Abstract
Background and purpose:
Heterologous expression of α1, β2 and γ2S(γ1) subunits produces a mixed population of GABAA receptors containing α1β2 or α1β2γ2S(γ1) subunits. GABA sensitivity (lower in receptors containing γ1 or γ2S subunits) and the potentiation of GABA-activated chloride currents (IGABA) by benzodiazepines (BZDs) are dependent on γ2S(γ1) incorporation. A variable γ subunit incorporation may affect the estimation of IGABA potentiation by BZDs. We propose an approach for estimation of BZD efficiency that accounts for mixed population of α1β2 and α1β2γ2S(γ1) receptors.
Experimental approach:
We investigated the relation between GABA sensitivity (EC50) and BZD modulation by analysing triazolam-, clotiazepam- and midazolam-induced potentiation of IGABA in Xenopus oocytes under two-microelectrode voltage clamp.
Key results:
Plotting EC50 versus BZD-induced shifts of GABA concentration-response curves (ΔEC50(BZD)) of oocytes injected with different amounts of α1, β2 and γ2S(γ1) cRNA (1:1:1–1:1:10) revealed a linear regression between γ2S(γ1)-mediated reduction of GABA sensitivity (EC50) and ΔEC50(BZD). The slope factors of the regression were always higher for oocytes expressing α1β2γ1 subunit receptors (1.8±0.1 (triazolam), 1.6±0.1 (clotiazepam), 2.3±0.2 (midazolam)) than for oocytes expressing α1β2γ2S receptors (1.4±0.1 (triazolam), 1.4±0.1 (clotiazepam), 1.3±0.1 (midazolam)). Mutant GABAA receptors (α1β2-R207Cγ2S) with lower GABA sensitivity showed higher drug efficiencies (slope factors=1.1±0.1 (triazolam), 1.1±0.1 (clotiazepam), 1.2±0.1 (midazolam)).
Conclusions and implications:
Regression analysis enabled the estimation of BZD efficiency when variable mixtures of α1β2 and α1β2γ2S(γ1) receptors are expressed and provided new insights into the γ2S(γ1) dependency of BZD action.
Keywords: GABAA receptor, benzodiazepine modulation, concentration–response curve, Xenopus oocytes, two-microelectrode voltage clamp
Introduction
GABA (γ-aminobutyric acid) mediates fast inhibitory transmission by interacting with GABA type A (GABAA) receptors in the central nervous system. These ligand-gated ion channels are assembled from individual subunits forming a pentameric structure. A total of 19 isoforms of mammalian GABAA receptor subunits have been cloned: α1–6, β1–3, γ1–3, δ, ɛ, π, ρ1–3 and θ (Barnard et al., 1998; Simon et al., 2004). The subunit composition determines GABA sensitivity, sensitivity for benzodiazepines (BZDs), barbiturates, neurosteroids and anaesthetics (Sieghart, 1995; Hevers and Luddens, 1998; Sigel, 2002; Ernst et al., 2003, 2005) and also the gating properties of GABAA receptors (Feng et al., 2004; Boileau et al., 2003, 2005). The N-terminal parts of α- and β-subunits participate in the formation of the two agonist sites (Sigel et al., 1992; Amin and Weiss, 1993; Boileau and Czajkowski, 1999; Wagner and Czajkowski, 2001; Newell and Czajkowski, 2003). GABA binding to these sites leads to pore opening.
BZDs interact with amino-acid residues located at the interface between α- and γ-subunits (Macdonald and Barker, 1978; Sigel and Buhr, 1997). These drugs allosterically modulate activation of GABAA receptors either by increasing apparent affinity of at least one agonist-binding site (Gallager and Tallman, 1983; Serfozo and Cash, 1992; Lavoie and Twyman, 1996) or affecting the pore opening (Baur and Sigel, 2005). GABAA receptors carry two GABA-binding sites at the respective αβ interfaces (see Twyman et al., 1990). By selective disruption of the one or the other of these sites in concatenated GABAA receptors, it recently has been demonstrated (Baur and Sigel, 2005) that chloride currents were potentiated by diazepam in both cases.
Heterologous expression systems are the basis for drug development and pharmacological characterization of different GABAA receptor subtypes. Transfection of mammalian cells or cRNA injection into Xenopus oocytes with α1, β2 and γ2 subunits may, however, result in different mixtures of α1β2 and α1β2γ2 receptors (Baumann et al., 2001; Boileau et al., 2002, 2003), which complicates the unequivocal estimation of potencies and efficiencies of BZDs in heterologous expression systems. This problem can be partially overcome by injecting larger amounts of γ2 subunit cRNA (Boileau et al., 2002) or, alternatively, by making use of concatenated GABAA receptor subunits (Baumann et al., 2002; Minier and Sigel, 2004; Boileau et al., 2005).
We have previously reported that different efficiencies of BZDs to enhance chloride currents through GABAA receptors comprising γ1 or γ2S subunits are related to their ability to shift the GABA concentration–response curves towards higher GABA sensitivities (Khom et al., 2006). Here we analyse the relation between γ-induced inhibition of GABA sensitivity and GABA-induced chloride current (IGABA) potentiation by three BZDs (triazolam, clotiazepam and midazolam) in oocytes expressing different populations of α1β2 and α1β2γ2S(γ1) subunit receptors. Correlation analysis yielded regression lines with slope factors reflecting higher drug efficiency in γ2S than in γ1 subunit-comprising receptors. GABA sensitivity and IGABA potentiation of concatenated subunits (γ2-β2-α1 and β2-α1) fitted the regression lines of non-concatenated receptors supporting the hypothesis that their lower GABA sensitivity is due to complete γ2S incorporation (rather than forced subunit arrangement). A simulation supports the hypothesis that even under conditions where a higher ratio of γ2S cRNA relative to α1 and β2 cRNA is injected oocytes contain a significant population of α1β2 receptors.
