Abstract
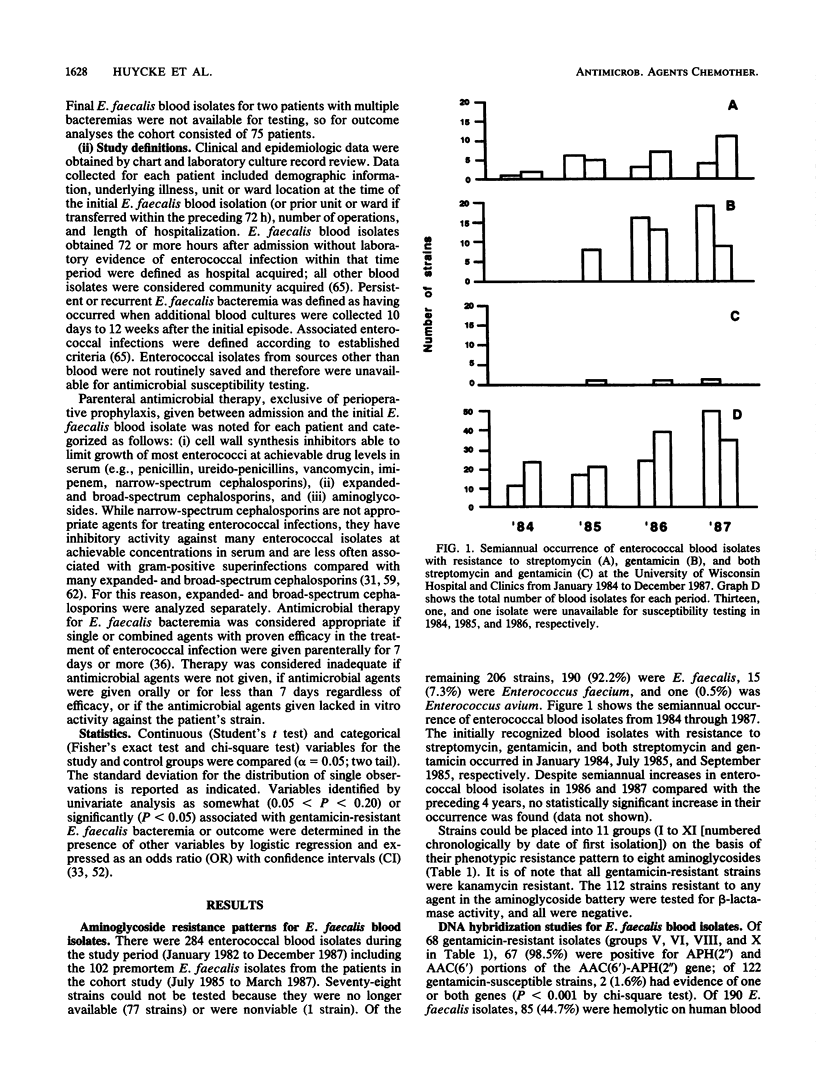
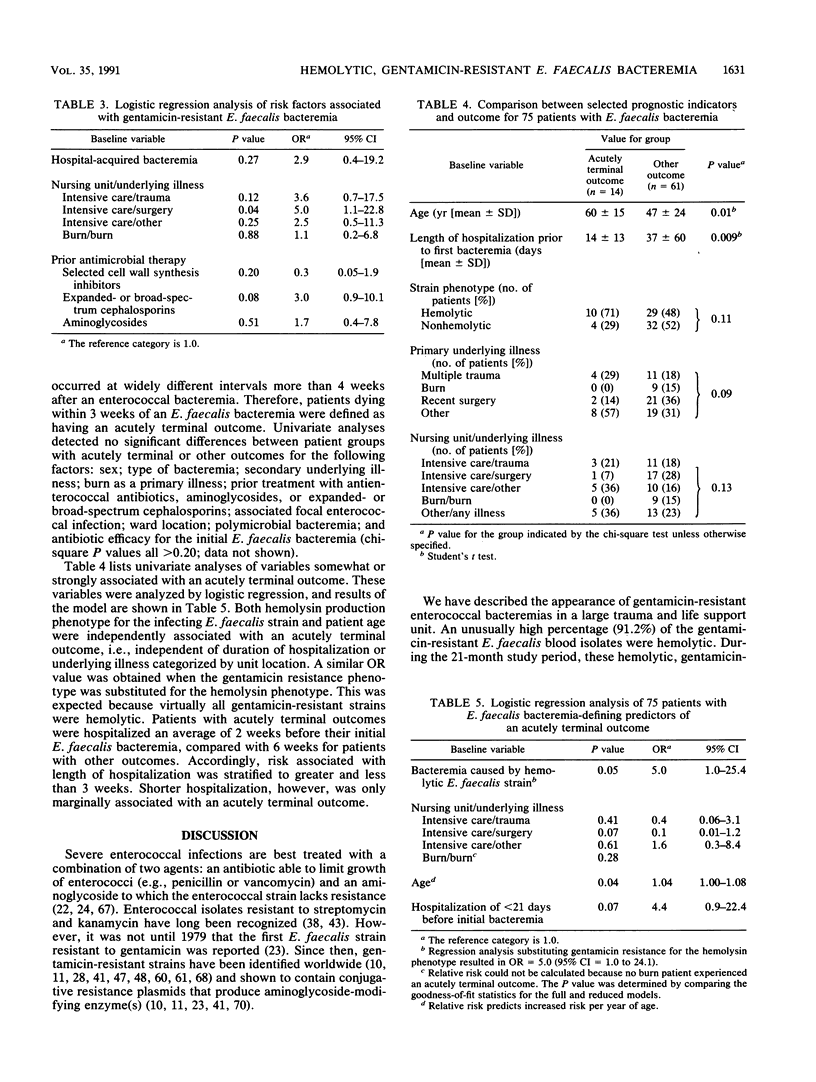
Between 1 January 1984 and 31 December 1987, 206 enterococcal blood isolates at the University of Wisconsin Hospital and Clinics were analyzed for high-level aminoglycoside resistance (hereafter high-level aminoglycoside resistance is simply referred to as "resistance") and hemolysin production. Of 190 Enterococcus faecalis isolates, 68 (35.8%) were resistant to gentamicin. Of these 68 strains, 67 (98.5%) contained a gene coding for the bifunctional aminoglycoside-modifying 6'-aminoglycoside acetyltransferase-2"-aminoglycoside phosphotransferase [AAC(6')-APH(2")] enzyme. Of 190 isolates, 85 (44.7%) were hemolytic and contained a gene coding for component A of the enterococcal hemolysin. Sixty-two of 68 (91.2%) gentamicin-resistant isolates but only 23 of 122 (18.8%) gentamicin-susceptible isolates were hemolytic (P less than 0.001). Twelve of the hemolytic, gentamicin-resistant E. faecalis blood isolates, but only 2 of 9 nonhemolytic or gentamicin-susceptible isolates, had identical chromosomal DNA restriction endonuclease digestion patterns, suggesting a common derivation for these strains. A historical cohort study from 1 July 1985 to 31 March 1987 identified by regression analysis postsurgical intensive care unit status (odds ratio [OR], 5.0; 95% confidence interval [CI], 1.1 to 22.8) and prior treatment with an expanded- or broad-spectrum cephalosporin (OR, 3.0; 95% CI, 0.9 to 10.1) as risk factors for gentamicin-resistant E. faecalis bacteremia. Patients with hemolytic, gentamicin-resistant E. faecalis bacteremia had a fivefold-increased risk for death within 3 weeks of their bacteremia compared with patients with nonhemolytic, gentamicin-susceptible strains (95% CI, 1.0 to 25.4).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Raynaud M. Purification and some properties of streptolysin O. Biochimie. 1973;55(10):1187–1193. doi: 10.1016/s0300-9084(74)80322-6. [DOI] [PubMed] [Google Scholar]
- Axelrod P., Talbot G. H. Risk factors for acquisition of gentamicin-resistant enterococci. A multivariate analysis. Arch Intern Med. 1989 Jun;149(6):1397–1401. [PubMed] [Google Scholar]
- Barrall D. T., Kenney P. R., Slotman G. J., Burchard K. W. Enterococcal bacteremia in surgical patients. Arch Surg. 1985 Jan;120(1):57–63. doi: 10.1001/archsurg.1985.01390250049008. [DOI] [PubMed] [Google Scholar]
- Basinger S. F., Jackson R. W. Bacteriocin (hemolysin) of Streptococcus zymogenes. J Bacteriol. 1968 Dec;96(6):1895–1902. doi: 10.1128/jb.96.6.1895-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brown J. J. Mortality associated with enterococcal bacteremia. Surg Gynecol Obstet. 1985 Jun;160(6):557–561. [PubMed] [Google Scholar]
