (P=0.006). Neither the prolongation of QTcV on cooling nor the shortening on warming were associated with arrhythmias or pro-arrhythmic biomarkers, such as extrasystoles, early after depolarisations or enhanced J waves (data not shown). The values of Tc, QT and QTcV of the treated dogs were significantly different from the control group over the same time frame. During this time course, no significantly different changes of Tc, HR, QT or QTcV from baseline (time=0) were evident in the control group (Table 1).
Table 1.
Core body temperature (Tc), heart rate (HR), QT interval (QT) and QTcV interval (QTcV) in cooled dogs (n=4), heated dogs (n=4) and control dogs (n=8)
| Time (min) | Tc (°C) | HR (b.p.m.) | QT (ms) | QTcV (ms) |
|---|---|---|---|---|
| Cooled | ||||
| 0 | 37.7±0.8 | 96±24‡ | 241±12 | 269±9 |
| 63±13 | 34.2±0.3†‡ | 61±13† | 323±35†‡ | 321±19†‡ |
| Heated | ||||
| 0 | 37.3±1.0 | 80±24 | 239±13 | 259±9 |
| 148±41 | 42.1±0.8†‡ | 132±71‡ | 171±27†‡ | 208±11†‡ |
| Control | ||||
| 0 | 37.7±1.2 | 72±13 | 257±22 | 264±20 |
| 60 | 37.6±1.1 | 76±12 | 252±18 | 265±10 |
| 150 | 37.5±0.9 | 71±11 | 256±17 | 262±20 |
Values are expressed as mean±s.d.
Comparisons within a group: †P-value<0.05; t-test (two sided, paired).
Intergroup comparisons: ‡P-value<0.05; ANOVA/Dunnett's test (two sided, unpaired).
To investigate the relationship between the duration of the QTcV interval and the Tc, the slope and the correlation coefficient (R2) of the relationship between QTcV interval and Tc, of all individual dogs (cooled and heated), were examined (Table 2). Over the range of temperatures examined (34.2–42.1 °C), the relation between QTcV and Tc appeared to be linear (Figure 1a); therefore, linear regression was therefore used. No significant difference (P=0.242) was observed between the slopes in the two procedures: cooling the dogs resulted in a slope of −14.85±2.08 with an R2 of 0.90±0.01 and heating the dogs resulted in a slope of −13.12±3.46 with an R2 of 0.97±0.01. This gave an average slope for cooling and heating of −14.0, that is, a decrease or increase in Tc of 1 °C induced an increase or decrease in the QTcV interval of 14 ms.
To correct the duration of the QTcV interval for changes in Tc to a temperature of 37.5 °C (normal body temperature in conscious telemetered dogs), the following formula was derived:
Plotting QTcVcT values against Tc (Figure 1b) produced a horizontal regression line with a slope of −0.06 and without any correlation (R2=0.0001, P=0.758).
