Abstract
Early cleavages of Xenopus embryos were oriented in strong, static magnetic fields. Third-cleavage planes, normally horizontal, were seen to orient to a vertical plane parallel with a vertical magnetic field. Second cleavages, normally vertical, could also be oriented by applying a horizontal magnetic field. We argue that these changes in cleavage-furrow geometries result from changes in the orientation of the mitotic apparatus. We hypothesize that the magnetic field acts directly on the microtubules of the mitotic apparatus. Considerations of the length of the astral microtubules, their diamagnetic anisotropy, and flexural rigidity predict the required field strength for an effect that agrees with the data. This observation provides a clear example of a static magnetic-field effect on a fundamental cellular process, cell division.
Keywords: cell division/mitotic apparatus/microtubules/Xenopus
The well defined and consistent early cleavages of the Xenopus embryo recommend it for analysis of the establishment of cleavage geometries. Shortly after fertilization, eggs orient their animal–vegetal (AV) axis parallel with gravity (see Fig. 1) and initiate cleavage, a series of synchronous cell divisions (see Fig. 2 a and b). The first three cleavages are typically orthogonal to each other. The first cleavage plane is vertical and passes through the AV axis. The second cleavage plane is also vertical, and passes through the AV axis, but at right angles to the first cleavage plane. The third cleavage plane is horizontal, parallel with and slightly above the egg equator, and perpendicular to the first two cleavage planes (1).
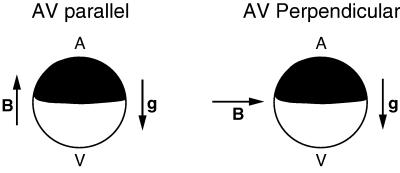
Figure 1.
Schematic diagrams of embryos in AV-parallel and AV-perpendicular magnetic fields. g is the gravity vector, and B is the magnetic-field vector.
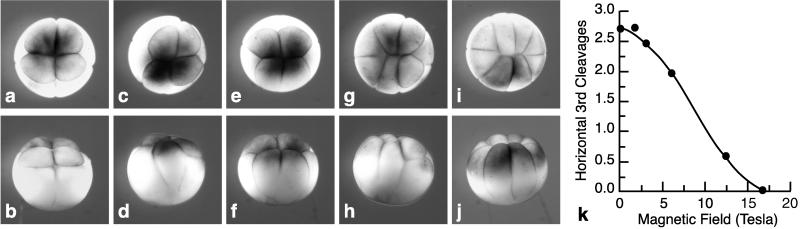
Figure 2.
Third cleavage in an AV-parallel magnetic field. Top (a, c, e, g, and i) and side (b, d, f, h, and j) views of eight-cell embryos from an AV-parallel field, showing the classes of third cleavage reorientation. For the side views, the embryo in the top view was rotated with the animal pole away from the viewer. The numbers of horizontal cleavages depicted are four (normal; a and b), three (c and d), two (e and f), one (g and h), and zero (i and j). (k) The average number of horizontal third cleavages per embryo as a function of field strength.
The shape of a dividing cell strongly influences the orientation of its mitotic apparatus (MA) and, hence, the orientation of the cell-division plane (2, 3). Hertwig (4) proposed an empirical rule that the MA aligns along the direction of “greatest protoplasmic mass” (5). In accord with Hertwig’s rule, investigators have manipulated cell shape to change MA orientation (3, 6). Evidence indicates that the MA aligns in response to forces exerted on it by the dynamic astral microtubules that extend to the cell membrane (7–11), and these forces depend on cell shape through the length dependence of the microtubule buckling force (7, 9).
Here, we show the reorientation of early cleavage planes in Xenopus, without altering cell shape or volume, by using a static magnetic field. We infer that the MA aligns perpendicularly to a strong magnetic field. We speculate that field-induced bending of astral microtubules can modify the MA-alignment process to yield the observed effects. By using recent measurements of the diamagnetic anisotropy (12) and flexural rigidity of microtubules (13), we estimate the field required to bend microtubules and find it agrees with our experimental observations.