- Chen H. Y., Williams J. D. Transferable resistance and aminoglycoside-modifying enzymes in enterococci. J Med Microbiol. 1985 Oct;20(2):187–196. doi: 10.1099/00222615-20-2-187. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty S. H., Flohr A. B., Simmons R. L. 'Breakthrough' enterococcal septicemia in surgical patients. 19 cases and a review of the literature. Arch Surg. 1983 Feb;118(2):232–238. doi: 10.1001/archsurg.1983.01390020076013. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison R. N., Fry D. E., Berberich S., Polk H. C., Jr Enterococcal bacteremia: clinical implications and determinants of death. Ann Surg. 1982 Jul;196(1):43–47. doi: 10.1097/00000658-198207000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatell J. M., Trilla A., Latorre X., Almela M., Mensa J., Moreno A., Miro J. M., Martinez J. A., Jimenez de Anta M. T., Soriano E. Nosocomial bacteremia in a large Spanish teaching hospital: analysis of factors influencing prognosis. Rev Infect Dis. 1988 Jan-Feb;10(1):203–210. doi: 10.1093/clinids/10.1.203. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Characterization of the A component of Streptococcus zymogenes lysin. J Bacteriol. 1971 Aug;107(2):551–556. doi: 10.1128/jb.107.2.551-556.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Purification and characterization of the L component of Streptococcus zymogenes lysin. J Bacteriol. 1971 Nov;108(2):804–808. doi: 10.1128/jb.108.2.804-808.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg R. M., Homann S. R., Phair J. P. Enterococcal bacteremia: analysis of 75 episodes. Rev Infect Dis. 1989 Jan-Feb;11(1):74–85. doi: 10.1093/clinids/11.1.74. [DOI] [PubMed] [Google Scholar]
- Hoffmann S. A., Moellering R. C., Jr The enterococcus: "putting the bug in our ears". Ann Intern Med. 1987 May;106(5):757–761. doi: 10.7326/0003-4819-106-5-757. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B., Segarra R. A., Gilmore M. S. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J Bacteriol. 1990 Jan;172(1):155–163. doi: 10.1128/jb.172.1.155-163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984 Aug;45(2):528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987 Aug;25(8):1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda D. P., Barry A. L., Andersen S. G. Emergence of Streptococcus faecalis isolates with high-level resistance to multiple aminocyclitol aminoglycosides. Diagn Microbiol Infect Dis. 1984 Jun;2(3):171–177. doi: 10.1016/0732-8893(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Jackson R. W. Bacteriolysis and inhibition of gram-positive bacteria by components of Streptococcus zymogenes lysin. J Bacteriol. 1971 Jan;105(1):156–159. doi: 10.1128/jb.105.1.156-159.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Moseley S. L., Roberts P. L., Stamm W. E. Aerobactin and other virulence factor genes among strains of Escherichia coli causing urosepsis: association with patient characteristics. Infect Immun. 1988 Feb;56(2):405–412. doi: 10.1128/iai.56.2.405-412.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N. Gram-positive superinfections following beta-lactam chemotherapy: the significance of the enterococcus. Infection. 1985;13 (Suppl 1):S81–S88. doi: 10.1007/BF01644225. [DOI] [PubMed] [Google Scholar]
