Abstract
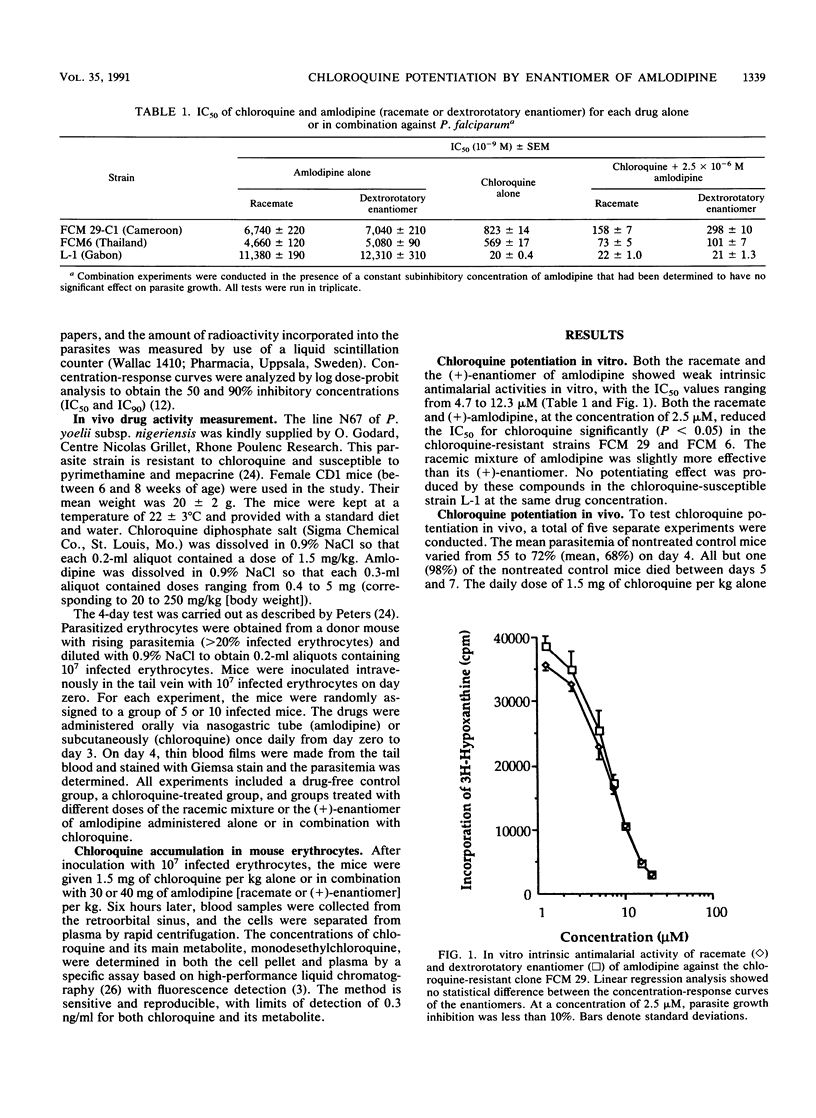
A combination of chloroquine and amlodipine, a derivative of 1,4-dihydropyridine calcium channel blocker, was tested against Plasmodium falciparum in vitro and P. yoelii in mice. The dextrorotary enantiomer of amlodipine, practically devoid of calcium channel blocking action, increased chloroquine accumulation inside the infected mouse erythrocytes and potentiated chloroquine action against the resistant strains of P. falciparum in vitro and P. yoelii in mice. Unlike the racemate, the dextrorotary amlodipine was not toxic to the host animal, even at the highest dose of 250 mg/kg. No potentiating effect was noted in the chloroquine-susceptible strains of P. falciparum. The results of this study indicate that chloroquine potentiation of amlodipine is probably independent of calcium channels and that a combination therapy of the dextrorotary enantiomer of amlodipine and chloroquine might be a potentially useful therapeutic strategy against chloroquine-resistant falciparum malaria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrowsmith J. E., Campbell S. F., Cross P. E., Stubbs J. K., Burges R. A., Gardiner D. G., Blackburn K. J. Long-acting dihydropyridine calcium antagonists. 1. 2-Alkoxymethyl derivatives incorporating basic substituents. J Med Chem. 1986 Sep;29(9):1696–1702. doi: 10.1021/jm00159a022. [DOI] [PubMed] [Google Scholar]
- Bayer R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. II. Pattern of inotropic effects of the optical isomers. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):69–80. doi: 10.1007/BF00499990. [DOI] [PubMed] [Google Scholar]
- Bergqvist Y., Frisk-Holmberg M. Sensitive method for the determination of chloroquine and its metabolite desethyl-chloroquine in human plasma and urine by high-performance liquid chromatography. J Chromatogr. 1980 Nov 14;221(1):119–127. doi: 10.1016/s0378-4347(00)81013-0. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Sjoerdsma A., McCann P. P., Kyle D. E., Oduola A. M., Rossan R. N., Milhous W. K., Davidson D. E., Jr Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science. 1988 Dec 2;242(4883):1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- Björkman A., Phillips-Howard P. A. The epidemiology of drug-resistant malaria. Trans R Soc Trop Med Hyg. 1990 Mar-Apr;84(2):177–180. doi: 10.1016/0035-9203(90)90246-b. [DOI] [PubMed] [Google Scholar]
- Echizen H., Brecht T., Niedergesäss S., Vogelgesang B., Eichelbaum M. The effect of dextro-, levo-, and racemic verapamil on atrioventricular conduction in humans. Am Heart J. 1985 Feb;109(2):210–217. doi: 10.1016/0002-8703(85)90585-x. [DOI] [PubMed] [Google Scholar]
