Abstract
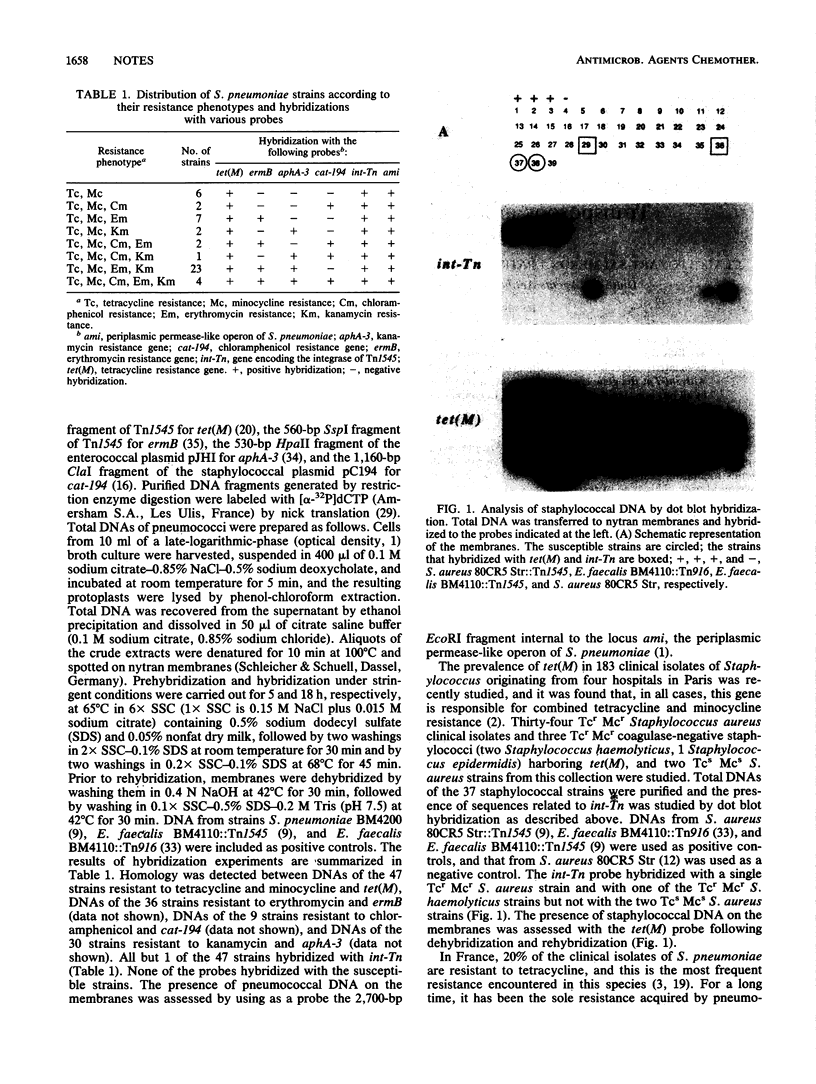
The distributions of tet(M) and conjugative transposons related to Tn1545 were studied by hybridization in 47 clinical isolates of Streptococcus pneumoniae resistant to tetracycline. Resistance to tetracycline was always associated with resistance to minocycline and was due to the presence of the tet(M) gene. Association of tet(M) with int-Tn, the gene encoding the protein required for the movements of Tn1545-like transposons, was found in all but one strain of S. pneumoniae. In contrast, int-Tn was detected in only 2 of 37 strains of Staphylococcus spp. harboring tet(M).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloing G., Trombe M. C., Claverys J. P. The ami locus of the gram-positive bacterium Streptococcus pneumoniae is similar to binding protein-dependent transport operons of gram-negative bacteria. Mol Microbiol. 1990 Apr;4(4):633–644. doi: 10.1111/j.1365-2958.1990.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Bismuth R., Zilhao R., Sakamoto H., Guesdon J. L., Courvalin P. Gene heterogeneity for tetracycline resistance in Staphylococcus spp. Antimicrob Agents Chemother. 1990 Aug;34(8):1611–1614. doi: 10.1128/aac.34.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A. Y., Goldstein F. W., Acar J. F. A seventeen-year epidemiological survey of antimicrobial resistance in pneumococci in two hospitals. J Antimicrob Chemother. 1988 Jul;22 (Suppl B):41–52. doi: 10.1093/jac/22.supplement_b.41. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï A., Horodniceanu T. Conjugative transfer of multiple antibiotic resistance markers in Streptococcus pneumoniae. J Bacteriol. 1980 Jul;143(1):313–320. doi: 10.1128/jb.143.1.313-320.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Korman R. Z., Zahler S. A., Adsit J. C., Dunny G. M. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtilis. J Bacteriol. 1987 Jun;169(6):2529–2536. doi: 10.1128/jb.169.6.2529-2536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey R. C., Swenson J. M., Clark N. C., Thornsberry C. DNA hybridization studies of a nucleotide sequence homologous to transposon Tn1545 in the "Minnesota" strain of multiresistant Streptococcus pneumoniae isolated in 1977. Diagn Microbiol Infect Dis. 1989 Jan-Feb;12(1):13–16. doi: 10.1016/0732-8893(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol Gen Genet. 1986 Nov;205(2):291–297. doi: 10.1007/BF00430441. [DOI] [PubMed] [Google Scholar]
- Doucet-Populaire F., Trieu-Cuot P., Dosbaa I., Andremont A., Courvalin P. Inducible transfer of conjugative transposon Tn1545 from Enterococcus faecalis to Listeria monocytogenes in the digestive tracts of gnotobiotic mice. Antimicrob Agents Chemother. 1991 Jan;35(1):185–187. doi: 10.1128/aac.35.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel H. W., Soedirman N., Rost J. A., van Leeuwen W. J., van Embden J. D. Transferability of macrolide, lincomycin, and streptogramin resistances between group A, B, and D streptococci, Streptococcus pneumoniae, and Staphylococcus aureus. J Bacteriol. 1980 May;142(2):407–413. doi: 10.1128/jb.142.2.407-413.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. F., Clewell D. B. A conjugative transposon (Tn919) in Streptococcus sanguis. Infect Immun. 1985 Feb;47(2):415–420. doi: 10.1128/iai.47.2.415-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hächler H., Kayser F. H., Berger-Bächi B. Homology of a transferable tetracycline resistance determinant of Clostridium difficile with Streptococcus (Enterococcus) faecalis transposon Tn916. Antimicrob Agents Chemother. 1987 Jul;31(7):1033–1038. doi: 10.1128/aac.31.