Abstract
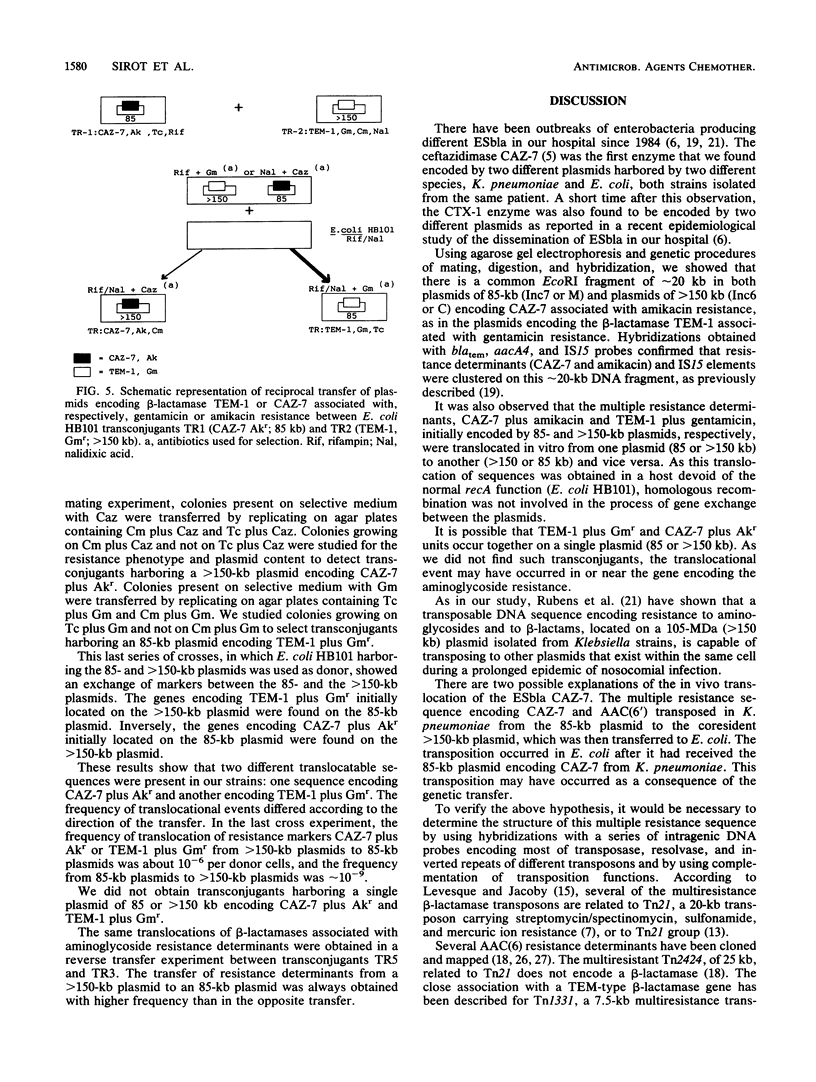
The extended-spectrum beta-lactamase CAZ-7, derived from TEMs, was produced by two different strains of the family Enterobacteriaceae, Klebsiella pneumoniae and Escherichia coli, isolated from the same patient. Both isolates were resistant to amikacin. In addition, the K. pneumoniae strain was TEM-1 producing and resistant to gentamicin. An E. coli HB101 transconjugant obtained from K. pneumoniae, selected on ceftazidime, showed that CAZ-7 and amikacin resistance were encoded by an 85-kb Inc7 or M plasmid, while an E. coli HB101 transconjugant obtained from E. coli under the same conditions showed that CAZ-7 and amikacin resistance were encoded by a greater than 150-kb Inc6 or C plasmid. Two other E. coli HB101 transconjugants obtained from K. pneumoniae, selected on gentamicin or chloramphenicol, showed that TEM-1 and gentamicin resistance could be encoded either by a greater than 150-kb Inc6 or C plasmid or by an 85-kb Inc7 or M plasmid. It was hypothesized that the genes for beta-lactam and aminoglycoside resistances were located on translocatable sequences. EcoRI digestion and hybridizations obtained with blatem, aacA4, and IS15 probes demonstrated that the CAZ-7 gene, amikacin resistance gene, and IS15 element were clustered on an approximately 20-kb fragment common to 85- and greater than 150-kb plasmids. E. coli HB101 transconjugants from K. pneumoniae and E. coli isolates were used to obtain translocations of CAZ-7 and amikacin resistance and of TEM-1 and gentamicin resistance between the 85- and greater than 150-kb plasmids. This study shows a typical example of in vivo gene dissemination involving transposable elements which translocate multiresistance genes, including an extended-spectrum beta-lactamase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanal C. M., Sirot D. L., Labia R., Petit A., Morand A., Sirot J. L., Cluzel R. A. Comparative study of a novel plasmid-mediated beta-lactamase, CAZ-2, and the CTX-1 and CAZ-1 enzymes conferring resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1988 Nov;32(11):1660–1665. doi: 10.1128/aac.32.11.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanal C. M., Sirot D. L., Petit A., Labia R., Morand A., Sirot J. L., Cluzel R. A. Multiplicity of TEM-derived beta-lactamases from Klebsiella pneumoniae strains isolated at the same hospital and relationships between the responsible plasmids. Antimicrob Agents Chemother. 1989 Nov;33(11):1915–1920. doi: 10.1128/aac.33.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L. Properties of plasmids responsible for production of extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Jan;35(1):164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Labia R., Andrillon J., Le Goffic F. Computerized microacidimetric determination of beta lactamase Michaelis-Menten constants. FEBS Lett. 1973 Jun 15;33(1):42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Courvalin P. IS15, a new insertion sequence widely spread in R plasmids of gram-negative bacteria. Mol Gen Genet. 1983;189(1):102–112. doi: 10.1007/BF00326061. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Witchitz J., Courvalin P. Modular evolution of disseminated Inc 7-M plasmids encoding gentamicin resistance. Plasmid. 1982 Nov;8(3):215–231. doi: 10.1016/0147-619x(82)90060-9. [DOI] [PubMed] [Google Scholar]