Smaller slope factors of the regression lines were estimated for oocytes expressing receptors with lower GABA sensitivity (mutant β2-R207C; Wagner et al., 2004).
Materials and methods
Expression of GABAA receptors
Stage V–VI oocytes from Xenopus laevis were prepared and cRNA was injected as previously described by Khom et al. (2006). Female X. laevis (NASCO, WI, USA) were anaesthetized by exposing them for 15 min to a 0.2% MS-222 (methanesulphonate salt of 3-aminobenzoic acid ethyl ester; Sandoz, Germany) solution before surgically removing parts of the ovaries. Follicle membranes from isolated oocytes were enzymically digested with 2 mg mL−1 collagenase (Type 1A; Sigma, Vienna, Germany). Synthesis of capped run-off poly(A+) cRNA transcripts was obtained from linearized cDNA templates (pCMV vector). At 1 day after enzymatic isolation, the oocytes were injected with 50 nL of water treated with diethylpyrocarbonate (Sigma) containing the different rat cRNAs at a concentration of approximately 300–3000 pg nL−1 per subunit. The amount of cRNA was determined by means of a NanoDrop ND-1000 (Kisker-Biotech, Steinfurt, Germany). To ensure expression of the γ-subunit with different incorporation in the case of α1β2γ1 and α1β2γ2S receptors cRNAs were mixed in a ratios: 1:1:1, 1:1:3 and 1:1:10. The double β2-23-α1 (β2-α1) and triple γ2-26-β2-23-α1 (γ2-β2-α1) concatemers (kindly provided by E Sigel) and the β2-R207C mutant (kindly provided by C Czajkowski) have been described previously (Baumann et al., 2001, 2002; Wagner et al., 2004). Oocytes were stored at 18 °C in ND96 solution (Methfessel et al., 1986). Voltage clamp measurements were performed between days 1 and 5 after cRNA injection.
Two-microelectrode voltage clamp studies
Electrophysiological experiments were performed by the two-microelectrode voltage clamp method making use of a TURBO TEC 01C amplifier (npi electronic GmbH, Tamm, Germany) at a holding potential of −70 mV. The bath solution contained 90 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2 and 5 mM HEPES (pH 7.4).
Perfusion system
GABA was applied by means of an automated fast perfusion system according to Baburin et al. (2006). To elicit IGABA the chamber was perfused with 120 μL of GABA-containing solution at volume rates of 300 μL s−1. Duration of washout periods was extended from 3 to up to 20 min with increasing concentrations of applied GABA to account for slow recovery from increasing levels of desensitization (see Khom et al., 2007 for details).
Analysing concentration–response curves
Concentration–response curves were generated and the data were fitted by non-linear regression analysis using Origin software (OriginLab Corporation, Northampton, MA, USA). Data were fitted to the equation: 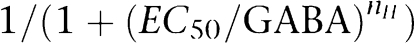 , where EC50 is the concentration of the GABA that induces half-maximal GABA-evoked current and nH is the Hill coefficient. Concentration–response data for each oocyte were normalized to the maximum control GABA current for that oocyte. Statistical significance was calculated using unpaired Student's t-test with a confidence interval of P<0.05.
, where EC50 is the concentration of the GABA that induces half-maximal GABA-evoked current and nH is the Hill coefficient. Concentration–response data for each oocyte were normalized to the maximum control GABA current for that oocyte. Statistical significance was calculated using unpaired Student's t-test with a confidence interval of P<0.05.
Calculation of EC50 and ΔEC50 of mixed population
The EC50 values and BZD-induced shifts in GABA sensitivity in oocytes expressing mixed receptor populations (different fractions of high GABA sensitive α1β2 versus low-sensitive α1β2γ2S receptors) are described by the concentration–response curves for each receptor population by:
 |
and
 |
where Rαβ and Rαβγ are peak IGABA currents (number of open channels in each population) at a given GABA concentration, iαβ and iαβγ, amplitudes of single channel currents, Nαβ and Nαβγ are numbers of channels characterized by EC50,αβ and EC50,αβγ, midpoints of concentration–response curves, Hαβ and Hαβγ—Hill coefficients.
The total normalized IGABA is a weighted sum of partial current responses:
where F is the fraction (0<F<1) of current through γ-containing GABAA receptors at saturating GABA concentrations: F=(iαβγNαβγ)/(iαβNαβ+iαβγNαβγ).
Under-application of a saturating concentration of BZD the receptor population consists of two subpopulations:
(1) αβ receptors with unchanged EC50,αβ and Hαβ
and
(2) modulated αβγ receptors with enhanced GABA sensitivity (midpoint, EC50,mod and Hill coefficient, Hmod):
 |
The total current response is described now by:
The apparent midpoints of the GABA concentration–response curves (EC50) for oocytes expressing a given fractions (F) of α1β2γ2S receptors and the shift of this curve (ΔEC50(BZD)) at saturating concentrations of triazolam, clotiazepam and midazolam can be obtained by varying the fractions (F) from 0 to 1 by maximum likelihood.