- Jones W. G., Barie P. S., Yurt R. W., Goodwin C. W. Enterococcal burn sepsis. A highly lethal complication in severely burned patients. Arch Surg. 1986 Jun;121(6):649–653. doi: 10.1001/archsurg.1986.01400060043004. [DOI] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., McCabe W. R. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980 Mar;68(3):344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- Landry S. L., Kaiser D. L., Wenzel R. P. Hospital stay and mortality attributed to nosocomial enterococcal bacteremia: a controlled study. Am J Infect Control. 1989 Dec;17(6):323–329. doi: 10.1016/0196-6553(89)90001-1. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Agger W. A. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988 Jul;67(4):248–269. [PubMed] [Google Scholar]
- Malone D. A., Wagner R. A., Myers J. P., Watanakunakorn C. Enterococcal bacteremia in two large community teaching hospitals. Am J Med. 1986 Oct;81(4):601–606. doi: 10.1016/0002-9343(86)90544-9. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Kaye D., Levison M. E., Hook E. W. Enterococcal endocarditis. An analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med. 1970 Feb;125(2):258–264. doi: 10.1001/archinte.125.2.258. [DOI] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Barnes M. W., Finland M. Bacteremia at Boston City Hospital: Occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis. 1975 Sep;132(3):316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr Enterococcal infections in patients treated with moxalactam. Rev Infect Dis. 1982 Nov-Dec;4 (Suppl):S708–S711. doi: 10.1093/clinids/4.supplement_3.s708. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Medrek T., Weinberg A. N. Prevalence of high-level resistance to aminoglycosides in clinical isolates of enterococci. Antimicrob Agents Chemother (Bethesda) 1970;10:335–340. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I., Axelrod P., Talbot G. H., Fischer S. H., Wennersten C. B., Moellering R. C., Jr, MacGregor R. R. Multiply high-level-aminoglycoside-resistant enterococci isolated from patients in a university hospital. J Clin Microbiol. 1988 Jul;26(7):1287–1291. doi: 10.1128/jcm.26.7.1287-1291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimailho A., Lampl E., Riou B., Richard C., Rottman E., Auzepy P. Enterococcal bacteremia in a medical intensive care unit. Crit Care Med. 1988 Feb;16(2):126–129. doi: 10.1097/00003246-198802000-00006. [DOI] [PubMed] [Google Scholar]
- Segarra R. A., Booth M. C., Morales D. A., Huycke M. M., Gilmore M. S. Molecular characterization of the Enterococcus faecalis cytolysin activator. Infect Immun. 1991 Apr;59(4):1239–1246. doi: 10.1128/iai.59.4.1239-1246.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Levy J., Wolinsky E. Enterococcal bacteremia without endocarditis. Arch Intern Med. 1981 Apr;141(5):578–581. [PubMed] [Google Scholar]
- Spengler R. F., Greenough 3d W. B., Stolley P. D. A descriptive study of nosocomial bacteremias at The Johns Hopkins Hospital, 1968--1974. Johns Hopkins Med J. 1978 Mar;142(3):77–84. [PubMed] [Google Scholar]
- Spiegel C. A., Huycke M. Endocarditis due to streptomycin-susceptible Enterococcus faecalis with high-level gentamicin resistance. Arch Intern Med. 1989 Aug;149(8):1873–1875. [PubMed] [Google Scholar]
