Abstract
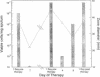
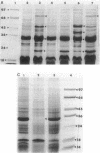
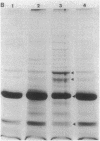
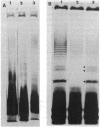
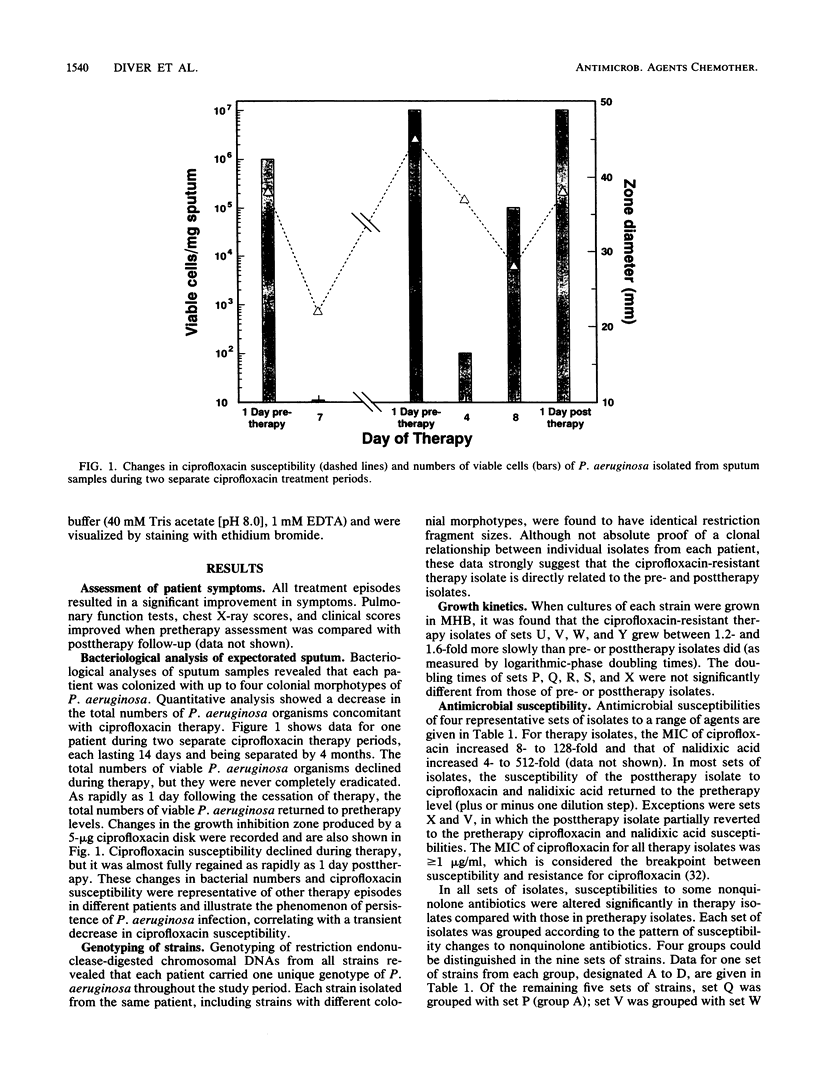
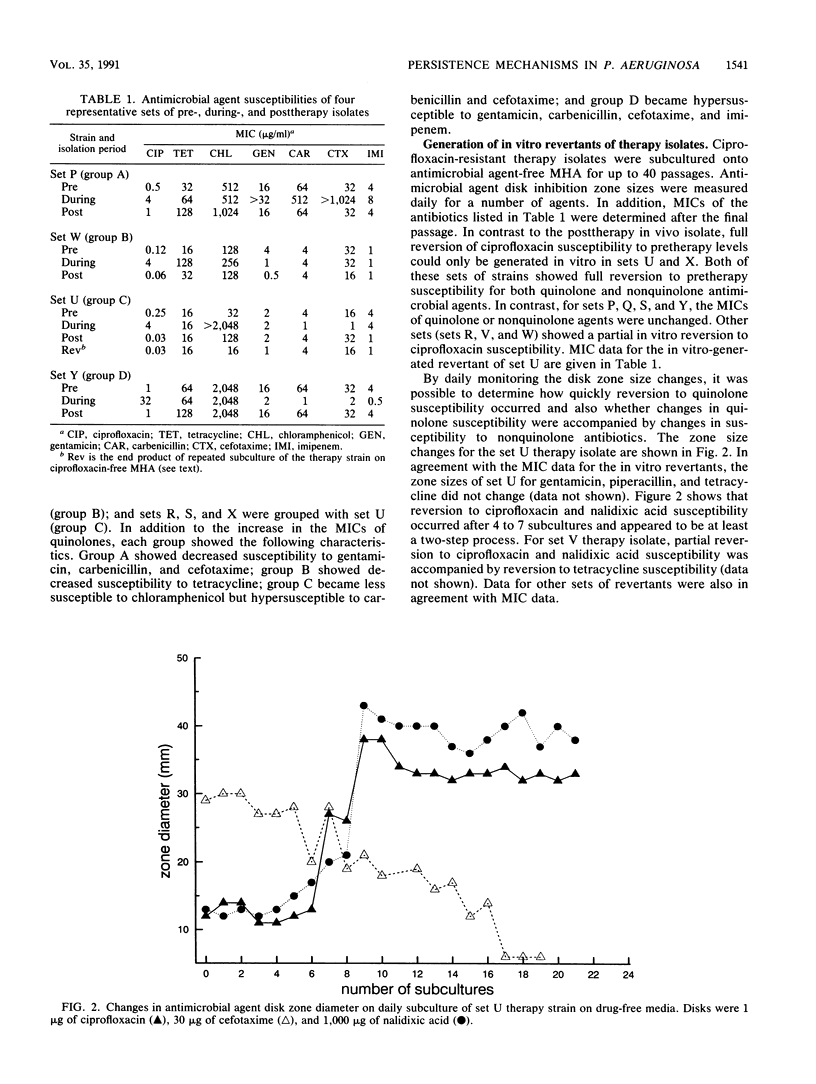
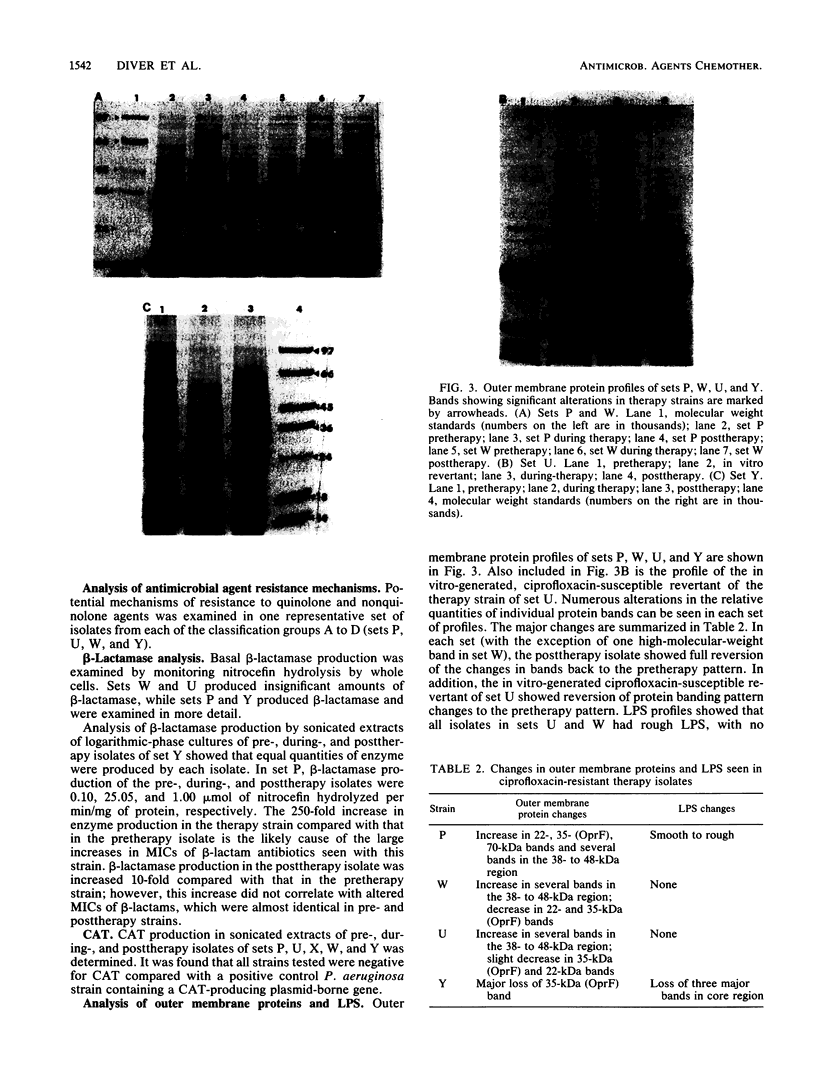
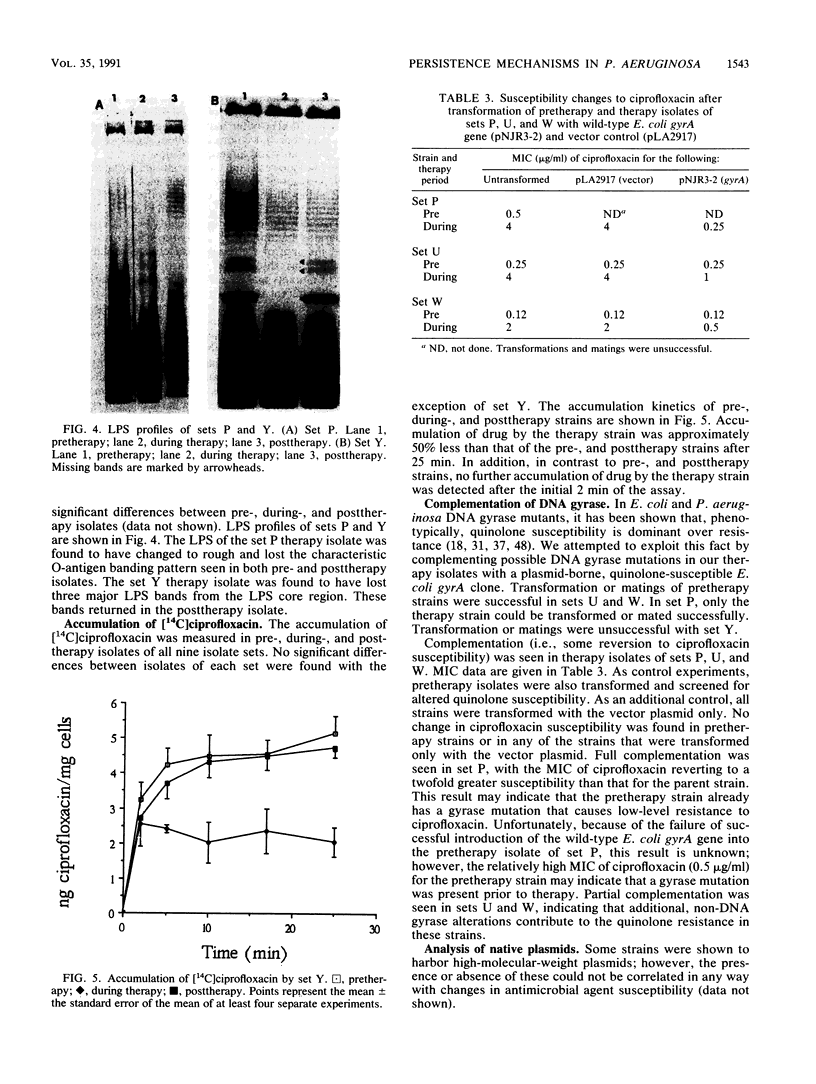
The mechanisms of persistence to ciprofloxacin in nine sets of Pseudomonas aeruginosa strains isolated during ciprofloxacin therapy of chronic lung infections in cystic fibrosis patients were studied. Low to moderate levels of ciprofloxacin resistance developed in each case. Each set of pretherapy ciprofloxacin-susceptible, during-therapy ciprofloxacin-resistant, and posttherapy ciprofloxacin-susceptible isolates were shown to be genotypically related by using a radiolabeled epidemiological gene probe. All ciprofloxacin-resistant isolates were found to have altered susceptibilities to both nalidixic acid and various chemically unrelated antibiotics. Analysis of possible resistance mechanisms showed that the strains had altered outer membrane protein or lipopolysaccharide profiles. Complementation of possible DNA gyrase mutations with a plasmid-borne, wild-type Escherichia coli gyrA gene indicated that altered DNA gyrase was at least partly responsible for ciprofloxacin resistance in all strains tested. Attempts to generate ciprofloxacin-susceptible revertants in vitro showed that in some strains reversion was rapid in the absence of ciprofloxacin, while in other strains it was not possible to generate revertants. These data indicate that persistence of Pseudomonas aeruginosa to ciprofloxacin involves changes in DNA gyrase and is associated with pleiotropic changes in outer membrane proteins and lipopolysaccharide.