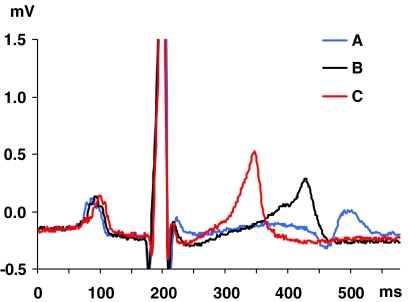
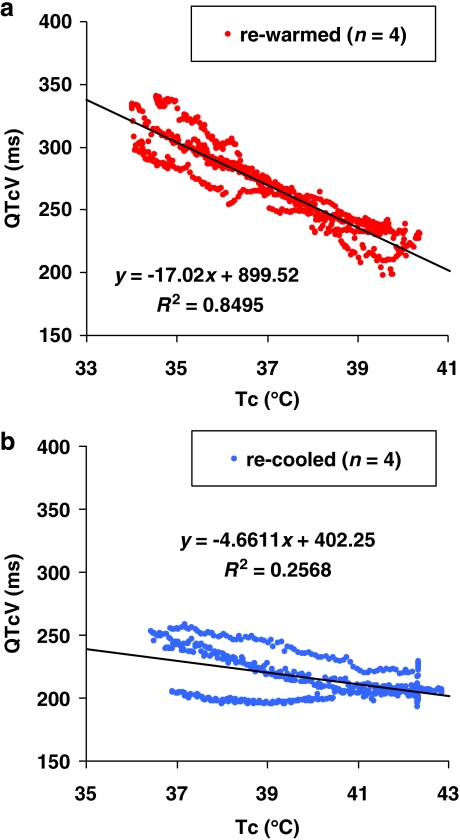
The four cooled dogs were re-warmed (from 34 to 40 °C; Table 3, rows A) and showed not only a clear decrease in QT interval (P=0.002) and QTcV (P=0.002) without significant changes in HR (P=0.684) or QTcVcT (P=0.326), but we observed an increase of the small T wave in the cooled dog to a tall, narrow based, peaked and symmetrical (‘tented') T wave (see Webster et al., 2002) at a high Tc (from 0.09±0.08 to 0.52±0.21 mV, P=0.024; Figure 2). Furthermore, an increase in serum potassium concentrations (from 3.1±0.3 to 4.3±0.2 mmol L−1, P=0.004) was noted throughout this temperature range. The slope and the correlation coefficient (R2) of the linear relationship (P<0.001) between QTcV time and Tc of pooled data in this group are shown in Figure 3a.
Table 3.
Core temperature (Tc), heart rate (HR), QT interval (QT) and QTcV interval (QTcV), temperature corrected QTcV (QTcVcT), T-wave amplitude (T wave) and serum potassium concentration [K+] in re-warmed dogs (A; n=4), warmed and subsequently re-cooled dogs (B; n=4) and control dogs (C; n=8)
| Tc (°C) | HR (b.p.m.) | QT (ms) | QTcV (ms) | QTcVcT (ms) | T wave (mV) | [K+] (mmol L−1) |
|---|---|---|---|---|---|---|
| A | ||||||
| 34.2±0.3‡ | 61±12 | 326±33‡ | 324±15‡ | 277±17 | 0.09±0.08‡ | 3.1±0.3 |
| 40.0±0.4†‡ | 65±17 | 227±17†‡ | 229±7†‡ | 264±8 | 0.52±0.21† | 4.3±0.2†‡ |
| B | ||||||
| 42.1±0.8‡ | 124±58‡ | 174±22‡ | 211±7‡ | 275±13 | 0.60±0.40 | 4.8±0.4‡ |
| 36.8±0.2† | 130±52‡ | 198±43‡ | 238±23 | 228±22†‡ | 0.35±0.31 | 5.1±1.2‡ |
| C | ||||||
| 37.7±1.2 | 72±13 | 257±22 | 264±20 | 264±20 | 0.35±0.11 | 3.4±0.4 |
| 37.5±0.9 | 72±10 | 253±14 | 261±20 | 261±20 | 0.42±0.16 | 3.6±0.3 |
Values are expressed as mean±s.d.
Comparisons within a group: †P-value<0.05; t-test (two sided, paired).
Intergroup comparisons: ‡P-value<0.05; ANOVA/Dunnett's test (two sided, unpaired).
Figure 2.
Example of T-wave morphology changes during 34.7 °C (A), 37.0 °C (B) and 41.3 °C (C). Note the narrow based, peaked and symmetric T wave (so-called tented T wave) at high core temperature.
Figure 3.
Plot of core temperature (Tc) against QTcV interval (QTcV) of (a) re-warmed dogs (n=4); linear correlation with slope=−17.02, R2=0.85 and P<0.001 and of (b) re-cooled dogs (n=4); linear correlation with slope=−4.66, R2=0.26 and P<0.001.