- Spiegel C. A. Laboratory detection of high-level aminoglycoside-aminocyclitol resistance in Enterococcus spp. J Clin Microbiol. 1988 Nov;26(11):2270–2274. doi: 10.1128/jcm.26.11.2270-2274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. L., Mitten J., Henry C. Effects of alpha and theta toxins from Clostridium perfringens on human polymorphonuclear leukocytes. J Infect Dis. 1987 Aug;156(2):324–333. doi: 10.1093/infdis/156.2.324. [DOI] [PubMed] [Google Scholar]
- Thornsberry C. Review of in vitro activity of third-generation cephalosporins and other newer beta-lactam antibiotics against clinically important bacteria. Am J Med. 1985 Aug 9;79(2A):14–20. doi: 10.1016/0002-9343(85)90255-4. [DOI] [PubMed] [Google Scholar]
- Weems J. J., Jr, Lowrance J. H., Baddour L. M., Simpson W. A. Molecular epidemiology of nosocomial, multiply aminoglycoside resistant Enterococcus faecalis. J Antimicrob Chemother. 1989 Aug;24(2):121–130. doi: 10.1093/jac/24.2.121. [DOI] [PubMed] [Google Scholar]
- Weinstein A. J., Lentnek A. L. Cephalosporin-aminoglycoside synergism in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1976 Jun;9(6):983–987. doi: 10.1128/aac.9.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Reller L. B., Murphy J. R., Lichtenstein K. A. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983 Jan-Feb;5(1):35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- Wenzel R. P., Osterman C. A., Hunting K. J., Gwaltney J. M., Jr Hospital-acquired infections. I. Surveillance in a university hospital. Am J Epidemiol. 1976 Mar;103(3):251–260. doi: 10.1093/oxfordjournals.aje.a112223. [DOI] [PubMed] [Google Scholar]
- Whiteside M., Moore J., Ratzan K. An investigation of enterococcal bacteremia. Am J Infect Control. 1983 Aug;11(4):125–129. doi: 10.1016/0196-6553(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Wilkowske C. J., Wright A. J., Sande M. A., Geraci J. E. Treatment of streptomycin-susceptible and streptomycin-resistant enterococcal endocarditis. Ann Intern Med. 1984 Jun;100(6):816–823. doi: 10.7326/0003-4819-100-6-816. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Dembinski S., Mikesell T., Schaberg D. R. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986 Jun;153(6):1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Mikesell T. S., Schaberg D. R. Heterogeneity of plasmids determining high-level resistance to gentamicin in clinical isolates of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Jul;30(1):78–81. doi: 10.1128/aac.30.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Patterson J. E., Edberg S., Pierson C., Kauffman C. A., Mikesell T. S., Schaberg D. R. Single-concentration broth microdilution test for detection of high-level aminoglycoside resistance in enterococci. J Clin Microbiol. 1987 Dec;25(12):2443–2444. doi: 10.1128/jcm.25.12.2443-2444.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Terpenning M. S., Schaberg D. R., Therasse P. M., Medendorp S. V., Kauffman C. A. High-level aminoglycoside-resistant enterococci. Colonization of nursing home and acute care hospital patients. Arch Intern Med. 1987 Sep;147(9):1591–1594. doi: 10.1001/archinte.147.9.1591. [DOI] [PubMed] [Google Scholar]