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosso J. A., Black P. G., Matsen J. M. Ciprofloxacin versus tobramycin plus azlocillin in pulmonary exacerbations in adult patients with cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):180–184. [PubMed] [Google Scholar]
- Brasfield D., Hicks G., Soong S., Tiller R. E. The chest roentgenogram in cystic fibrosis: a new scoring system. Pediatrics. 1979 Jan;63(1):24–29. [PubMed] [Google Scholar]
- Bryan L. E. Two forms of antimicrobial resistance: bacterial persistence and positive function resistance. J Antimicrob Chemother. 1989 Jun;23(6):817–820. doi: 10.1093/jac/23.6.817. [DOI] [PubMed] [Google Scholar]
- Chamberland S., Malouin F., Rabin H. R., Schollaardt T., Parr T. R., Jr, Bryan L. E. Persistence of Pseudomonas aeruginosa during ciprofloxacin therapy of a cystic fibrosis patient: transient resistance to quinolones and protein F-deficiency. J Antimicrob Chemother. 1990 Jun;25(6):995–1010. doi: 10.1093/jac/25.6.995. [DOI] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. Alterations in outer membrane proteins of Pseudomonas aeruginosa associated with selective resistance to quinolones. Antimicrob Agents Chemother. 1988 May;32(5):785–787. doi: 10.1128/aac.32.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrandchamps D., Munzinger J. Increasing rates of in vitro resistance to ciprofloxacin and norfloxacin in isolates from urine specimens. Antimicrob Agents Chemother. 1989 Apr;33(4):595–596. doi: 10.1128/aac.33.4.595-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver J. M., Bryan L. E., Sokol P. A. Transformation of Pseudomonas aeruginosa by electroporation. Anal Biochem. 