Abstract
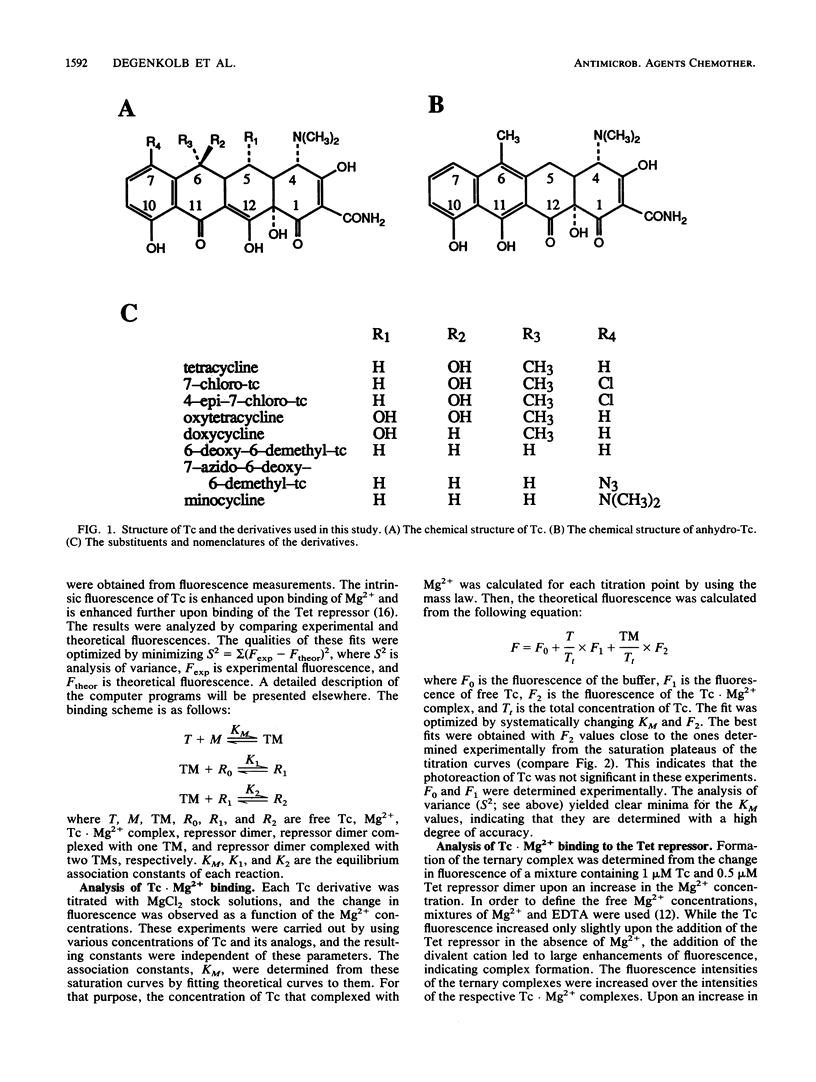
We used the Tn10-encoded Tet repressor, which has a highly specific binding capacity for tetracycline, to probe contacts between the drug and protein by chemical interference studies of the antibiotic. For that purpose, the equilibrium association constants of modified tetracyclines with the Tet repressor and Mg2+ cations were determined quantitatively. The results confirm the previous notion that Mg2+ probably binds with the oxygens at positions 11 and 12 and is absolutely required for protein-drug recognition. Modifications were introduced at positions seven, six, five, and four of the drug, and anhydrotetracycline was also studied. Substitutions or eliminations of functions at these positions influenced binding to the Tet repressor up to 35-fold. The introduction of an azido function at position seven in 7-azidotetracycline and epimerization of the substituents at position four in 4-epitetracycline lead to a 2- or 25-fold reduction, respectively, of Tet repressor affinity in those compounds. Anhydrotetracycline bound about 35-fold more strongly than tetracycline did, indicating that the oxygen at position 11 may be involved in Tet repressor recognition. This increased binding is in contrast to the lower antibiotic activity of anhydrotetracycline and indicates that the Tet repressor and ribosomes recognize the drug differently.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck C. F., Mutzel R., Barbé J., Müller W. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J Bacteriol. 1982 May;150(2):633–642. doi: 10.1128/jb.150.2.633-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Construction of a single-copy promoter vector and its use in analysis of regulation of the transposon Tn10 tetracycline resistance determinant. J Bacteriol. 1984 Jun;158(3):910–919. doi: 10.1128/jb.158.3.910-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Overlapping divergent promoters control expression of Tn10 tetracycline resistance. Gene. 1983 Aug;23(2):149–156. doi: 10.1016/0378-1119(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Hillen W., Gatz C., Altschmied L., Schollmeier K., Meier I. Control of expression of the Tn10-encoded tetracycline resistance genes. Equilibrium and kinetic investigation of the regulatory reactions. J Mol Biol. 1983 Sep 25;169(3):707–721. doi: 10.1016/s0022-2836(83)80166-1. [DOI] [PubMed] [Google Scholar]
- Hillen W., Klock G., Kaffenberger I., Wray L. V., Reznikoff W. S. Purification of the TET repressor and TET operator from the transposon Tn10 and characterization of their interaction. J Biol Chem. 1982 Jun 10;257(11):6605–6613. [PubMed] [Google Scholar]
- Hillen W., Schollmeier K., Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J Mol Biol. 1984 Jan 15;172(2):185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt C., Tovar K., Hillen W., Porschke D. Dynamics of repressor-operator recognition: the Tn10-encoded tetracycline resistance control. Biochemistry. 1988 Feb 23;27(4):1094–1104. doi: 10.1021/bi00404a003. [DOI] [PubMed] [Google Scholar]
- Oehmichen R., Klock G., Altschmied L., Hillen W. Construction of an E. coli strain overproducing the Tn10-encoded TET repressor and its use for large scale purification. EMBO J. 1984 Mar;3(3):539–543. doi: 10.1002/j.1460-2075.1984.tb01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parge H. E., Schneider M., Hahn V., Saenger W., Altschmied L., Hillen W. Crystallization of and preliminary X-ray diffraction data for TET-repressor and the TET-repressor-tetracycline complex. J Mol Biol. 1984 Dec 25;180(4):1189–1191. doi: 10.1016/0022-2836(84)90279-1. [DOI] [PubMed] [Google Scholar]
- Postle K., Nguyen T. T., Bertrand K. P. Nucleotide sequence of the repressor gene of the TN10 tetracycline resistance determinant. Nucleic Acids Res. 1984 Jun 25;12(12):4849–4863. doi: 10.1093/nar/12.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Altschmied L., Hillen W. Kinetic and equilibrium characterization of the Tet repressor-tetracycline complex by fluorescence measurements. Evidence for divalent metal ion requirement and energy transfer. J Mol Biol. 1986 Feb 5;187(3):341–348. doi: 10.1016/0022-2836(86)90437-7. [DOI] [PubMed] [Google Scholar]
- Traub B., Beck C. F. Resistance to various tetracyclines mediated by transposon Tn10 in Escherichia coli K-12. Antimicrob Agents Chemother. 1985 May;27(5):879–881. doi: 10.1128/aac.27.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]



