Abstract
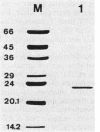
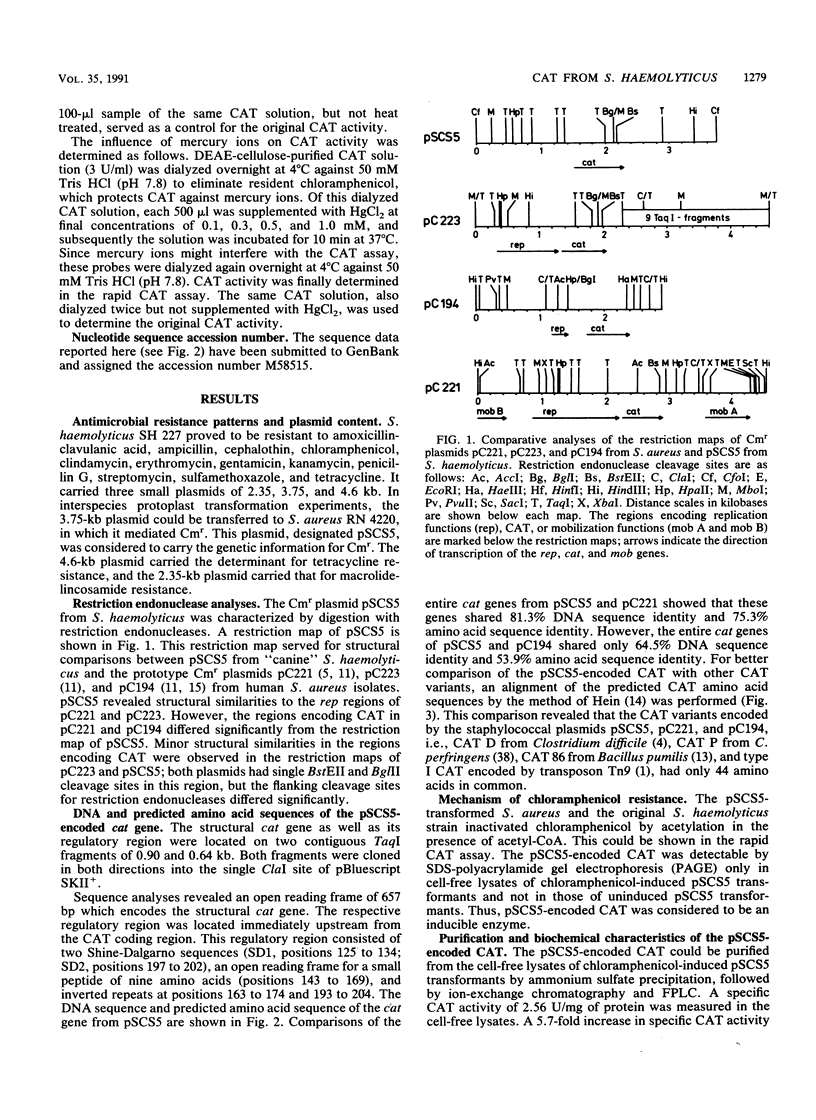
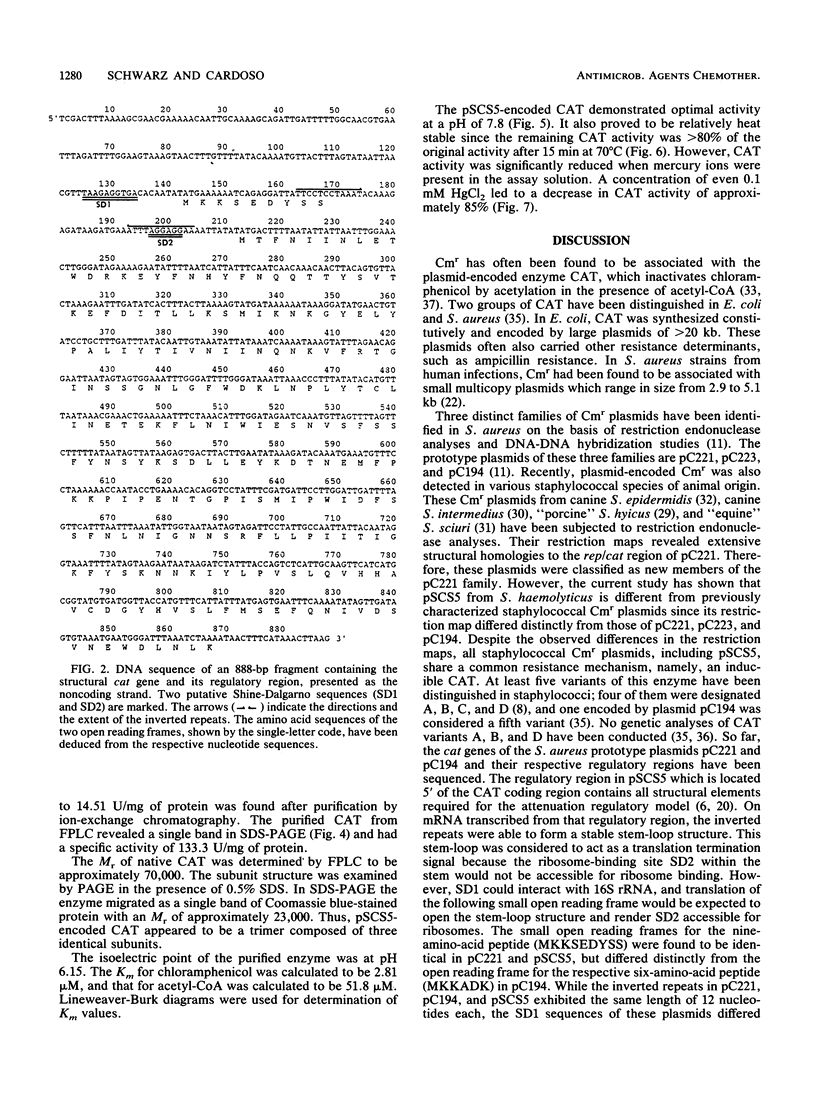
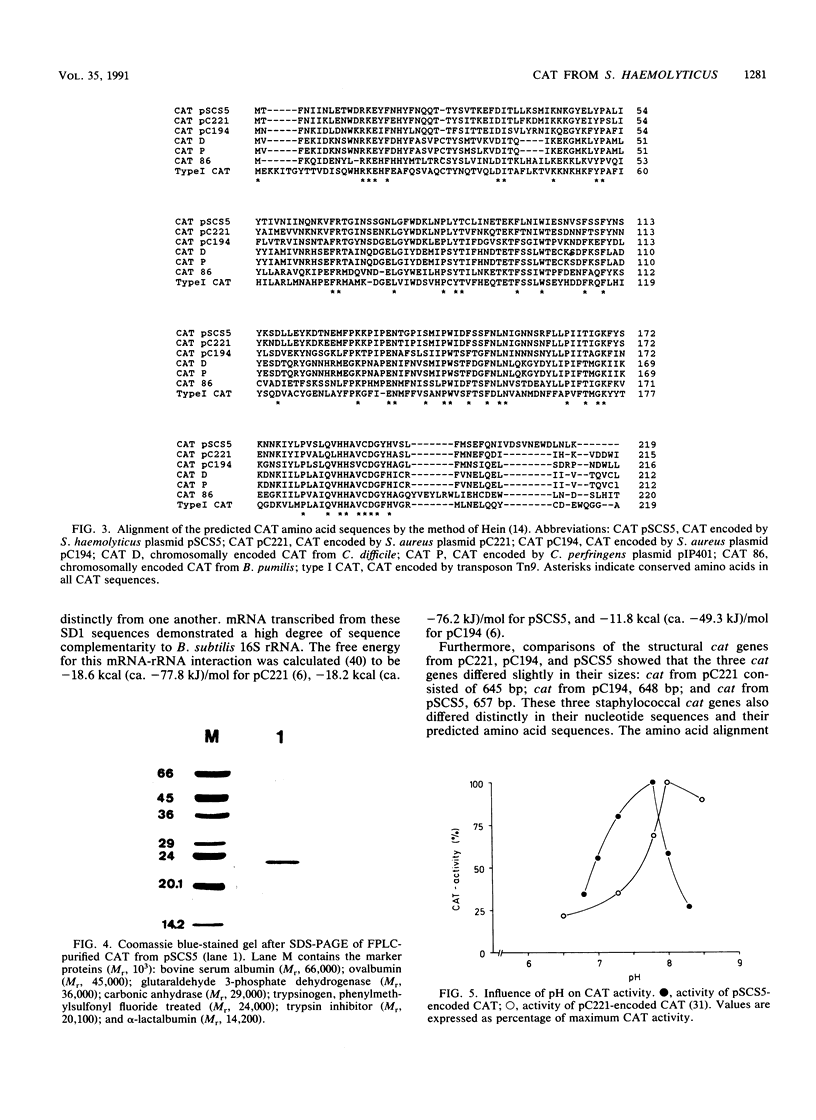
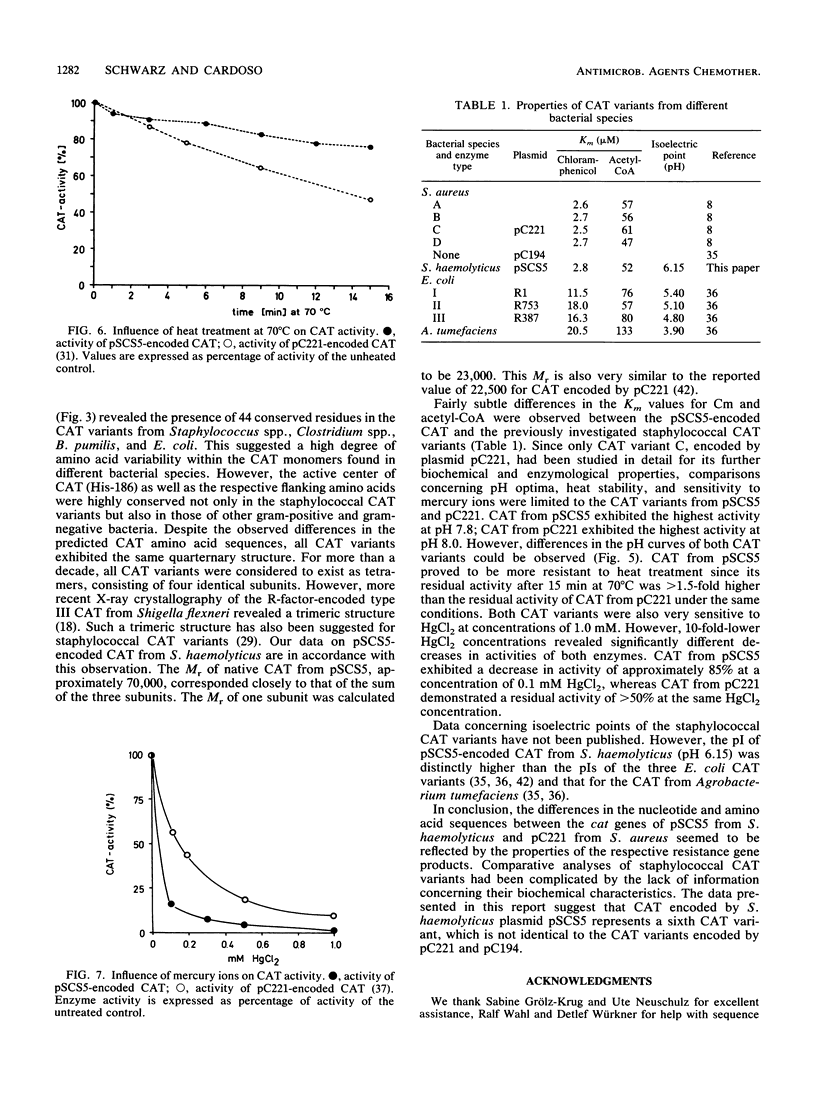
A small chloramphenicol resistance (Cmr) plasmid of approximately 3.75 kb, designated pSCS5, was isolated from Staphylococcus haemolyticus. This plasmid encoded an inducible chloramphenicol acetyltransferase (CAT; EC 2.3.1.28). The cat gene of pSCS5 was cloned into the Escherichia coli plasmid vector pBluescript SKII+. It differed in its nucleotide sequence and deduced amino acid sequence from the cat genes described previously in staphylococci and other gram-positive bacteria. The CAT enzyme was purified from cell-free lysates by ammonium sulfate precipitation, ion-exchange chromatography, and fast protein liquid chromatography. The native enzyme had an Mr of 70,000 and was composed of three identical subunits, each with an Mr of approximately 23,000. Its isoelectric point was at pH 6.15. CAT from pSCS5 exhibited Km values of 2.81 and 51.8 microM for chloramphenicol and acetyl coenzyme A, respectively. The optimum pH for activity was 7.8. CAT encoded by pSCS5 proved to be relatively heat stable, but sensitive to mercury ions. The observed differences in the nucleotide sequence and the biochemical characteristics of the enzyme allowed the identification of the pSCS5-encoded CAT from S. haemolyticus as a CAT variant different from those described previously in gram-positive bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. G., Shaw W. V. The use of synthetic oligonucleotides with universal templates for rapid