1990 Aug 15;189(1):75–79. doi: 10.1016/0003-2697(90)90046-c. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. J., Harvey L., Hixon D. L., Poretz D. M. Ciprofloxacin therapy of infections caused by Pseudomonas aeruginosa and other resistant bacteria. Antimicrob Agents Chemother. 1985 Aug;28(2):308–310. doi: 10.1128/aac.28.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follath F., Bindschedler M., Wenk M., Frei R., Stalder H., Reber H. Use of ciprofloxacin in the treatment of Pseudomonas aeruginosa infections. Eur J Clin Microbiol. 1986 Apr;5(2):236–240. doi: 10.1007/BF02013997. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E. Penetration of beta-lactams through Pseudomonas aeruginosa porin channels. Antimicrob Agents Chemother. 1987 Aug;31(8):1216–1221. doi: 10.1128/aac.31.8.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood K., To M., Rabin H. R., Woods D. E. Subinhibitory antibiotics reduce Pseudomonas aeruginosa tissue injury in the rat lung model. J Antimicrob Chemother. 1989 Dec;24(6):937–945. doi: 10.1093/jac/24.6.937. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Siehnel R., Martin N. Outer membrane proteins of Pseudomonas. Mol Microbiol. 1990 Jul;4(7):1069–1075. doi: 10.1111/j.1365-2958.1990.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Jensen T., Pedersen S. S., Nielsen C. H., Høiby N., Koch C. The efficacy and safety of ciprofloxacin and ofloxacin in chronic Pseudomonas aeruginosa infection in cystic fibrosis. J Antimicrob Chemother. 1987 Oct;20(4):585–594. doi: 10.1093/jac/20.4.585. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M. Mechanism of ciprofloxacin resistance in Pseudomonas aeruginosa. J Infect Dis. 1988 Sep;158(3):537–541. doi: 10.1093/infdis/158.3.537. [DOI] [PubMed] [Google Scholar]