- Fitch C. D. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science. 1970 Jul 17;169(3942):289–290. doi: 10.1126/science.169.3942.289. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Kyle D. E., Martin R. K., Oduola A. M., Forsyth K., Kemp D. J., Cowman A. F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990 May 17;345(6272):255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Geary T. G., Divo A. A., Jensen J. B. Effect of calmodulin inhibitors on viability and mitochondrial potential of Plasmodium falciparum in culture. Antimicrob Agents Chemother. 1986 Nov;30(5):785–788. doi: 10.1128/aac.30.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H. Effect of calcium antagonists on malaria susceptibility to chloroquine. Parasitol Today. 1988 Aug;4(8):209–211. doi: 10.1016/0169-4758(88)90159-7. [DOI] [PubMed] [Google Scholar]
- Gruber A., Peterson C., Reizenstein P. D-verapamil and L-verapamil are equally effective in increasing vincristine accumulation in leukemic cells in vitro. Int J Cancer. 1988 Feb 15;41(2):224–226. doi: 10.1002/ijc.2910410211. [DOI] [PubMed] [Google Scholar]
- Herwaldt B. L., Schlesinger P. H., Krogstad D. J. Accumulation of chloroquine by membrane preparations from Plasmodium falciparum. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):257–267. doi: 10.1016/0166-6851(90)90169-m. [DOI] [PubMed] [Google Scholar]
- Higgins C. Transport proteins. Export-import family expands. Nature. 1989 Aug 3;340(6232):342–342. doi: 10.1038/340342a0. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K., Schlesinger P. H. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987 Nov 27;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K. Plasmodium falciparum: modulation by calcium antagonists of resistance to chloroquine, desethylchloroquine, quinine, and quinidine in vitro. Trans R Soc Trop Med Hyg. 1990 Jul-Aug;84(4):474–478. doi: 10.1016/0035-9203(90)90004-x. [DOI] [PubMed] [Google Scholar]
- Le Bras J., Deloron P. In vitro study of drug sensitivity of Plasmodium falciparum: evaluation of a new semi-micro test. Am J Trop Med Hyg. 1983 May;32(3):447–451. doi: 10.4269/ajtmh.1983.32.447. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Macomber P. B., O'Brien R. L., Hahn F. E. Chloroquine: physiological basis of drug resistance in Plasmodium berghei. Science. 1966 Jun 3;152(3727):1374–1375. doi: 10.1126/science.152.3727.1374. [DOI] [PubMed] [Google Scholar]
- Martin S. K., Oduola A. M., Milhous W. K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987 Feb 20;235(4791):899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. Multiple-drug resistance in human cancer. N Engl J Med. 1987 May 28;316(22):1388–1393. doi: 10.1056/NEJM198705283162207. [DOI] [PubMed] [Google Scholar]
- Peters W., Ekong R., Robinson B. L., Warhurst D. C., Pan X. Q. Antihistaminic drugs that reverse chloroquine resistance in Plasmodium falciparum. Lancet. 1989 Aug 5;2(8658):334–335. doi: 10.1016/s0140-6736(89)90522-9. [DOI] [PubMed] [Google Scholar]
- Pussard E., Verdier F., Blayo M. C. Simultaneous determination of chloroquine, amodiaquine and their metabolites in human plasma, red blood cells, whole blood and urine by column liquid chromatography. J Chromatogr. 1986 Jan 10;374(1):111–118. doi: 10.1016/s0378-4347(00)83258-2. [DOI] [PubMed] [Google Scholar]