The four heated dogs were then cooled (Table 3, rows B), but now showed inconsistent changes in QT interval (P=0.141) or QTcV (P=0.071), and the increased HR (P=0.770) did not return to normothermic baseline values. Furthermore, the increased serum potassium concentrations did not return to baseline values (P=0.724), only the T-wave amplitude was slightly decreased (P=0.269). The slope and the correlation coefficient (R2) of the relationship between QTcV time and Tc of pooled data in this group after re-cooling from 42 °C were now much lower (Figure 3b). Indeed, after re-cooling these four warmed dogs to normal Tc, two of them showed a further increase in serum potassium concentration (from 4.9 and 4.7 to 6.7 and 5.1 mmol L−1, respectively), and QTcV did not return to baseline values (from 206 and 208 ms to 205 and 239 ms; slopes: −1.31 and −0.02 and R2: 0.4 and 0.15, respectively). The other two dogs showed only partial decreases in serum potassium concentrations (from 5.3 and 4.4 mmol L−1 to 4.6 and 3.8 mmol L−1, respectively), and QTcV did not fully increase to baseline values (from 221 and 207 ms to 255 and 253 ms; slopes: −7.53 and −8.07 and R2: 0.97 and 0.93, respectively).
In the normothermic control group, as expected, no significant changes were measured in HR, QT, QTcV, etc., during a constant Tc of 37.7 to 37.5 °C over the same time period as the cooling and warming experiments reported above (Table 3, rows C). In addition, experiments with atrially paced normothermic dogs showed that QTcV was not significantly altered at rates up to 120 b.p.m. (unpublished data).
In freely moving telemetered dogs (Table 4), the exercise procedure (from walking to running over 40 min) induced a small increase in Tc (P<0.05) and HR (P<0.05), and a decrease in QT (P<0.05). The rate-correcting formula by Van de Water did not fully correct for HR: exercise induced a decrease in QTcV (P<0.05) and showed a slope of −15.7. Using our Tc correction formula, we calculated a fully HR- and body temperature-independent parameter, QTcVcT of 244±11ms (P=0.824).
Table 4.
Core temperature (Tc), heart rate (HR), QT interval (QT) and QTcV interval (QTcV), temperature corrected QTcV (QTcVcT), in freely moving telemetered beagle dogs (n=36) at rest and after exercise
| Tc (°C) | HR (b.p.m.) | QT (ms) | QTcV (ms) | QTcVcT (ms) | |
|---|---|---|---|---|---|
| Rest | 37.6±0.6 | 109±23 | 206±16 | 244±14 | 244±16 |
| Exercise | 38.3±0.8† | 152±22† | 181±14† | 233±10† | 244±11 |
Values are expressed as mean±s.d.
†P-value<0.05; t-test (two sided, paired).
Estimated slopes of changes in Tc and QTc in dogs and humans, extracted from already published data showed similar results compared to our own data (Table 5). Cooling, heating, re-warming or fever induced changes in Tc, but all studies showed after calculation (ΔQTc/ΔTc) an estimated slope between −11.0 and −17.1.
Table 5.