MATERIALS AND METHODS
All experiments were performed with a two-turn Bitter solenoid at the Francis Bitter National Magnet Laboratory (MIT, Cambridge, MA). At the center of the solenoid, the field is maximal and homogenous, but, away from the center, the field varies continuously along the axis, creating a field with a field gradient. In a single experimental run, sets of eggs were placed in different positions along the axis of the solenoid, thereby subjecting them to different field strengths as well as different field/field gradients. The current through the solenoid, and hence the field itself, was regulated to ±0.3%. Magnetic fields were applied parallel or perpendicularly to the AV axis of the egg by orienting the solenoid axis parallel or perpendicularly to the direction of gravity (see Fig. 1). Eggs were held in position in a water-cooled copper sleeve with glass or plastic dishes on copper plates. Temperature was monitored with thermocouples at each sample position, periodically confirmed with an alcohol thermometer and held between 18°C and 21°C. Temperature of the discharge was also monitored. This apparatus prevented measurement of the aspect ratio of eggs in the bore. In a related experiment, however, aspect ratios of embryos in a 14-T field parallel to the AV axis were measured with an apparatus specifically designed to allow visualization of their tops and sides (14).
Ovulation and fertilization were performed as described (15). Eggs were placed into the magnetic field before 0.3 of a normalized first cell cycle and removed when control embryos had reached third cleavage (or as indicated). Half of the samples were fixed, and the remainder were left to develop to tadpole stage 34 (1). Eggs left to develop were maintained at 18°C until returned to the laboratory, where they were maintained at 16°C in 33.3 mM NaCl/0.67 mM KCl/0.67 mM CaCl2/0.33 mM MgCl2/1.67 mM Hepes, pH 7.4, and gentamicin. During development, embryos were held to a maximum of 20 embryos per dish. Samples were fixed in Bouin’s solution (16), washed in 50% ethanol/5 mM ammonium hydroxide, then brought to 100% ethanol. For each experiment, eggs were pooled from five females. Results are pooled from two separate experiments on 2 separate days.
RESULTS
Magnetic fields were first applied parallel to the AV axis (AV-parallel; Fig. 1 Right). Sets of embryos were placed in magnetic fields of strengths of 1.74, 3.07, 6.05, 12.4, and 16.7 T. At the 16.7-T position, the field was homogenous. Photographs of the sides and tops of embryos in a 14-T AV-parallel field obtained in a related experiment (14) showed that the shapes of the embryos were unaltered along any axis to within our resolution of 10%. The cleavage geometries of samples removed at the end of first and second cleavage were normal. By contrast, at the end of third cleavage, a striking alteration in the orientation of the cleavage planes was seen. Many third cleavages, normally horizontal, were vertical (i.e., parallel with the magnetic field). Moreover, the number of normal horizontal cleavages decreased with the increasing strength of the magnetic field. To quantitate this effect, we scored each individual third cleavage among the eight cells of a fixed embryo as horizontal or vertical. Fig. 2 shows top and side views of embryos with four (Fig. 2 a and b), three (Fig. 2 c and d), two (Fig. 2 e and f), one (Fig. 2 g and h), or zero (Fig. 2 i and j) horizontal third cleavages. Most oblique cleavages created a cell whose body spanned the AV tiers as in Fig. 2d and were scored as vertical. The average number of horizontal third cleavages per embryo are graphed as a function of magnetic-field strength (Fig. 2k). With increasing field strength, the average number of normal horizontal third cleavages per embryo decreases from the control-embryo value of 2.7 to 0; in short, virtually all third cleavages are vertical at 16.7 T. The percentage of embryos that developed to normal tadpoles (50%) was independent of the number of vertical third cleavages per embryo and did not vary over the range of 1.7–16.7 T. The remainder exhibited field-induced gastrulation defects (J.M.D., J.M.V., Jr., K.L., and K.L.M., unpublished work).