- Kresken M., Wiedemann B. Development of resistance to nalidixic acid and the fluoroquinolones after the introduction of norfloxacin and ofloxacin. Antimicrob Agents Chemother. 1988 Aug;32(8):1285–1288. doi: 10.1128/aac.32.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Legakis N. J., Tzouvelekis L. S., Makris A., Kotsifaki H. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob Agents Chemother. 1989 Jan;33(1):124–127. doi: 10.1128/aac.33.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masecar B. L., Celesk R. A., Robillard N. J. Analysis of acquired ciprofloxacin resistance in a clinical strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Feb;34(2):281–286. doi: 10.1128/aac.34.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Nakamura M., Kojima T., Yoshida H. gyrA and gyrB mutations in quinolone-resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):254–255. doi: 10.1128/aac.33.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle J. W., Janda J. M., Woods D. E., Vasil M. L. Characterization and use of a DNA probe as an epidemiological marker for Pseudomonas aeruginosa. J Infect Dis. 1987 Jan;155(1):119–126. doi: 10.1093/infdis/155.1.119. [DOI] [PubMed] [Google Scholar]
- Ogle J. W., Reller L. B., Vasil M. L. Development of resistance in Pseudomonas aeruginosa to imipenem, norfloxacin, and ciprofloxacin during therapy: proof provided by typing with a DNA probe. J Infect Dis. 1988 Apr;157(4):743–748. doi: 10.1093/infdis/157.4.743. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Wijnands W. J., Wise R. Quinolone/ureidopenicillin cross-resistance. Lancet. 