Estimated slopes of changes in body temperature (Tc) and QTc in dogs and humans, extracted from published data
| Treatment | Species | Tc (°C) range | QTc (ms) range | Estimated slope | Literature |
|---|---|---|---|---|---|
| Heated | Dog | 38.0–41.5 | 276–233 | −12.3 | Geller et al. (1952) |
| Cooled | Human | 36.0–29.0 | 460–570 | −15.7 | Fleming and Muir (1956) |
| Cooled | Human | 37.0–31.0 | 353–435 | −13.7 | Hicks et al. (1956) |
| Cooled | Dog | 38.0–32.0 | 248–329 | −13.4 | West et al. (1959) |
| Cooled | Dog | 38.4–26.7 | 340–490 | −12.8 | Beyda et al. (1960) |
| Cooled | Infant | 37.0–30.0 | 388–508 | −17.1 | Wakusawa et al. (1977) |
| Fever | Human | 37.2–39.4 | 411–373 | −16.9 | Karjalainen and Viitasalo (1986) |
| Re-warmed | Human | 29.4–33.3 | 545–483 | −16.0 | Mattu et al. (2002) |
| Re-warmed | Human | 25.6–30.7 | 609–522 | −17.0 | Mattu et al. (2002) |
| Cooled | Infant | 37.0–34.0 | 431–465 | −11.3 | Horan et al. (2007) |
| Re-warmed | Human | 31.7–35.8 | 505–460 | −11.0 | Yaganti et al. (2007) |
Discussion and conclusions
In this study, we were able to cool the Tc of anaesthetized dogs to 34.2 °C (hypothermic conditions), and to warm the Tc to 42.1 °C (hyperthermic conditions) with only minor changes in HR. Hypothermia prolonged the QT interval and conversely, hyperthermia shortened the QT interval, both without obvious pro-arrhythmic signs. The reliable Van de Water formula (King et al., 2006), often used in dog studies, did not properly correct these large changes in QT intervals. The prolongation of the QT interval in hypothermic conditions has been previously described before in dogs (Beyda et al., 1960) and humans (Mattu et al., 2002; Aslam et al., 2006), but the shortening of the QT interval in hyperthermic conditions has been poorly studied (Geller et al., 1952). The relationship between QTcV and Tc over the temperature range investigated, cooling (from 37.7 to 34.2 °C) and heating (from 37.3 to 42.1 °C) in this study was linear for all individual dogs (R2 between 0.88 and 0.98), and a slope was calculated, showing an average decrease of 14 ms per degree change. The correction formula for the Tc was calculated and validated for different circumstances (re-warming, re-cooling and exercise), for different models (anaesthetized and telemetered dogs) and from cases in the literature (dogs and humans).
The mechanisms of temperature change on the ECG in general, and on the QT interval in particular, are almost certainly multi-factorial. Metabolic rate varies with body temperature in both homoeotherms and poikilotherms, and affects most bodily processes. Biologists are familiar with the marked changes in contractility and spontaneous activity in isolated organs produced by different bath temperatures (for example, Smith et al., 1951). However, under such conditions in vitro, extracellular ionic concentrations are constant. In whole animals, however, other factors may also be involved. It has been reported that hypothermia is accompanied by hypokalaemia, a shift of potassium from extracellular to intracellular or extra-vascular spaces (Koht et al., 1983). Hypokalaemia causes a decrease in the resting membrane potential in ventricular cells, reduces the T-wave amplitude and induces QT prolongation (Slovis and Jenkins, 2002). Other investigators have noted that enhancement of the body temperature, induced by exercise-dependent malignant hyperthermia (Wappler et al., 2000), or by artificially induced hyperthermia in dogs (Spurr and Barlow, 1959), can lead to an increase in serum potassium concentrations. Hyperkalaemic states have an effect on the ECG, and the early signs are tall, narrow based, peaked and symmetric T waves (so called ‘tented' T waves), and the QT interval is decreased (Webster et al., 2002). In our study, we found increases in Tc to be associated with increases in T-wave amplitudes and in serum potassium concentration in re-warmed dogs. As such, changes in serum potassium concentrations could be considered to contribute towards the effects on QTcV reported here. However, it is important to note that the changes in serum potassium concentrations do not affect the QTcV/Tc slope in our anaesthetized dogs, and the proposed formula fully corrected the QTcV interval for the increase in Tc. Indeed, action potential durations have also been shown to be markedly altered in vitro in guinea-pigs (Duker et al., 1987; Lathrop et al., 1998), rabbits (unpublished data from Langendorff hearts) and pigs (Roscher et al., 2001), where electrolyte levels are controlled through perfused physiological salt solutions.
