Abstract
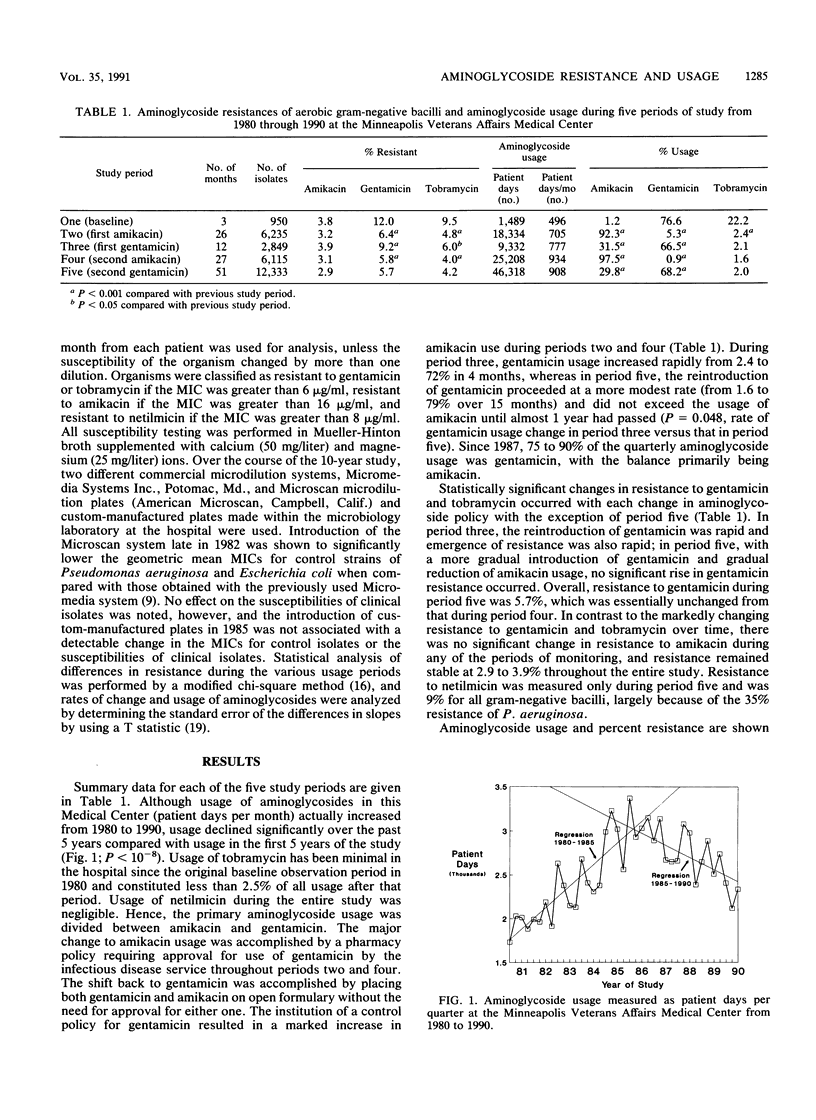
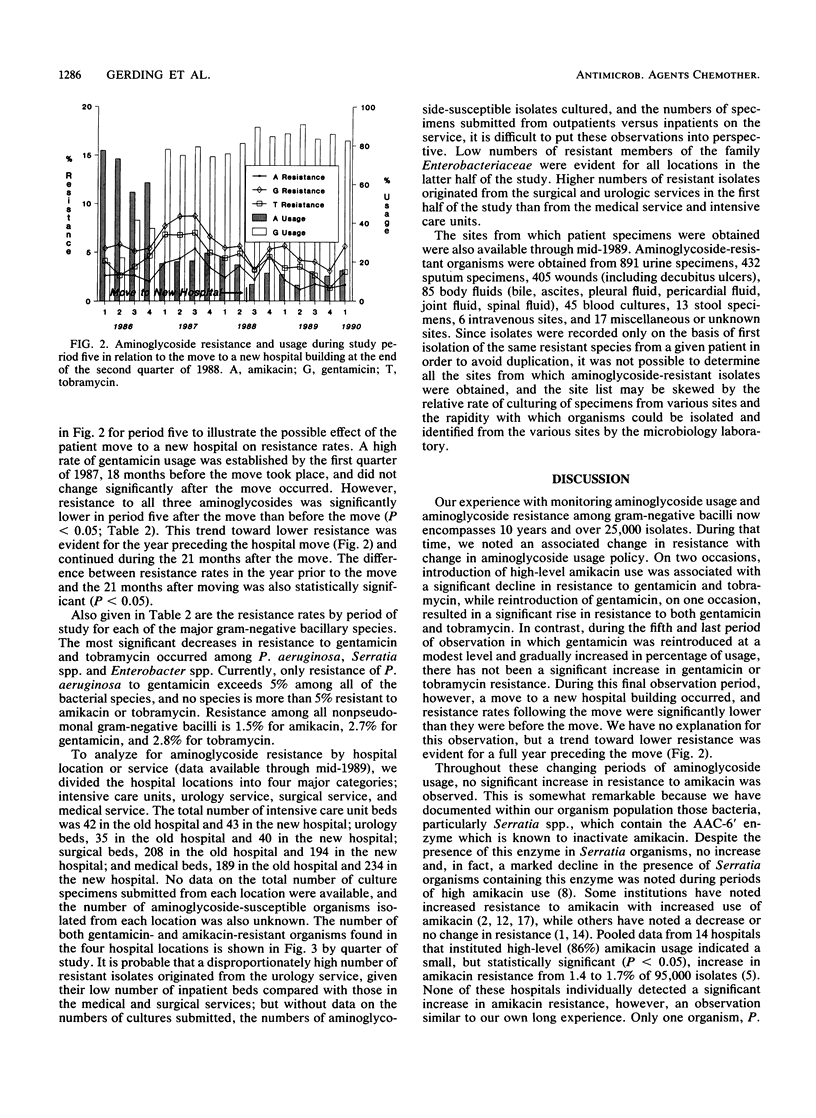
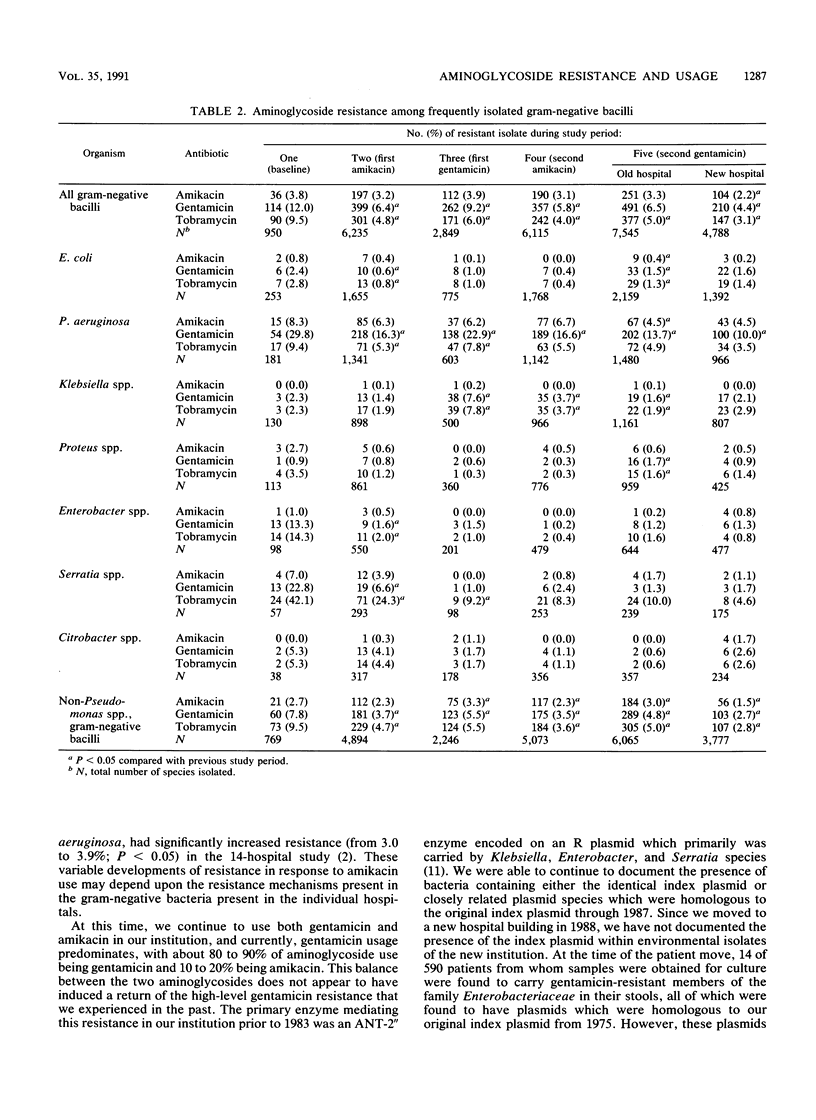
For 10 years the 700-bed Minneapolis Veterans Affairs Medical Center has conducted a policy of carefully controlled aminoglycoside usage and monitoring of resistance of over 25,000 aerobic and facultative gram-negative bacillary isolates to the aminoglycosides. On two occasions during the 1980s, our experience of introducing amikacin at a high level of usage was associated with a significant reduction in resistance to gentamicin and tobramycin among gram-negative bacilli. Rapid reintroduction of gentamicin usage in 1982 after the first amikacin period was associated with a significant and rapid increase in gentamicin and tobramycin resistance. However, in 1986, gentamicin was again reintroduced to this institution at an initially modest level, and the percentage of usage of gentamicin was gradually increased over a 15-month period without a significant change in resistance to gentamicin, tobramycin, or amikacin while maintaining an overall 68% gentamicin usage and 30% amikacin usage. Aminoglycoside usage (measured as patient days) rose steadily from under 2,000 patient days per quarter in 1980 and 1981 to over 3,000 days per quarter in 1985. Since 1985, usage has declined to under 2,500 patient days per quarter in 1990. This usage rise and fall occurred during a steadily declining daily patient census that was 590 in 1980 and 465 in 1989. A move to a new hospital building in June 1988 was associated with an additional significant decline in resistance to all aminoglycosides (P less than 0.05), continuing a trend that was evident