1987 Oct 17;2(8564):907–907. doi: 10.1016/s0140-6736(87)91387-0. [DOI] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J. Broad-host-range gyrase A gene probe. Antimicrob Agents Chemother. 1990 Oct;34(10):1889–1894. doi: 10.1128/aac.34.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SHWACHMAN H., KULCZYCKI L. L. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child. 1958 Jul;96(1):6–15. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen H. J., Scott E. M., Stevenson M. I., Black A. E., Redmond A. O., Collier P. S. Clinical and pharmacokinetic aspects of ciprofloxacin in the treatment of acute exacerbations of pseudomonas infection in cystic fibrosis patients. J Antimicrob Chemother. 1989 Nov;24(5):787–795. doi: 10.1093/jac/24.5.787. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg S. L., Wang J. C. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987 Oct 20;197(4):729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- Taussig L. M., Kattwinkel J., Friedewald W. T., Di Sant'Agnese P. A. A new prognostic score and clinical evaluation system for cystic fibrosis. J Pediatr. 1973 Mar;82(3):380–390. doi: 10.1016/s0022-3476(73)80110-6. [DOI] [PubMed] [Google Scholar]
- Yamano Y., Nishikawa T., Komatsu Y. Outer membrane proteins responsible for the penetration of beta-lactams and quinolones in Pseudomonas aeruginosa. J Antimicrob Chemother. 1990 Aug;26(2):175–184. doi: 10.1093/jac/26.2.175. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Nakamura M., Bogaki M., Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jun;34(6):1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E., Nakae T. Identification of porins in the outer membrane of Pseudomonas aeruginosa that form small diffusion pores. J Biol Chem. 1989 Apr 15;264(11):6297–6301. [PubMed] [Google Scholar]