- Lafond M., Couture F., Vézina G., Levesque R. C. Evolutionary perspectives on multiresistance beta-lactamase transposons. J Bacteriol. 1989 Dec;171(12):6423–6429. doi: 10.1128/jb.171.12.6423-6429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage D. D., Gerbaud G. R., Chabbert Y. A. Carte génétique et strucutre chez Escherichia coli K12 d'un plasmide de résistance isolé de Salmonella ordonez. Ann Microbiol (Paris) 1975 May-Jun;126A(4):435–448. [PubMed] [Google Scholar]
- Levesque R. C., Jacoby G. A. Molecular structure and interrelationships of multiresistance beta-lactamase transposons. Plasmid. 1988 Jan;19(1):21–29. doi: 10.1016/0147-619x(88)90059-5. [DOI] [PubMed] [Google Scholar]
- Levesque R. C., Medeiros A. A., Jacoby G. A. Molecular cloning and DNA homology of plasmid-mediated beta-lactamase genes. Mol Gen Genet. 1987 Feb;206(2):252–258. doi: 10.1007/BF00333581. [DOI] [PubMed] [Google Scholar]
- Meyer J. F., Nies B. A., Wiedemann B. Amikacin resistance mediated by multiresistance transposon Tn2424. J Bacteriol. 1983 Aug;155(2):755–760. doi: 10.1128/jb.155.2.755-760.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A., Gerbaud G., Sirot D., Courvalin P., Sirot J. Molecular epidemiology of TEM-3 (CTX-1) beta-lactamase. Antimicrob Agents Chemother. 1990 Feb;34(2):219–224. doi: 10.1128/aac.34.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A., Sirot D. L., Chanal C. M., Sirot J. L., Labia R., Gerbaud G., Cluzel R. A. Novel plasmid-mediated beta-lactamase in clinical isolates of Klebsiella pneumoniae more resistant to ceftazidime than to other broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1988 May;32(5):626–630. doi: 10.1128/aac.32.5.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Farrar W. E., Jr, McGee Z. A., Schaffner W. Evolution of a plasmid mediating resistance to multiple antimicrobial agents during a prolonged epidemic of nosocomial infections. J Infect Dis. 1981 Feb;143(2):170–181. doi: 10.1093/infdis/143.2.170. [DOI] [PubMed] [Google Scholar]
- Sirot D., Chanal C., Labia R., Meyran M., Sirot J., Cluzel R. Comparative study of five plasmid-mediated ceftazidimases isolated in Klebsiella pneumoniae. J Antimicrob Chemother. 1989 Oct;24(4):509–521. doi: 10.1093/jac/24.4.509. [DOI] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Sirot J., Chanal C., Petit A., Sirot D., Labia R., Gerbaud G. Klebsiella pneumoniae and other Enterobacteriaceae producing novel plasmid-mediated beta-lactamases markedly active against third-generation cephalosporins: epidemiologic studies. Rev Infect Dis. 1988 Jul-Aug;10(4):850–859. doi: 10.1093/clinids/10.4.850. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tolmasky M. E., Crosa J. H. Tn1331, a novel multiresistance transposon encoding resistance to amikacin and ampicillin in Klebsiella pneumoniae. Antimicrob Agents Chemother. 1987 Dec;31(12):1955–1960. doi: 10.1128/aac.31.12.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran van Nhieu G., Collatz E. Primary structure of an aminoglycoside 6'-N-acetyltransferase AAC(6')-4, fused in vivo with the signal peptide of the Tn3-encoded beta-lactamase. J Bacteriol. 1987 Dec;169(12):5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Champs C., Sirot D., Chanal C., Poupart M. C., Dumas M. P., Sirot J. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991 Apr;27(4):441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Grinsted J. Genetic and molecular characterization of Tn21, a multiple resistance transposon from R100.1. J Bacteriol. 1982 Jul;151(1):222–228. doi: 10.1128/jb.151.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]