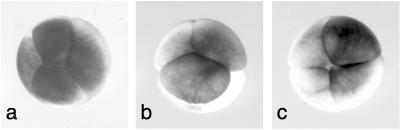
Next, the magnetic field was applied perpendicularly to the AV axis of the egg (AV-perpendicular) to determine whether the first or second cleavage could be reoriented in an analogous fashion. Eggs were positioned in a homogeneous 15-T AV-perpendicular field of a horizontal-bore solenoid. Samples were withdrawn at the end of the first, second, and third cleavage. No alteration was observed for the first-cleavage orientation. The second cleavage, however, was reoriented in a significant number of cases (17–24%). Fig. 3 illustrates the two classes of reorientation that were noted. In the first class, the second cleavages are still vertical but are not orthogonal to the first-cleavage plane. Rather, they are at oblique angles, resulting in two large and two small blastomeres (Fig. 3a). In the second class, one of the two second cleavages is reoriented to a horizontal plane, parallel to the magnetic field, resulting in a small animal-hemisphere blastomere on top of a large vegetal blastomere and a normal-looking half embryo (Fig. 3b). For comparison, a control four-cell embryo is also shown (Fig. 3c). AV-perpendicular fields did not affect third cleavages. All embryos from the AV-perpendicular field developed normally.
Figure 3.
Views of embryos from an AV-perpendicular field, showing the classes of second-cleavage reorientation. (a) The second cleavage is oblique to the first. (b) A single second cleavage is horizontal. (c) The second cleavage is normal.
DISCUSSION
Our results indicate that cleavage furrows align parallel to a strong magnetic field. This parallel alignment leads to the complete reorientation, from horizontal to vertical, of the third cleavage in an AV-parallel 16.7-T field without affecting the first and second cleavages. No effect is expected on first and second cleavage, because their planes are already oriented parallel to the applied field. By contrast, in an AV-perpendicular field, only the planes of the first and second cleavages are expected to be turned by the field, because horizontal third cleavages are already parallel to the field. The absence of reoriented first cleavages is explained easily, because the first cleavage can orient along any meridian about the AV axis (17). The finding that reoriented second cleavages occurred much less frequently than reoriented third cleavages in both the controls and in a magnetic field suggests that the cell shape at second cleavage strongly constrains the orientation of the cleavage furrow compared with cell-shape constraints at third cleavage. This conclusion agrees with Bjerknes’ calculations of MA orientation in Xenopus. He found that the third-cleavage MA was the most susceptible to reorientation (7). Our data also support this finding, as one of four third cleavages in control embryos is vertical (Fig. 2k). Quantifying the changes from the orthogonal aspect shows that there is a notable deviation from standardized descriptions of third-cleavage planes in normal Xenopus embryos (1).
It is significant that the maximum effect on the third and second cleavage furrows occurred in the homogeneous-field region and not in the inhomogeneous (gradient) field. Magnetic fields couple to diamagnetic materials, such as those in frog embryos, in two ways. A diamagnetic material in an inhomogeneous magnetic field experiences a translational force that is proportional to the field/field-gradient product and directed toward lower field. If a material has intrinsic anisotropies in the susceptibilities of its molecules, then a magnetic field, homogeneous or inhomogeneous, exerts torque on it. Therefore, the effect is related directly to field strength rather than the field/field-gradient product. That is, cleavage-furrow reorientation results from magnetic field-induced torques and not translational forces.
The target structures on which the torques act must consist of a large number of diamagnetically anisotropic molecules. Even though most biological molecules are diamagnetic and diamagnetically anisotropic, the randomizing effects of thermal energy (kT, where k is Boltzmann’s constant and T is the thermodynamic temperature) reduce the net translation or alignment of single molecules induced by magnetic fields (B) in the 1.7- to 16.7-T range (18, 19). However, the thermal energy cannot prevent the alignment of very large molecules, polymers, or groups of molecules, because their diamagnetic susceptibilities are cumulative (18–22). For example, the estimated diamagnetic anisotropy per unit length of a microtubule is χ′ = 2 × 10−23 m2 so that a 3-μm long microtubule will orient parallel to a 10-T field with an orientation energy equivalent to kT (12).