for the year preceding the move. Resistance to aminoglycoside antibiotics is now at the lowest level in 10 years at this institution, with only one gram-negative organism, Pseudomonas aeruginosa, that exhibits more than 5% resistance to gentamicin and no gram-negative species that are more than 5% resistant to amikacin and tobramycin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betts R. F., Valenti W. M., Chapman S. W., Chonmaitree T., Mowrer G., Pincus P., Messner M., Robertson R. Five-year surveillance of aminoglycoside usage in a university hospital. Ann Intern Med. 1984 Feb;100(2):219–222. doi: 10.7326/0003-4819-100-2-219. [DOI] [PubMed] [Google Scholar]
- Camus D., Carlier Y., Bina J. C., Borojevic R., Prata A., Capron A. Sensitization to Schistosoma mansoni antigen in uninfected children born to infected mothers. J Infect Dis. 1976 Oct;134(4):405–408. doi: 10.1093/infdis/134.4.405. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Opal S., Kopecko D. J. Progressive increase in antibiotic resistance of gram-negative bacterial isolates. Walter Reed Hospital, 1976 to 1980: specific analysis of gentamicin, tobramycin, and amikacin resistance. Arch Intern Med. 1983 Nov;143(11):2075–2080. [PubMed] [Google Scholar]
- Gerding D. N., Buxton A. E., Hughes R. A., Cleary P. P., Arbaczawski J., Stamm W. E. Nosocomial multiply resistant Klebsiella pneumoniae: epidemiology of an outbreak of apparent index case origin. Antimicrob Agents Chemother. 1979 Apr;15(4):608–615. doi: 10.1128/aac.15.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Larson T. A. Aminoglycoside resistance in gram-negative bacilli during increased amikacin use. Comparison of experience in 14 United States hospitals with experience in the Minneapolis Veterans Administration Medical Center. Am J Med. 1985 Jul 15;79(1A):1–7. doi: 10.1016/0002-9343(85)90184-6. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, McNeill W. F. Characteristics of Serratia marcescens containing a plasmid coding for gentamicin resistance in nosocomial infections. J Infect Dis. 1981 Jun;143(6):810–817. doi: 10.1093/infdis/143.6.810. [DOI] [PubMed] [Google Scholar]
- Kauffman C. A., Ramundo N. C., Williams S. G., Dey C. R., Phair J. P., Watanakunakorn C. Surveillance of gentamicin-resistant gram-negative bacilli in a general hospital. Antimicrob Agents Chemother. 1978 Jun;13(6):918–923. doi: 10.1128/aac.13.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. A., Garrett C. R., Gerding D. N. Frequency of aminoglycoside 6'-N-acetyltransferase among Serratia species during increased use of amikacin in the hospital. Antimicrob Agents Chemother. 1986 Jul;30(1):176–178. doi: 10.1128/aac.30.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. A., Peterson L. R., Gerding D. N. Microdilution aminoglycoside susceptibility testing of Pseudomonas aeruginosa and Escherichia coli: correlation between MICs of clinical isolates and quality control organisms. J Clin Microbiol. 1985 Nov;22(5):819–821. doi: 10.1128/jcm.22.5.819-821.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Gerding D. N., Cleary P. P. Hospital distribution, persistence, and reintroduction of related gentamicin R plasmids. Antimicrob Agents Chemother. 1986 Apr;29(4):654–659. doi: 10.1128/aac.29.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Gerding D. N., Cleary P. P. Plasmid macroevolution in a nosocomial environment: demonstration of a persistent molecular polymorphism and construction of a cladistic phylogeny on the basis of restriction data. Mol Gen Genet. 1984;194(1-2):173–178. doi: 10.1007/BF00383513. [DOI] [PubMed] [Google Scholar]