Chemicals
Compounds were obtained from the following sources: triazolam (8-chloro-6-(2-chlorophenyl)-1-methyl-4H-1,2,4-triazolo[4,3-a][1,4]benzodiazepine; Sigma), clotiazepam (5-(2-chlorophenyl)-7-ethyl-1,3-dihydro-1-methyl-2H-thieno[2,3-e][1,4]diazepin-2-one; Troponwerke, Köln, Germany), midazolam (8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine; Hoffmann La Roche, Basel, Switzerland).
Results
Modulation by BZDs of chloride currents through GABAA receptors expressed in Xenopus oocytes is dependent on the incorporation of a γ-subunit (Boileau et al., 2002). On the one hand injection of increasing amounts of γ-encoding cRNA (that is, α1:β2:γ2S ratios of 1:1:3 or 1:1:10) gradually reduces GABA sensitivity and, on the other hand, the increased fraction of α1β2γ2S receptors results in an increased IGABA potentiation by BZDs. To quantify the relation between γ-subunit-mediated inhibition of GABA sensitivity and BZD action we have analysed modulation of GABA-induced chloride currents (IGABA) in a large population of oocytes injected with different mixtures (1:1:1, 1:1:3, 1:1:10) of wild-type or mutated α1, β2 and γ2S or γ1 GABAA receptor subunits, or γ2-β2-α1/β2-α1 concatenated constructs. Triazolam, clotiazepam and midazolam were chosen because these drugs modulate GABAA receptors incorporating γ2S and γ1 subunits that would allow us to compare their action at different efficiencies.
Modulation of IGABA by BZDs in oocytes expressing variable ratios of α1β2 versus α1β2γ1 or α1β2γ2S receptors
Figure 1a illustrates the effect of triazolam on the concentration–response curves of IGABA through GABAA receptors in oocytes injected with different ratios of cRNAs encoding for α1, β2 and γ2S subunits. A saturating concentration (1 μM) of triazolam shifted the GABA concentration–response curves leftwards without significant effects on the maximal response. Higher γ2S expression and thus, presumably higher γ2S incorporation resulted in reduced GABA sensitivity and larger triazolam-induced shifts of the GABA EC50 values (ΔEC50(triazolam): 16 μM (cRNA ratio α1:β2:γ2S 1:1:1), 24 μM (1:1:3), 50 μM (1:1:10; Figure 1a, see also legend to Figure 1 for individual EC50 values).
Figure 1.
(a) Typical GABA concentration–response curves of oocytes injected with cRNAs of α1, β2 and γ2S subunits (cRNA stoichiometry: 1:1:1, left panel; 1:1:3, middle panel and 1:1:10, right panel) in the absence (control) and presence of 1 μM triazolam. The corresponding EC50 values for 1:1:1 were 24 μM (control), 8 μM (triazolam); for 1:1:3: 38 μM (control), 14 μM (triazolam) and for 1:1:10: 71 μM (control), 21 μM (triazolam). (b) Corresponding IGABA through α1β2γ2S (1:1:3) channels modulated by 1 μM triazolam at indicated GABA concentrations. (c) GABA concentration–response curves of oocytes injected with cRNAs of α1, β2 and γ1 subunits (1:1:1, left panel; 1:1:3, middle panel and 1:1:10, right panel) in the absence (control) and presence of 1 μM triazolam. The corresponding EC50 values for 1:1:1 were: 21 μM (control), 12 μM (triazolam); for 1:1:3: 31 μM (control), 16 μM (triazolam); for 1:1:10: 66 μM (control), 29 μM (triazolam). Each graph in (a) and (c) represents one experiment on one oocyte.
The typical pattern of IGABA modulation of an oocyte (α1:β2:γ2S cRNA ratio 1:1:3) is illustrated in Figure 1b. Triazolam enhanced IGABA predominantly at low GABA concentrations corresponding to EC5−10 (concentrations of GABA that induce 5–10% of maximal GABA-evoked current) and had almost no effects at a saturating GABA concentration. Similar observations were made for α1β2γ1 receptors (Figure 1c).
Different efficiencies of IGABA potentiation in oocytes expressing α1β2γ1 or α1β2γ2S receptors
Figure 1 illustrates that GABA potency (EC50) and IGABA modulation by triazolam were both dependent on the amount of γ2S (or γ1) incorporation (see also Boileau et al., 2002). These experiments confirmed that apparent higher γ-expression and correspondingly lower GABA sensitivity were always associated with larger BZD-induced shifts of the curves. In line with Boileau et al. (2002, 2003) we observed a higher GABA sensitivity at cRNA ratios 1:1:1 and 1:1:3 compared to 1:1:10. The range of GABA EC50s for a given cRNA ratio reflected differences in γ-incorporations.