DNA sequencing: results with staphylococcal replicon pC221. EMBO J. 1985 Feb;4(2):561–568. doi: 10.1002/j.1460-2075.1985.tb03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner R., Matzura H. Regulation of the inducible chloramphenicol acetyltransferase gene of the Staphylococcus aureus plasmid pUB112. EMBO J. 1985 Sep;4(9):2295–2300. doi: 10.1002/j.1460-2075.1985.tb03929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Fitton J. E., Shaw W. V. Comparison of chloramphenicol acetyltransferase variants in staphylococci. Purification, inhibitor studies and N-terminal sequences. Biochem J. 1979 Feb 1;177(2):575–582. doi: 10.1042/bj1770575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt J. W., Johnston J. L., Galetto D. W., Archer G. L. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn B. A., Davis C. E., Jr Staphylococcus haemolyticus urinary tract infection in a male patient. J Clin Microbiol. 1988 May;26(5):1055–1057. doi: 10.1128/jcm.26.5.1055-1057.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. R., Williams D. M., Lovett P. S. Nucleotide sequence of a Bacillus pumilus gene specifying chloramphenicol acetyltransferase. Gene. 1983 Oct;24(2-3):163–169. doi: 10.1016/0378-1119(83)90076-8. [DOI] [PubMed] [Google Scholar]
- Hein J. Unified approach to alignment and phylogenies. Methods Enzymol. 1990;183:626–645. doi: 10.1016/0076-6879(90)83041-7. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E., Orban B. S., Walker D. D. Plasmid composition of Staphylococcus species. Can J Microbiol. 1981 Mar;27(3):271–278. doi: 10.1139/m81-043. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leslie A. G., Moody P. C., Shaw W. V. Structure of chloramphenicol acetyltransferase at 1.75-A resolution. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4133–4137. doi: 10.1073/pnas.85.12.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. Translational attenuation as the regulator of inducible cat genes. J Bacteriol. 1990 Jan;172(1):1–6. doi: 10.1128/jb.172.1.1-6.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle L. E., Hoban S. A., Harding G. K. Characterization of coagulase-negative staphylococci from urinary tract specimens. J Clin Microbiol. 1983 Feb;17(2):267–271. doi: 10.1128/jcm.17.2.267-271.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeg W., Bingöl R., Blobel H. Purification of penicillinase ( -lactamase) and acid phosphatase from Staphylococcus aureus in one procedure. Biochim Biophys Acta. 1972 May 12;268(2):542–549. doi: 10.1016/0005-2744(72)90351-8. [DOI] [PubMed] [Google Scholar]
- Schwarz S., Blobel H. Plasmids and resistance to antimicrobial agents and heavy metals in Staphylococcus hyicus from pigs and cattle. Zentralbl Veterinarmed B. 1989 Nov;36(9):669–673. doi: 10.1111/j.1439-0450.1989.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Schwarz S., Cardoso M., Blobel H. Detection of a novel chloramphenicol resistance plasmid from "equine" Staphylococcus sciuri. Zentralbl Veterinarmed B. 1990 Nov;37(9):674–679. doi: 10.1111/j.1439-0450.1990.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Schwarz S., Cardoso M., Blobel H. Plasmid-mediated chloramphenicol resistance in Staphylococcus hyicus. J Gen Microbiol. 1989 Dec;135(12):3329–3336. doi: 10.1099/00221287-135-12-3329. [DOI] [PubMed] [Google Scholar]