The small slopes and lack of linearity between Tc and QTc in dogs warmed to over 42 °C and then re-cooled to 36.8 °C are probably caused by the very high body temperatures induced in these dogs. Indeed, it was noted that when the body temperature was raised to over 42 °C, the return to normothermia was associated with lower QT intervals, and higher HRs, serum potassium concentration and T waves in these dogs. The critical thermal maximum (CTM) in dogs is between 43.0 and 44.5 °C, and in heatstroke models dogs die after CTM has been reached for a longer period (Bynum et al., 1977), so these temperatures are by themselves pathological. In our study, four dogs were increased to a Tc close to the CTM. During re-cooling to normal Tc, two of them showed only a partial decrease in serum potassium concentrations, and QTcV did not fully increase to baseline values. The other two dogs showed a further increase in serum potassium concentrations, and the increases in QTcV were even smaller than those seen in the two dogs whose potassium concentrations decreased.
It should be emphasized that these apparently pathological influences of high temperature on serum potassium concentrations and on QTcV intervals (Table 3 (rows B) and Figure 3b) were obtained subsequent to the data shown in Tables 1 and 2, which were used to derive the proposed correction factor.
As mentioned above, the effects of drug-induced temperature changes on QT (QTc) have been poorly studied, and to our knowledge, have not been systematically examined within cardiovascular safety studies. However, there are many existing drugs that are known to affect body temperature (Clark, 1979; Clark and Clark, 1980a, 1980b), and there is increasing interest in certain classes of developmental drugs (for example, 5-HT1A receptor agonists (Seletti et al., 1995), dopamine D3 receptor agonists (Boulay et al., 1998), TRPV1 antagonists (Szallasi et al., 2007)) and biologic drugs (interferon-alpha (Kentner et al., 2006), interleukin (Gatti et al., 2002)), which have documented effects on body temperature homoeostasis. Therefore, any drug that affects Tc can be expected to have temperature effects on QTc (∼14 ms per degree) whether or not the drug has actual direct effects on cardiac repolarization. Thus in a CV safety study, a new chemical entity with no effect on IKr (HERG) can easily appear to prolong QTc interval, simply by a decrease in body temperature. Conversely, the effects of a new chemical entity, with a direct blocking effect on IKr, could be masked by the shortening of QTc intervals resulting from an increase in body temperature.
Drugs may also affect locomotor activity, which itself can affect Tc (Tontodonati et al., 2007). In conscious animal safety studies, modern technology (for example, ITS systems) now allows accurate online measurement of Tc and locomotor activity in addition to cardiovascular parameters, but the former parameters are rarely examined in conventional study protocols. Our study in conscious, exercising dogs showed an apparent shortening of QTcV by 11 ms (4.5%), which was fully compensated by taking the Tc rise into account. This emphasizes the importance of investigating possible drug-induced effects on locomotor activity as well as on Tc, both to examine a new drug's safety profile, and to distinguish between direct and indirect changes on Tc.
Limitations
First, the results were achieved in the anaesthetized animal, so may not be directly applicable to the conscious state using a similar method. However, both the exercise study with freely moving telemetered dogs showed a complete correction for HR and temperature by the proposed correction formula, and the results recalculated from literature studies tabulated in this study are qualitatively supportive of the results reported—in animals and in humans—in cases of both hypo- and hyperthermia.
Second, we have investigated this relationship over an adequate range for drug- or exercise-induced changes in Tc (∼34–42 °C), but it may not be valid for lower temperatures. The apparently pathological body changes (for example, massive increase in serum potassium concentrations, irreversible effects on ECG) seen in some dogs warmed to near the CTM of ∼43 °C, should be taken into account and avoided in further studies.
Lastly, although this correction formula has been obtained in anaesthetized dogs, and validated in one example in freely moving exercising dogs, it may not be appropriate for other species (including humans) and circumstances. Further studies will be required to check and/or improve this formula. In conclusion, this study has demonstrated a marked dependence of the QT interval (QTc) on temperature in the anaesthetized dog, and has determined for the first time, to our knowledge, a correction formula to allow normalization to normothermic values. The use of this correction formula was validated in different situations, such as re-warming and re-cooling, and in exercise tests in conscious telemetered beagle dogs, and the applicability of the formula in dogs and humans under different circumstances was further investigated by a comparison with published data.