EC50s of individual oocytes were plotted versus the BZD-induced shifts of the GABA concentration–response curves (ΔEC50(BZD)s; Figure 2). For all three compounds applied at saturating concentrations, we obtained a clear correlation between EC50s and ΔEC50(BZD)s. The slope factors for oocytes expressing α1β2γ1 subunit receptors (1.8±0.1 (triazolam; Figure 2a), 1.6±0.1 (clotiazepam; Figure 2b) and 2.3±0.2 (midazolam; Figure 2c)) were always higher than for oocytes expressing α1β2γ2S receptors (1.4±0.1 (triazolam; Figure 2d), 1.4±0.1 (clotiazepam; Figure 2e) and 1.3±0.1 (midazolam; Figure 2f)). The regression line approached the ordinate in the range of the EC50 of α1β2 receptors. The data suggest that the slope factors may reflect the efficiency of IGABA potentiation for these compounds (see Table 1). For clotiazepam, we estimated similar slopes for γ1- and γ2S-incorporating receptors (difference statistically not significant; P>0.05) whereas for triazolam and midazolam the slopes of the regression lines for the two receptor subtypes were significantly different (P<0.05).
Figure 2.
Correlation between EC50 values of the GABA concentration–response curves (EC50s) of oocytes expressing α1β2γ1 receptors (a–c), α1β2γ2S receptors (d–f) and shifts of these EC50 values (ΔEC50(BZD)s) by modulation of the GABA concentration–response curve by 1 μM triazolam (a, d), 10 μM clotiazepam (b, e) and 10 μM midazolam (c, f); α-, β- and γ-subunit cRNA stoichiometries are 1:1:1, 1:1:3 and 1:1:10. Correlation coefficients were 0.94 (triazolam), 0.94 (clotiazepam), 0.94 (midazolam) (P<0.0001 in all cases, α1β2γ1) and 0.97 (triazolam), 0.94 (clotiazepam), 0.90 (midazolam) (P<0.0001 in all cases, α1β2γ2S). Each data point represents one experiment on one oocyte.
Table 1.
Comparison of slope factors and maximal potentiation by BZDs
| BZD |
α1β2γ1 |
α1β2γ2S |
||
|---|---|---|---|---|
| Slope | Maximum IGABA potentiation (%)a | Slope | Maximum IGABA potentiation (%)a | |
| Triazolam | 1.8±0.1 | 85±7 | 1.4±0.1 | 253±12 |
| Clotiazepam | 1.6±0.1 | 172±24 | 1.4±0.1 | 260±27 |
| Midazolam | 2.3±0.2 | 92±8 | 1.3±0.1 | 342±64 |
Abbreviations: BZD, benzodiazepine; IGABA, GABA-activated chloride currents.
Data from Khom et al. (2006).
Pharmacological properties of concatenated subunits fit the extrapolated regression lines
If the inhibition of GABA sensitivity caused by γ-incorporation correlates with IGABA potentiation, then complete γ-incorporation in concatenated subunits is expected to result in larger EC50s. To test this hypothesis we first injected higher amounts of γ1 and γ2S subunit cRNA (1:1:20; see Figure 3). We obtained, however, only a non-significant (P<0.05) further reduction in GABA sensitivity (α1β2γ1: EC50=61±3 μM, n=4 and α1β2γ2S: EC50=62±4 μM, n=4, see dashed lines in Figure 3). Next we expressed a mixture of concatenated subunit constructs (γ2-β2-α1 and β2-α1, 1:1 ratio; Baumann et al., 2002) and analysed the GABA concentration–response curves. Figure 3 compares the mean GABA concentration–response curves obtained for oocytes injected with cRNAs for α1β2 (1:1, n=6), α1β2γ1 (1:1:10, n=24), α1β2γ1 (1:1:20, n=4), α1β2γ2S (1:1:10, n=27), α1β2γ2S (1:1:20, n=4) or concatenated γ2-β2-α1/β2-α1 receptors (1:1, n=18). In line with previous studies (Baumann et al., 2002) oocytes expressing concatenated subunits in Xenopus oocytes displayed a lower GABA sensitivity. The EC50 of concatenated subunits was substantially shifted to the right (EC50=186±13 μM, n=18; Figures 3 and 4). The EC50s and corresponding shifts of the concentration–response curve (ΔEC50(BZD)s) for all three compounds are shown in Figures 4a–c. When EC50s and corresponding ΔEC50(BZD)s were added to the graph shown in Figure 2 (Figures 4d–f) they fitted the extended linear correlation obtained for non-concatenated subunits.
Figure 3.
GABA concentration–response curves for oocytes expressing α1β2 (cRNA injection 1:1), α1β2γ1 (1:1:10), α1β2γ1 (1:1:20), α1β2γ2S (1:1:10), α1β2γ2S (1:1:20) and γ2-β2-α1/β2-α1 (1:1) receptors. The corresponding mean EC50 values and Hill coefficients were α1β2 (1:1): 8±2 μM, nH=1.0±0.2 (n=6); α1β2γ1 (1:1:10): 48±3 μM, nH=1.3±0.1 (n=24); α1β2γ1 (1:1:20): 61±3 μM, nH=1.5±0.1 (n=4); α1β2γ2S (1:1:10): 51±3 μM, nH=1.4±0.1 (n=27); α1β2γ2S (1:1:20): 62±4 μM, nH=1.5±0.1 (n=4); γ2-β2-α1/β2-α1 (1:1): 186±13 μM, nH=1.3±0.1 (n=18).
Figure 4.