- Levine J. F., Maslow M. J., Leibowitz R. E., Pollock A. A., Hanna B. A., Schaefler S., Simberkoff M. S., Rahal J. J., Jr Amikacin-resistant gram-negative bacilli: correlation of occurrence with amikacin use. J Infect Dis. 1985 Feb;151(2):295–300. doi: 10.1093/infdis/151.2.295. [DOI] [PubMed] [Google Scholar]
- Moody M. M., de Jongh C. A., Schimpff S. C., Tillman G. L. Long-term amikacin use. Effects on aminoglycoside susceptibility patterns of gram-negative bacilli. JAMA. 1982 Sep 10;248(10):1199–1202. doi: 10.1001/jama.248.10.1199. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Ross D. G., Guzman M. A., Medeiros A. A., Hedges R. W., Botstein D. Dissemination of an antibiotic resistance plasmid in hospital patient flora. Antimicrob Agents Chemother. 1980 Apr;17(4):537–543. doi: 10.1128/aac.17.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra S., Vera D., Ramírez-Ronda C. H. Susceptibility of aerobic gram-negative bacilli to aminoglycosides. Effects of 45 months of amikacin as first-line aminoglycoside therapy. Am J Med. 1986 Jun 30;80(6B):65–70. doi: 10.1016/0002-9343(86)90481-x. [DOI] [PubMed] [Google Scholar]
- Sadowski P. L., Peterson B. C., Gerding D. N., Cleary P. P. Physical characterization of ten R plasmids obtained from an outbreak of nosocomial Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 1979 Apr;15(4):616–624. doi: 10.1128/aac.15.4.616. [DOI] [PMC free article] [PubMed] [Google Scholar]