We have considered the three main structures involved in establishing cleavage-furrow orientation as potential primary magnetic-field targets: the plasma membrane, the contractile ring, and the MA. All consist of large assemblies of aligned molecules or polymers that are known to be diamagnetically anisotropic (12, 18). If the interaction of an AV-parallel magnetic field with the phospholipid bilayer (18) of the plasma membrane causes significant flattening of the embryo, vertical third cleavages may be induced. We observed no deformations of the embryos in the magnet to within 10%. By comparison, mechanical deformations of 30% or more are necessary for orienting first cleavage (6); thus, this mechanism seems unlikely. The second structure, the contractile ring, could also be turned directly by the magnetic field, because it contains aligned actin filaments (23). However, functional mitoses resulted in embryos that all had vertical third cleavages, and even embryos that did not gastrulate properly did not exhibit any evidence of cell death. Consequently, the contractile ring was not uncoupled from the MA in the field, ruling out its potential as a target.
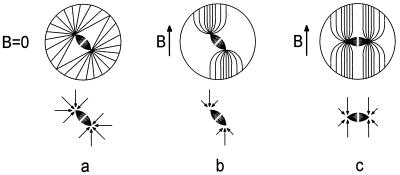
Perhaps the most obvious structure is the MA, because it is known to dictate the position of the cleavage furrow (3) and consists largely of diamagnetically anisotropic microtubules (12). It is believed that under normal conditions, the MA is driven into its final orientation by torques exerted on the spindle by astral microtubules that grow to and push against the cell membrane (3, 7–9). We speculate that an applied magnetic field changes this process in two ways. It exerts an additional, direct magnetic torque on the spindle (τBS) that, according to in vitro measurements (12), tends to align the spindle with the field. It also changes the torque exerted on the spindle by the astral microtubules (τAS) by altering the distribution and effective mechanical properties of the astral microtubules. If τAS > τBS, the spindle will align perpendicularly to the field, resulting in vertical third cleavages. A model for how the magnetic field can affect τAS is illustrated in Fig. 4. For B = 0 (Fig. 4a), the distribution of the astral microtubules is uniform, and the symmetry dictates that τAS = 0. For B ≠ 0, growing astral microtubules bend to align with the field, creating a nonuniform distribution. Moreover, the maximum force that an individual microtubule can exert is larger when it is parallel to an applied magnetic field, because the field helps to stabilize it, preventing it from buckling. The forces exerted by this nonuniform distribution on the spindle poles create a net torque that tends to turn the spindle as in Fig. 4b. Calculations (J.M.V., Jr., and W.L.J., unpublished work) with in vitro measurements of the flexural rigidity (13) and χ′ (12) suggest that such a qualitative rearrangement of the astral microtubules is reasonable. Specifically, they indicate that at 10 T, the field strength at which half of the third cleavages were vertical, microtubules as long as those in the asters of Xenopus (>200 μm) bend to align with the field. Predicting whether or not τAS > τBS requires knowing the amount of astral and spindle microtubules, because we expect τAS and τBS to be roughly proportional to these quantities, respectively. Images of first-cleavage MAs in the later stages of mitosis (24) suggest that the amount of astral microtubules can exceed the amount of spindle microtubules so that τAS > τBS is plausible.
Figure 4.
Model of the magnetic-field effect on the MA. (Upper) Representation of cells and the MA. The small fusiform shape represents the spindle. (Lower) Free-body diagrams of the spindle. The arrows represent the forces, exerted on the spindle poles by the astral microtubules. At maximum, these forces are equivalent to the force at which each of the microtubules buckle. (a) Cell in which the mitotic spindle has formed in zero magnetic field. The sum of the torques on the spindle caused by the astral microtubules (τAS) is zero for the spindle in the center of the cell as shown. (b) In a large magnetic field, the shapes of the asters change as the microtubules bend to align with the magnetic-field vector B. This bending changes the distribution of forces acting on the spindle poles and makes τAS such that the spindle tends to be turned perpendicularly to the applied magnetic-field vector (B). If τAS exceeds the torque exerted directly on the spindle by B then the spindle will tend to rotate into the configuration shown in c, which will be stable.
The effect that cleavage planes align parallel with a strong magnetic field must result from the interaction between the magnetic field and a diamagnetically anisotropic structure(s). At present, we consider the MA the most likely candidate, but we cannot rule out other targets or mechanisms.
Acknowledgments
We thank Dr. S. Inoué and the referees for helpful comments and insights. We gratefully acknowledge the expert assistance of the personnel at the Francis Bitter National Magnet Laboratory of MIT. This work was supported in part by a Brown University Salomon Faculty Research Award.