GABA concentration–response curves of oocytes injected with concatenated subunits (γ2-β2-α1/β2-α1) in the absence (control) and presence of 1 μM triazolam (a), 10 μM clotiazepam (b) and 10 μM midazolam (c). The corresponding EC50 values were: 158 μM (control), 34 μM (triazolam); 150 μM (control), 60 μM (clotiazepam); 132 μM (control), 43 μM (midazolam). EC50 values of the GABA concentration–response curves of oocytes expressing concatenated subunit constructs (γ2-β2-α1 and β2-α1 in 1:1 ratio) and ΔEC50(BZD)s induced by (d) 1 μM triazolam, (e) 10 μM clotiazepam and (f) 10 μM midazolam were added to the regression lines from Figures 2d–f. Corresponding regression lines (dashed) were taken from Figures 2d–f and extended towards the values of the concatenated subunits. Solid lines (d–f) represent the non-linear fit of Equations 1–5 (see Materials and methods) by the maximal likelihood. Calculated current fractions of γ-incorporating receptors (0, 50, 70 and 100%) are indicated. Each graph (a, b and c) and each data point (d, e and f) represent one experiment on one oocyte.
Estimation of α1β2 and α1β2γ2S receptor fractions
In an independent approach we theoretically calculated the behaviour of a mixed population of GABAA receptors with a high (α1β2) and a low (α1β2γ2S) GABA sensitivity. The total normalized IGABA is the weighted sum of partial current responses of the individual receptors and only α1β2γ2S are sensitive to BZDs (see Materials and methods). The simulated curves are shown in Figure 4 (solid lines). To calculate the putative fractions of α1β2 and α1β2γ2S receptors for oocytes displaying different GABA sensitivities we made use of the concentration of half-maximal activation of the simulated curve of αβ receptors (EC50,α1β2=8±2 μM, n=6; see also Sigel and Baur, 2000; Baumann et al., 2001) and EC50,α1β2γ2S (186 μM) of concatenated receptors and corresponding Hill coefficients (Hα1β2=1.0, Hα1β2γ2S=1.3) from our experiments (see Figure 3).
The maximum likelihood fit of the EC50 versus ΔEC50 relation predicts 100% γ2S incorporation for the concatenated receptors (Figures 4d–f). Our calculations suggest, however, that even at a 1:1:10 cRNA ratio the fraction (F) of current through α1β2γ2S receptors accounts for only between 50 and 70% of the total current. If we assume that the single channel currents through γ incorporating receptors is n times larger than through α1β2 receptors, then the fraction (f) of γ incorporating receptors is given by f=F/(n−F(1−n)).
This formula accounts for differences in single channel conductance. The frequency of openings and mean open time also influences mean conductance of both subpopulations. The equation describing concentration–response curve used in our calculation (see Materials and methods) estimates the fraction of open channels that in turn reflects the mean open time of single channels at different GABA concentrations and thus accounts for differences in open time. Assuming a twofold greater single channel conductance for α1β2γ2S receptors (that is, n=2, see Angelotti and Macdonald, 1993; Fisher and Macdonald, 1997) and F≈70% would predict f≈54%. In other words, our analysis suggests that even under conditions where α1:β2:γ2S were injected at a ratio of 1:1:10 only about 50% of the expressed receptors contain a γ2S subunit. This may explain the large difference in EC50s of concatenated and non-concatenated receptors (even at a ratio of 1:1:20; Figure 3).
Relation between EC50 and ΔEC50(BZD) on a GABAA receptor mutant with reduced GABA sensitivity
Larger slope factors of γ1- versus lower slope factors of γ2S-containing receptors indicated that this parameter provides a measure of drug efficiency. To further validate the sensitivity of this parameter, we made use of a mutation (β2-R207C; located in the GABA-binding site), known to induce a reduction in GABA sensitivity (see also Wagner and Czajkowski, 2001; Wagner et al., 2004). GABAA receptors composed of α1 and β2-R207C subunits displayed a reduced GABA sensitivity (Figure 5a; EC50 for α1β2-R207C=486±81 μM, n=6; see also Wagner et al., 2004). Co-expression of α1, β2-R207C with the γ2S subunit induced a further reduction of GABA sensitivity (Figure 5a; EC50 for α1β2-R207Cγ2S=3217±378 μM, n=18). A saturating concentration of clotiazepam (10 μM) significantly increased chloride currents at GABA EC5−10 (Figure 5b). The corresponding correlation between individual EC50 values of the GABA concentration–response curves (EC50s) of oocytes expressing α1β2-R207Cγ2S receptors and shifts of these EC50 values (ΔEC50(BZD)s) caused by three BZDs is shown in Figures 6d–f. The regression lines approach the EC50 axis in a range (460–587 μM; Figures 6d–f) close to the EC50 of α1β2-R207C receptors (Figure 5a). Figures 6a–c illustrate typical shifts of the dose–response curves by a saturating concentration of triazolam (1 μM) for different α1:β2-R207C:γ2S cRNA ratios (1:1:1—ΔEC50=421 μM, (a); 1:1:3—ΔEC50=948 μM, (b) and 1:1:10—ΔEC50=2900 μM, (c); see also legend to Figure 6 for individual EC50 values). The slopes of regression lines were 1.1±0.1 (triazolam), 1.1±0.1 (clotiazepam) and 1.2±0.1 (midazolam). Interestingly, the three BZDs enhanced IGABA at saturating GABA concentrations (10–100 mM) where peak currents in wild-type receptors were hardly affected (compare triazolam action in Figures 1 and 6).
Figure 5.