- Schwarz S., Cardoso M., Grölz-Krug S., Blobel H. Common antibiotic resistance plasmids in Staphylococcus aureus and Staphylococcus epidermidis from human and canine infections. Zentralbl Bakteriol. 1990 Aug;273(3):369–377. doi: 10.1016/s0934-8840(11)80440-8. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Bacterial resistance to chloramphenicol. Br Med Bull. 1984 Jan;40(1):36–41. doi: 10.1093/oxfordjournals.bmb.a071945. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Bentley D. W., Sands L. Mechanism of Chloramphenicol Resistance in Staphylococcus epidermidis. J Bacteriol. 1970 Dec;104(3):1095–1105. doi: 10.1128/jb.104.3.1095-1105.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase: enzymology and molecular biology. CRC Crit Rev Biochem. 1983;14(1):1–46. doi: 10.3109/10409238309102789. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Steffen C., Matzura H. Nucleotide sequence analysis and expression studies of a chloramphenicol-acetyltransferase-coding gene from Clostridium perfringens. Gene. 1989 Feb 20;75(2):349–354. doi: 10.1016/0378-1119(89)90282-5. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wren B. W., Mullany P., Clayton C., Tabaqchali S. Nucleotide sequence of a chloramphenicol acetyl transferase gene from Clostridium difficile. Nucleic Acids Res. 1989 Jun 26;17(12):4877–4877. doi: 10.1093/nar/17.12.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidenzaig Y., Fitton J. E., Packman L. C., Shaw W. V. Characterization and comparison of chloramphenicol acetyltransferase variants. Eur J Biochem. 1979 Oct 15;100(2):609–618. doi: 10.1111/j.1432-1033.1979.tb04208.x. [DOI] [PubMed] [Google Scholar]