- Reynolds C. H., Claxton P. T. Inhibition of calmodulin-activated cyclic nucleotide phosphodiesterase: multiple binding-sites for tricyclic drugs on calmodulin. Biochem Pharmacol. 1982 Feb 1;31(3):419–421. doi: 10.1016/0006-2952(82)90192-7. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Colombani P. M., Hess A. D., Aikawa M., Atkinson C. T., Milhous W. K. Calcium and calmodulin antagonists inhibit human malaria parasites (Plasmodium falciparum): implications for drug design. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7310–7314. doi: 10.1073/pnas.84.20.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Kato M., Izumo A., Hagiwara A., Doi S. Plasmodium chabaudi: in vivo effects of Ca2+ antagonists on chloroquine-resistant and chloroquine-sensitive parasites. Exp Parasitol. 1990 May;70(4):419–426. doi: 10.1016/0014-4894(90)90126-w. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Verdier F., Le Bras J., Clavier F., Hatin I., Blayo M. C. Chloroquine uptake by Plasmodium falciparum-infected human erythrocytes during in vitro culture and its relationship to chloroquine resistance. Antimicrob Agents Chemother. 1985 Apr;27(4):561–564. doi: 10.1128/aac.27.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier F., Le Bras J., Clavier F., Hatin I. Blood levels and in vitro activity of desethylchloroquine against Plasmodium falciparum. Lancet. 1984 May 26;1(8387):1186–1187. doi: 10.1016/s0140-6736(84)91436-3. [DOI] [PubMed] [Google Scholar]
- Warhurst D. C. Mechanism of chloroquine resistance in malaria. Parasitol Today. 1988 Aug;4(8):211–213. doi: 10.1016/0169-4758(88)90160-3. [DOI] [PubMed] [Google Scholar]
- Watt G., Long G. W., Grogl M., Martin S. K. Reversal of drug-resistant falciparum malaria by calcium antagonists: potential for host cell toxicity. Trans R Soc Trop Med Hyg. 1990 Mar-Apr;84(2):187–190. doi: 10.1016/0035-9203(90)90248-d. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Panton L. J., Gluzman I. Y., do Rosario V. E., Gwadz R. W., Walker-Jonah A., Krogstad D. J. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990 May 17;345(6272):253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Serrano A. E., Wasley A., Bogenschutz M. P., Shankar A. H., Wirth D. F. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989 Jun 9;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- Ye Z. G., Van Dyke K. Reversal of chloroquine resistance in falciparum malaria independent of calcium channels. Biochem Biophys Res Commun. 1988 Aug 30;155(1):476–481. doi: 10.1016/s0006-291x(88)81111-2. [DOI] [PubMed] [Google Scholar]
- Ye Z. G., Van Dyke K., Spearman T., Safa A. R. 3H-azidopine photoaffinity labeling of high molecular weight proteins in chloroquine resistant falciparum malaria. Biochem Biophys Res Commun. 1989 Jul 31;162(2):809–813. doi: 10.1016/0006-291x(89)92382-6. [DOI] [PubMed] [Google Scholar]