(a) GABA concentration–response curves of oocytes expressing α1β2-R207C (1:1, EC50=486±81 μM, nH=1.2±0.2, n=6) and α1β2-R207Cγ2S (1:1:10, EC50=3217±378 μM, nH=0.9±0.1, n=18) receptors. (b) Representative traces for enhancement of IGABA through α1β2-R207Cγ2S. Control currents (GABA) in the absence of clotiazepam and corresponding currents elicited by co-application of GABA and clotiazepam are shown.
Figure 6.
GABA concentration–response curves of oocytes injected with cRNA ratios of α1, β2-R207C and γ2S subunits of 1:1:1 (a), 1:1:3 (b) and 1:1:10 (c) in the absence (control) and presence of 1 μM triazolam. The corresponding EC50 values for 1:1:1 were: 827 μM (control), 406 μM (triazolam); for 1:1:3: 1945 μM (control), 997 μM (triazolam); for 1:1:10: 3891 μM (control), 991 μM (triazolam). (d–f) Correlation between individual EC50 values of the GABA concentration–response curves (EC50s) of oocytes expressing α1β2-R207Cγ2S receptors and shifts of these EC50 values (ΔEC50(BZD)s) caused by modulation of the GABA concentration–response curve by 1 μM triazolam (d), 10 μM clotiazepam (e) and 10 μM midazolam (f). The slopes of regression lines were 1.1±0.1 (triazolam), 1.1±0.1 (clotiazepam) and 1.2±0.1 (midazolam). Correlation coefficients were 0.96 (triazolam), 0.98 (clotiazepam), 0.97 (midazolam) (P<0.0001 in all cases). Each graph (a, b and c) and each data point (d, e and f) represent one experiment on one oocyte.
Discussion
This study revealed a linear correlation between the γ-subunit-mediated suppression and BZD-induced increase of GABA sensitivity in α1β2γ2S and α1β2γ1 receptors. We made use of a statistical approach because direct evaluation of this relationship is complicated by variable γ-incorporation even under conditions where high ratios of γ-subunit cRNA relative to α1 and β2 are injected. Injection of higher cRNA ratio of α1:β2:γ2S than 1:1:10 induced only a slight further reduction in GABA sensitivity suggesting a non-linear relationship between cRNA ratios and γ-subunit incorporation (see 1:1:20 ratio in Figure 3 and also Boileau et al., 2002). We have therefore induced different γ-expression by injecting different cRNA ratios and analysed the relation between γ-mediated inhibition and BZD-induced enhancement of GABA sensitivity.
Slopes of regression lines are inversely related to BZD efficiency
An initially observed trend that larger BZD induced shifts of the concentration–response curves (ΔEC50(BZD)s) in oocytes expressing larger fractions of γ-subunit-containing receptors (induced by injection of increasing amounts of cRNA encoding for γ-subunits, Figure 1) was confirmed by correlation analysis (Figure 2). The linear relationship between EC50 values and ΔEC50(BZD) suggests that BZDs reduce a γ-subunit-mediated inhibition of GABA sensitivity. A slope of 1.0 would correspond to complete elimination of a γ-subunit-induced inhibition of GABA sensitivity and BZDs would correspondingly shift the concentration–response curve of α1β2γ1(2S) receptors to the position of α1β2 receptors. The observation that the tested BZDs never increased GABA sensitivity to that or above that of α1β2 receptors indicates that these drugs only partially can compensate for the γ-subunit-induced inhibition.
The estimated slope factors ranged from 1.1±0.1 in α1β2-R207Cγ2S (triazolam, clotiazepam) to 2.3±0.2 (midazolam) in α1β2γ1 subunit receptors. To evaluate the significance of the slopes we compared the slopes on oocytes expressing α1β2γ1 and α1β2γ2S subunit receptors. The present results support previously estimated maximal IGABA potentiation by midazolam (342±64%)>clotiazepam (260±27%)≈triazolam (253±12%) for oocytes expressing α1β2γ2S receptors (Khom et al., 2006) and indicate a reversed order of regression slopes (midazolam: 1.3±0.1<clotiazepam: 1.4±0.1=triazolam: 1.4±0.1). Furthermore, steeper slopes of the regression lines for α1β2γ1 compared to α1β2γ2S receptors specify that midazolam and to a lesser extent triazolam and clotiazepam increase GABA sensitivity less efficiently in α1β2γ1 than in α1β2γ2S receptors (Figure 2), which confirms our previous studies (Khom et al., 2006). Taken together our data suggest that the slopes of the regression lines between ΔEC50(BZD)s and EC50s are inversely related to drug efficiency.
The described approach is time consuming as it requires the injection of different cRNA ratios and the measurements of a large number of concentration–response curves. Our experiments under different conditions (including different BZDs, γ-subunits and a mutation in the β2 subunit; Figures 2, 4 and 6) revealed, however, that the regression lines intercept the y-axis close to the EC50 of α1β2 receptors. This finding prompted us to evaluate the possibility to use the y-intercept and three randomly selected data points (cRNA ratio 1:1:10; Figures 2d–f) for correlation analysis. The simplified procedure yielded regression lines with slopes that were statistically not significantly different from larger data sets (data not shown).
The relevance of the slope factor was further evaluated making use of the mutation β2-R207C that is known to induce a more than 60-fold reduction in GABA sensitivity (Wagner et al., 2004). Figure 5a illustrates that co-expression of γ2S decreased the GABA sensitivity analogously to wild-type receptors (Figure 3). The estimated slope factors (1.1±0.1 (triazolam), 1.1±0.1 (clotiazepam) and 1.2±0.1 (midazolam); Figure 6) were always smaller than in wild type (though only in the case of triazolam and clotiazepam that difference was significant, P<0.05), suggesting even higher BZD efficiencies compared to wild type.