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. R., Koornhof H. J., Robins-Browne R. M., Stevenson C. M., Vermaak Z. A., Freiman I., Miller G. B., Witcomb M. A., Isaäcson M., Ward J. I. Emergence of multiply resistant pneumococci. N Engl J Med. 1978 Oct 5;299(14):735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- Jenssen W. D., Thakker-Varia S., Dubin D. T., Weinstein M. P. Prevalence of macrolides-lincosamides-streptogramin B resistance and erm gene classes among clinical strains of staphylococci and streptococci. Antimicrob Agents Chemother. 1987 Jun;31(6):883–888. doi: 10.1128/aac.31.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990 Apr;3(2):171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Trieu-Cuot P., Courvalin P. Nucleotide sequence of the tetM tetracycline resistance determinant of the streptococcal conjugative shuttle transposon Tn1545. Nucleic Acids Res. 1986 Sep 11;14(17):7047–7058. doi: 10.1093/nar/14.17.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Johnson S. R., Biddle J. W., Roberts M. C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986 Nov;30(5):664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany P., Wilks M., Lamb I., Clayton C., Wren B., Tabaqchali S. Genetic analysis of a tetracycline resistance element from Clostridium difficile and its conjugal transfer to and from Bacillus subtilis. J Gen Microbiol. 1990 Jul;136(7):1343–1349. doi: 10.1099/00221287-136-7-1343. [DOI] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. Molecular characterization of two proteins involved in the excision of the conjugative transposon Tn1545: homologies with other site-specific recombinases. EMBO J. 1989 Aug;8(8):2425–2433. doi: 10.1002/j.1460-2075.1989.tb08373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. The integration-excision system of the conjugative transposon Tn 1545 is structurally and functionally related to those of lambdoid phages. Mol Microbiol. 1990 Sep;4(9):1513–1521. doi: 10.1111/j.1365-2958.1990.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Hillier S. L., Hale J., Holmes K. K., Kenny G. E. Tetracycline resistance and tetM in pathogenic urogenital bacteria. Antimicrob Agents Chemother. 1986 Nov;30(5):810–812. doi: 10.1128/aac.30.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Moncla B. J. Tetracycline resistance and TetM in oral anaerobic bacteria and Neisseria perflava-N. sicca. Antimicrob Agents Chemother. 1988 Aug;32(8):1271–1273. doi: 10.1128/aac.32.8.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C. Plasmid-mediated Tet M in Haemophilus ducreyi. Antimicrob Agents Chemother. 1989 Sep;33(9):1611–1613. doi: 10.1128/aac.33.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Shoemaker N. B., Burdett V., Guild W. R. Transfer of plasmids by conjugation in Streptococcus pneumonias. Plasmid. 1980 Jan;3(1):70–79. doi: 10.1016/s0147-619x(80)90035-9. [DOI] [PubMed] [Google Scholar]
- Storrs M. J., Poyart-Salmeron C., Trieu-Cuot P., Courvalin P. Conjugative transposition of Tn916 requires the excisive and integrative activities of the transposon-encoded integrase. J Bacteriol. 1991 Jul;173(14):4347–4352. doi: 10.1128/jb.173.14.4347-4352.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Poyart-Salmeron C., Carlier C., Courvalin P. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 1990 Jun 25;18(12):3660–3660. doi: 10.1093/nar/18.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilhao R., Papadopoulou B., Courvalin P. Occurrence of the Campylobacter resistance gene tetO in Enterococcus and Streptococcus spp. Antimicrob Agents Chemother. 1988 Dec;32(12):1793–1796. doi: 10.1128/aac.32.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]