Temperature is rarely studied in safety studies, and our findings suggest that
Some drugs may be incorrectly classified as having direct effects on repolarization (QT) if they also change body temperature by direct or indirect (for example, by changing locomotor activity) mechanisms;
Conversely, direct QT effects may be missed when testing drugs that change body temperature;
Altered plasma potassium levels are found in association with the phenomena reported, and
Identification or classification of patients (for example, long QT syndrome) by exercise tests may be improved by using a more accurate correction formula.
We recommend that all ICH S7A, conscious animal, safety studies should routinely measure body Tc and locomotor activity (which may indirectly influence Tc), and correct QTc using an appropriate formula if body temperature changes are detected. This will help to avoid generating misleading data and to explain false-positive or -negative results. Indeed, correction for temperature changes on the QT interval should also be considered in human trials, especially where stress testing is incorporated into the design, and repeated-dose large animal toxicological studies.
Acknowledgments
We gratefully acknowledge the excellent exercise dog data provided by Frank Cools on behalf of our conscious dog group.
Abbreviations
- ICH
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- QTcV
QT interval corrected according to the Van de Water formula
- QTcVcT
QTcV corrected for changes in core temperature
Conflict of interest
The authors state no conflict of interest.
References
- Algra A, Tijssen J, Roelandt J, Pool J, Lubsen J. QTc prolongation measured by standard 12 lead electrocardiogram is an independent risk factor for sudden death. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- Alhaddad IA, Khalil M, Brown EJ. Osborn waves of hypothermia. Circulation. 2000;101:233–244. doi: 10.1161/01.cir.101.25.e233. [DOI] [PubMed] [Google Scholar]
- Aslam AF, Aslam KA, Vasavada BC, Khan IA. Hypothermia: evaluation, electrocardiographic manifestations, and management. Am J Med. 2006;119:297–301. doi: 10.1016/j.amjmed.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Aytemir K, Maarouf N, Gallacher MM, Yap YG, Waktare JEP, Malik M. Comparison of formulae for heart rate correction of QT interval in exercise electrocardiograms. Pacing Clin Electrophysiol. 1999;22:1397–1401. doi: 10.1111/j.1540-8159.1999.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Benatar A, Decraene T. Comparison of formulae for heart rate correction of QT interval in exercise ECGs from healthy children. Heart. 2001;86:199–202. doi: 10.1136/heart.86.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyda EJ, Jung M, Bellet S. Effect of hypothermia on the tolerance of dogs to digitalis. Circ Res. 1960;9:129–135. [Google Scholar]
- Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology. 1998;38:555–565. doi: 10.1016/s0028-3908(98)00213-5. [DOI] [PubMed] [Google Scholar]
- Bynum G, Pattom J, Bowers W, Leav I, Wolfe D, Hamlet M, et al. An anesthetized dog heatstroke model. J Appl Physiol. 1977;43:292–296. doi: 10.1152/jappl.1977.43.2.292. [DOI] [PubMed] [Google Scholar]
- Clark WG. Changes in body temperature after administration of amino acids, peptides, dopamine, neuroleptics and related agents. Neurosc Biobehav Rev. 1979;3:179–231. doi: 10.1016/0149-7634(79)90010-1. [DOI] [PubMed] [Google Scholar]
- Clark WG, Clark YL. Changes in body temperature after administration of acetylcholine, histamine, morphine, prostaglandins and related agents. Neurosci Biobehav Rev. 1980a;4:175–240. doi: 10.1016/0149-7634(80)90015-9. [DOI] [PubMed] [Google Scholar]