Concatenated receptor subunits fit the regression lines
Forced γ2 subunit incorporation in oocytes was previously shown to be associated with a significant loss in GABA sensitivity compared to oocytes injected with 1:1:10 cRNA ratios (Baumann et al., 2002; see also Figure 3; but see Baur et al., 2006). Here we show that the higher EC50s and corresponding ΔEC50(BZD)s of concatenated subunits fit the predictions of the regression lines of non-concatenated subunits (Figures 4d–f, closed triangles). The mean EC50 of the concatenated γ2-β2-α1/β2-α1 subunits (Figure 3) corresponds to the data of Baumann et al. (2002). Lower GABA sensitivity and higher BZD efficiencies of concatenated subunits expressed in Xenopus oocytes may accordingly result from more complete γ-incorporation (rather than from forced subunit arrangement or interactions). Our data suggest that the studied GABAA receptor composed of two concatenated constructs may represent a model of completely assembled receptor with intact GABA- and BZD-binding sites. This hypothesis (based on the extrapolation of the regression line; Figure 4) requires, however, further studies.
These experimental data could be reproduced by a mathematical model describing BZD modulation in oocytes expressing different current fractions of high GABA sensitive α1β2 versus low-sensitive α1β2γ2S receptors. Maximum likelihood analysis predicted (in line with the experimental observations) a linear relationship that enables the calculation of a putative fraction of α1β2γ2S receptors at a given EC50 and ΔEC50(BZD). This calculation had, however, to account for the different single channel conductance of the two receptor subtypes (Angelotti and Macdonald, 1993; Fisher and Macdonald, 1997). Interestingly these independent calculations reproduced not only the linear correlation but predicted the expected 100% γ2S incorporation for the concatemers and thus would support the experimental findings of the concatenated receptors and our conclusions.
Percentage of α1β2γ2 receptors in oocytes expressing these subunits
Boileau et al. (2002) have clearly demonstrated that injecting higher ratios of γ2 subunit cRNA results in a purer population of α1β2γ2 subunit receptors. In addition, they observed a remarkable run-down of BZD modulation, suggesting a decay of γ2 subunit-incorporating receptors over time after injection into oocytes that makes it difficult to compare data from different labs. Furthermore, small changes in the applied effective GABA concentration (usually between EC3 and EC10) can substantially affect the apparent BZD efficiency, thus further confounding a calculation of the percentage of expressed α1β2γ2S receptors. The method suggested above for determining the slope of the regression line, however, also allows the determination of the percentage of α1β2γ2S receptors. After correction for single channel conductances (assuming a twofold difference in the single channel conductance of α1β2 and α1β2γ2S receptors, Angelotti and Macdonald, 1993; Fisher and Macdonald, 1997), a 70% current ratio would correspond to about 50% of γ2S subunit incorporation.
The regression slopes are independent from variations in expression of α1β2 versus α1β2γ2S(γ1) fractions or variations in experimental conditions (GABA concentration). The statistical analysis described here is based on the different shifts of the concentration–response curves and, therefore, independent of these possible experimental errors. The low percentage of α1β2γ2S receptors formed under the conditions used indicate that at least in the Xenopus oocyte system (but not necessarily in other heterologous expression systems or under native conditions) the efficiency of assembly of α1β2 receptors might be comparable to or even higher than that of α1β2γ2S receptors, resulting in comparable amounts of α1β2 or α1β2γ2S receptors even when high concentrations of γ-subunits are available.
Implications for the mechanism of BZD action
The different shifts in the GABA concentration–response curves for GABAA receptors with different subunit compositions or mutants can be interpreted in terms of a mechanism where BZDs increase the microscopic affinity of the GABA-binding site or, alternatively, by a mechanism where BZDs facilitate channel gating (for example, Jones-Davis et al., 2005). The second scenario is supported by previous findings suggesting that BZD-like modulators enhance the amplitude of the GABA response by stabilizing the open channel conformation (Downing et al., 2005; Rusch and Forman, 2005; Campo-Soria et al., 2006).
We are tempted to speculate that the BZD-induced IGABA increase reflects an increase in GABA efficacy (as defined by Colquhoun, 1998) by a direct transduction of the BZD effect to the channel region (Akabas, 2004; Ernst et al., 2005). An increase of efficacy from apparent low level (for example, in mutant β2-R207C or concatemers) could explain an increase of the maximal GABA response (‘over-potentiation' of the BZDs on the α1β2-R207Cγ2S isoform or concatenated receptors) and a simultaneous shift of the concentration–response curve (Figures 4a–c and 6a–c; Colquhoun, 1998; Downing et al., 2005; Rusch and Forman, 2005; Campo-Soria et al., 2006). These theoretical considerations need, however, more experimental validation including the investigation of further mutations (for example, in the putative GABA-binding site and/or pore region) and the use of BZDs with a broad range of efficiencies.
Acknowledgments
This work was supported by FWF grant 15914 (SH).
Abbreviations
- BZD
benzodiazepine
- DEPC
diethylpyrocarbonate
- IGABA
GABA-induced chloride current
Conflict of interest
The authors state no conflict of interest.