ABBREVIATIONS
- AV
animal–vegetal
- MA
mitotic apparatus
References
- 1.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North–Holland; 1967. [Google Scholar]
- 2.Rappaport R. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- 3.Rappaport R. Cytokinesis in Animal Cells. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 4.Hertwig O. Arch Mikrosk Anat. 1893;42:662–807. [Google Scholar]
- 5.Wilson E B. The Cell in Development and Heredity. New York: Macmillan; 1925. [Google Scholar]
- 6.Black S D, Vincent J-P. Dev Biol. 1988;128:65–71. doi: 10.1016/0012-1606(88)90267-9. [DOI] [PubMed] [Google Scholar]
- 7.Bjerknes M. Science. 1986;234:1413–1416. doi: 10.1126/science.3787253. [DOI] [PubMed] [Google Scholar]
- 8.Inoué S. Ann N Y Acad Sci. 1990;582:1–14. doi: 10.1111/j.1749-6632.1990.tb21662.x. [DOI] [PubMed] [Google Scholar]
- 9.Holy T E, Dogterom M, Yurke B, Leibler S. Proc Natl Acad Sci USA. 1997;94:6228–6231. doi: 10.1073/pnas.94.12.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gard D L, Cha B J, Schroeder M. Curr Top Dev Biol. 1995;31:383–431. doi: 10.1016/s0070-2153(08)60234-3. [DOI] [PubMed] [Google Scholar]
- 11.White J, Strome S. Cell. 1996;84:195–198. doi: 10.1016/s0092-8674(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 12.Bras W, Diakun G P, Diaz J F, Maret G, Kramer H, Bordas J, Medrano F J. Biophys J. 1998;74:1509–1521. doi: 10.1016/S0006-3495(98)77863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogterom M, Yurke B. Science. 1997;278:856–860. doi: 10.1126/science.278.5339.856. [DOI] [PubMed] [Google Scholar]
- 14.Valles J M, Jr, Lin K, Denegre J M, Mowry K L. Biophys J. 1997;73:1130–1133. doi: 10.1016/S0006-3495(97)78145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denegre J M, Ludwig E, Mowry K L. Dev Biol. 1997;192:446–454. doi: 10.1006/dbio.1997.8773. [DOI] [PubMed] [Google Scholar]
- 16.Humason G L. Animal Tissue Techniques. San Francisco: Freeman; 1979. [Google Scholar]
- 17.Danilchik M V, Black S D. Dev Biol. 1988;128:58–64. doi: 10.1016/0012-1606(88)90266-7. [DOI] [PubMed] [Google Scholar]
- 18.Maret G, Dransfield K. In: Strong and Ultrastrong Magnetic Fields and Their Applications, Topics in Applied Physics. Herlach F, editor. Vol. 57. New York: Springer; 1985. pp. 143–204. [Google Scholar]
- 19.Chabre M. In: Biophysical Effects of Steady Magnetic Fields, Springer Proceedings in Physics. Maret G, Kiepenheur J, Boccara N, editors. Vol. 11. New York: Springer; 1986. pp. 28–33. [Google Scholar]
- 20.Hong F T, Mauzerall D, Mauro A. Proc Natl Acad Sci USA. 1971;68:1283–1285. doi: 10.1073/pnas.68.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torbet J, Freyssinet J, Hudry-Clergeon G. Nature (London) 1981;289:91–93. doi: 10.1038/289091a0. [DOI] [PubMed] [Google Scholar]
- 22.Torbet J. In: Biophysical Effects of Steady Magnetic Fields, Springer Proceedings in Physics. Maret G, Kiepenheuer J, Boccara N, editors. Vol. 11. New York: Springer; 1986. pp. 23–27. [Google Scholar]
- 23.Bras W. Ph.D. thesis. Liverpool, U.K.: Liverpool John Moores University; 1995. p. 164. [Google Scholar]
- 24.Gard D L. Methods Cell Biol. 1993;38:241–264. doi: 10.1016/s0091-679x(08)61006-7. [DOI] [PubMed] [Google Scholar]