- Clark WG, Clark YL. Changes in body temperature after administration of adrenergic and serotonergic agents and related drugs including antidepressants. Neurosci Biobehav Rev. 1980b;4:281–375. doi: 10.1016/0149-7634(80)90002-0. [DOI] [PubMed] [Google Scholar]
- De Clerck F, Van de Water A, D'Aubioul J, Lu HR, van Rossem K, Hermans A, et al. In vivo measurement of QT prolongation, dispersion and arrhythmogenesis: application to the preclinical cardiovascular safety pharmacology of a new chemical entity. Fundam Clin Pharmacol. 2002;16:125–140. doi: 10.1046/j.1472-8206.2002.00081.x. [DOI] [PubMed] [Google Scholar]
- Duker G, Sjöquist P, Johansson BW. Monophasic action potentials during induced hypothermia in hedgehog and guinea pig hearts. Heart Circ Physiol. 1987;22:1083–1088. doi: 10.1152/ajpheart.1987.253.5.H1083. [DOI] [PubMed] [Google Scholar]
- Hicks CE, McCord MC, Blount SG. Electrocardiographic changes during hypothermia and circulatory occlusion. Circulation. 1956;13:21–28. doi: 10.1161/01.cir.13.1.21. [DOI] [PubMed] [Google Scholar]
- Horan M, Edwards AD, Firmin RK, Ablett T, Rawson H, Field D. The effect of temperature on the QTc interval in the newborn infant receiving extracorporeal membrane oxygenation (ECMO) Early Hum Dev. 2007;83:217–223. doi: 10.1016/j.earlhumdev.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF. Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans. Exp Physiol. 1996;81:685–693. doi: 10.1113/expphysiol.1996.sp003969. [DOI] [PubMed] [Google Scholar]
- Fleming PR, Muir FH. Electrocardiographic changes in induced hypothermia in man. Br Heart J. 1956;19:59–66. doi: 10.1136/hrt.19.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridericia LS. Die Systolendauer in Elektrokardiogram bei normalen Menschen und bei Herzkranken. Acta Med Scand. 1920;53:469–486. [Google Scholar]
- Gatti S, Beck J, Fantuzzi G, Bartfai T, Dinarello CA. Effect of interleukin-18 on mouse core body temperature. Am J Physiol Regul Integr Comp Physiol. 2002;282:702–709. doi: 10.1152/ajpregu.00393.2001. [DOI] [PubMed] [Google Scholar]
- Geller HM, Nahum LH, Sikand RS, Levine H. Effect of artificially induced hyperthermia on the electrogram of the dog. J Appl Physiol. 1952;4:584–592. doi: 10.1152/jappl.1952.4.7.584. [DOI] [PubMed] [Google Scholar]
- Karjalainen J, Viitasalo M. Fever and cardiac rhythm. Arch Intern Med. 1986;146:1169–1171. [PubMed] [Google Scholar]
- Kentner AC, Miguelez M, James JS, Bielajew C. Behaviour and physiological effects of a single injection of rat interferon-alpha on male Sprague–Dawley rats: a long-term evaluation. Brain Res. 2006;1095:96–106. doi: 10.1016/j.brainres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- King A, Bailie M, Bari Olivier N. Magnitude of error introduced by application of heart rate correction formulas to the canine QT interval. Ann Noninvasive Electrocardiol. 2006;11:289–298. doi: 10.1111/j.1542-474X.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- Koht A, Cane R, Cerullo LJ. Serum potassium levels during prolonged hypothermia. Clin Anesth. 1983;9:275–277. doi: 10.1007/BF01691254. [DOI] [PubMed] [Google Scholar]
- Lathrop DA, Contney SJ, Bosnjak ZJ, Stowe DF. Reversal of hyperthermia-induced action potential lengthening by the KATP channel agonist Bimakalim in isolated guinea pig ventricular muscle. Gen Pharmacol. 1998;31:125–131. doi: 10.1016/s0306-3623(97)00395-9. [DOI] [PubMed] [Google Scholar]
- Mattu A, Brady WJ, Perron AD. Electrocardiographic manifestations of hypothermia. Am J Emerg Med. 2002;20:314–326. doi: 10.1053/ajem.2002.32633. [DOI] [PubMed] [Google Scholar]