References
- Akabas MH. GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. doi: 10.1016/S0074-7742(04)62001-0. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993;13:1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baburin I, Beyl S, Hering S. Automated fast perfusion of Xenopus oocytes for drug screening. Pflugers Arch. 2006;453:117–123. doi: 10.1007/s00424-006-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Subunit arrangement of gamma-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Baur R, Minier F, Sigel E. A GABA(A) receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 2006;580:1616–1620. doi: 10.1016/j.febslet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Baur R, Sigel E. Benzodiazepines affect channel opening of GABA A receptors induced by either agonist binding site. Mol Pharmacol. 2005;67:1005–1008. doi: 10.1124/mol.104.008151. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABA(A) receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Czajkowski C. Identification of transduction elements for benzodiazepine modulation of the GABA(A) receptor: three residues are required for allosteric coupling. J Neurosci. 1999;19:10213–10220. doi: 10.1523/JNEUROSCI.19-23-10213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effects of gamma2S subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacology. 2003;44:1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Pearce RA, Czajkowski C. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci. 2005;25:11219–11230. doi: 10.1523/JNEUROSCI.3751-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing SS, Lee YT, Farb DH, Gibbs TT. Benzodiazepine modulation of partial agonist efficacy and spontaneously active GABA(A) receptors supports an allosteric model of modulation. Br J Pharmacol. 2005;145:894–906. doi: 10.1038/sj.bjp.0706251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Brauchart D, Boresch S, Sieghart W. Comparative modeling of GABA(A) receptors: limits, insights, future developments. Neuroscience. 2003;119:933–943. doi: 10.1016/s0306-4522(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bruckner S, Boresch S, Sieghart W. Comparative models of GABAA receptor extracellular and transmembrane domains: important insights in pharmacology and function. Mol Pharmacol. 2005;68:1291–1300. doi: 10.1124/mol.105.015982. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Bianchi MT, Macdonald RL. Pentobarbital differentially modulates alpha1beta3delta and alpha1beta3gamma2L GABAA receptor currents. Mol Pharmacol. 2004;66:988–1003. doi: 10.1124/mol.104.002543. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. Single channel properties of recombinant GABAA receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse L929 cells. J Physiol. 1997;505 Part 2:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallager DW, Tallman JF. Consequences of benzodiazepine receptor occupancy. Neuropharmacology. 1983;22:1493–1498. doi: 10.1016/0028-3908(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Jones-Davis DM, Song L, Gallagher MJ, Macdonald RL. Structural determinants of benzodiazepine allosteric regulation of GABA(A) receptor currents. J Neurosci. 2005;25:8056–8065. doi: 10.1523/JNEUROSCI.0348-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S, Baburin I, Timin EN, Hohaus A, Sieghart W, Hering S. Pharmacological properties of GABAA receptors containing gamma1 subunits. Mol Pharmacol. 2006;69:640–649. doi: 10.1124/mol.105.017236. [DOI] [PubMed] [Google Scholar]
- Khom S, Baburin I, Timin E, Hohaus A, Trauner G, Kopp B, et al. Valerenic acid potentiates and inhibits GABA(A) receptors: molecular mechanism and subunit specificity. Neuropharmacology. 2007;53:178–187. doi: 10.1016/j.neuropharm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Twyman RE. Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology. 1996;35:1383–1392. doi: 10.1016/s0028-3908(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barker JL. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature. 1978;271:563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Minier F, Sigel E. Techniques: use of concatenated subunits for the study of ligand-gated ion channels. Trends Pharmacol Sci. 2004;25:499–503. doi: 10.1016/j.tips.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Newell JG, Czajkowski C. The GABAA receptor alpha 1 subunit Pro174-Asp191 segment is involved in GABA binding and channel gating. J Biol Chem. 2003;278:13166–13172. doi: 10.1074/jbc.M211905200. [DOI] [PubMed] [Google Scholar]
- Rusch D, Forman SA. Classic benzodiazepines modulate the open-close equilibrium in alpha1beta2gamma2L gamma-aminobutyric acid type A receptors. Anesthesiology. 2005;102:783–792. doi: 10.1097/00000542-200504000-00014. [DOI] [PubMed] [Google Scholar]
- Serfozo P, Cash DJ. Effect of a benzodiazepine (chlordiazepoxide) on a GABAA receptor from rat brain. Requirement of only one bound GABA molecule for channel opening. FEBS Lett. 1992;310:55–59. doi: 10.1016/0014-5793(92)81145-c. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2:833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R. Electrophysiological evidence for the coexistence of alpha1 and alpha6 subunits in a single functional GABA(A) receptor. J Neurochem. 2000;74:2590–2596. doi: 10.1046/j.1471-4159.2000.0742590.x. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Kellenberger S, Malherbe P. Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J. 1992;11:2017–2023. doi: 10.1002/j.1460-2075.1992.tb05258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABA(A) receptor genes in the human genome. J Biol Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Rogers CJ, Macdonald RL. Intraburst kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1990;423:93–220. doi: 10.1113/jphysiol.1990.sp018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C. Structure and dynamics of the GABA binding pocket: a narrowing cleft that constricts during activation. J Neurosci. 2001;21:67–74. doi: 10.1523/JNEUROSCI.21-01-00067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C, Jones MV. An arginine involved in GABA binding and unbinding but not gating of the GABA(A) receptor. J Neurosci. 2004;24:2733–2741. doi: 10.1523/JNEUROSCI.4316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]