- Newbold P, Sanders N, Reele SB. Lack of correlation between exercise and sibenadet-induced changes in heart rate corrected measurements of the QT interval. Br J Clin Pharmacol. 2007;63:279–287. doi: 10.1111/j.1365-2125.2006.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo SM, Kennedy BW, Tullett WM, Blyth AS, Dougall JR. Drug-induced hyperthermia. Anaesthesia. 1993;10:892–895. doi: 10.1111/j.1365-2044.1993.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Roscher R, Arlock P, Sjöberg T, Steen S. Effects of dopamine on porcine myocardial action potentials and contractions at 37°C and 32 °C. Acta Anaesthesiol Scand. 2001;45:421–426. doi: 10.1034/j.1399-6576.2001.045004421.x. [DOI] [PubMed] [Google Scholar]
- Seletti B, Benkelfat C, Blier P, Annable L, Gilbert F, de Montigny C. Serotonin1A receptor activation by flesinoxan in humans, body temperature and neuroendocrine responses. Neuropsychopharmacology. 1995;13:93–104. doi: 10.1016/0893-133X(95)00025-9. [DOI] [PubMed] [Google Scholar]
- Slovis C, Jenkins R. ABC of clinical electrocardiography. Conditions not primarily affecting the heart. Br Med J. 2002;324:1320–1323. doi: 10.1136/bmj.324.7349.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Syverton JT, Coxe JW. In vitro studies of the coronary arteries of man and swine as demonstrated by a new technique, angioplethysmokymography. Circulation. 1951;4:890–898. doi: 10.1161/01.cir.4.6.890. [DOI] [PubMed] [Google Scholar]
- Sprung J, Cheng EY, Gamulin S, Kampine JP, Bosnjak ZJ. Effects of acute hypothermia and beta-adrenergic receptor blockade on serum potassium concentration in rats. Crit Care Med. 1991;19:1545–1551. doi: 10.1097/00003246-199112000-00018. [DOI] [PubMed] [Google Scholar]
- Spurr GB, Barlow G. Influence of prolonged hyperthermia and hypothermia on myocardial sodium, potassium and chloride. Circ Res. 1959;7:210–218. doi: 10.1161/01.res.7.2.210. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Tontodonati M, Fasdelli N, Moscardo E, Giarola A, Dorigatti R. A canine model used to simultaneously assess potential neurobehavioural and cardiovascular effect of candidate drugs. J Pharmacol Toxicol Methods. 2007;56:265–275. doi: 10.1016/j.vascn.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Van de Water A, Verheyen J, Xhonneux R, Reneman R. An improved method to correct the QT-interval of the electrocardiogram for changes in heart rate. J Pharmacol Methods. 1989;22:207–217. doi: 10.1016/0160-5402(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Wakusawa R, Shibata S, Okada K. Simple deep hypothermia for open heart surgery in infancy. Can Anaesth Soc. 1977;24:491–504. doi: 10.1007/BF03005454. [DOI] [PubMed] [Google Scholar]
- Wappler F, Fiege M, Antz M, Schulte am Esch J. Hemodynamic and metabolic alterations in response to graded exercise in a patient susceptible to malignant hyperthermia. Anesthesiology. 2000;92:268–272. doi: 10.1097/00000542-200001000-00042. [DOI] [PubMed] [Google Scholar]
- Webster A, Brady W, Morris F. Recognising signs of danger: ECG changes resulting from a abnormal serum potassium concentration. Emerg Med. 2002;19:74–77. doi: 10.1136/emj.19.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West TC, Frederickson EL, Amory DW. Single fiber recording of the ventricular response to induced hypothermia in the anesthetized dog. Circ Res. 1959;7:880–888. doi: 10.1161/01.res.7.6.880. [DOI] [PubMed] [Google Scholar]
- White K, Simpson G. The combined use of MAOIs and tricyclics. J Clin Psychiatry. 1984;45:67–69. [PubMed] [Google Scholar]
- Yaganti VM, Naik CA, Dhond AJ. Electrocardiographic manifestations in a hypothermic patient: review of Osborn waves. Indian Heart J. 2007;59:80–82. [PubMed] [Google Scholar]