Abstract
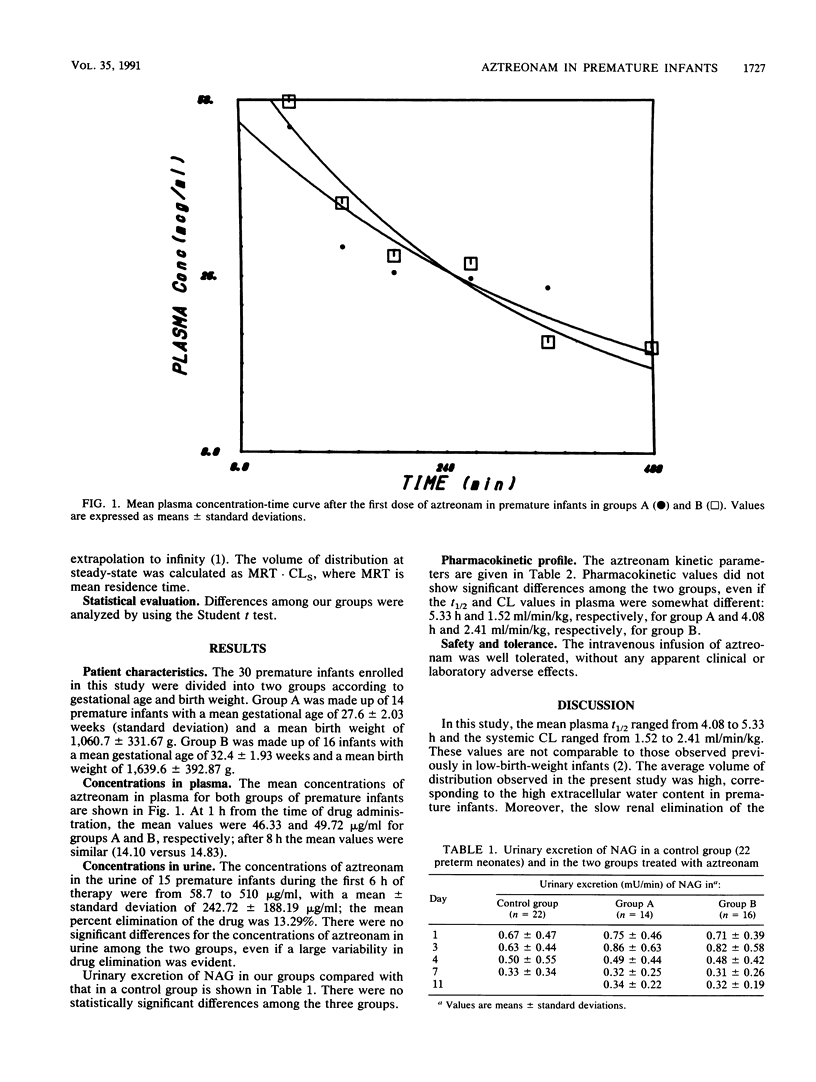
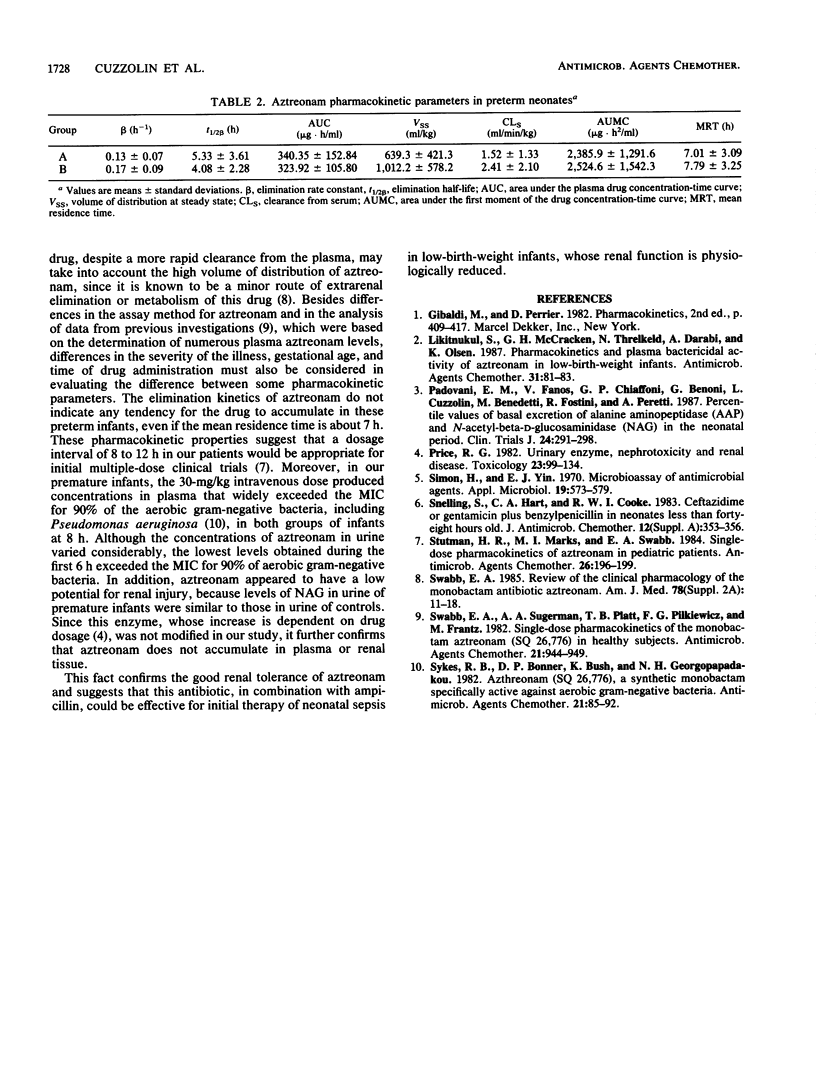
Aztreonam (30 mg/kg of body weight) was administered intravenously over 3 min every 12 h to 30 preterm neonates divided into two groups according to gestational age (mean age for group A was less than 30 weeks and mean age for group B was greater than 30 weeks) and birth weight (mean weight for group A was less than 1,500 g and mean weight for group B was greater than 1,500 g). Blood and urine samples were analyzed by microbiological assay. The pharmacokinetics were described by one-compartment and noncompartment models. The mean half-life and clearance for premature infants weighing less than 1,500 g were 5.33 +/- 3.61 h and 1.52 +/- 1.33 ml/min/kg, respectively; for those weighing more than 1,500 g, the values were 4.08 +/- 2.28 h and 2.41 +/- 2.10 ml/min/kg, respectively. The mean urinary concentration of aztreonam in 15 premature infants during the first 6 h of therapy was 242.72 +/- 188.19 micrograms/ml, with a mean percentage of elimination of 13.29%. Urinary excretion of N-acetyl-beta-D-glucosaminidase (a specific and sensitive test for the detection of drug-induced renal tubule damage) did not show significant differences in our group of premature infants compared with that in a control group. The dose of 30 mg/kg and a dosage interval of 8 to 12 h could be recommended for the treatment of suitable bacterial infections in all premature infants.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Likitnukul S., McCracken G. H., Jr, Threlkeld N., Darabi A., Olsen K. Pharmacokinetics and plasma bactericidal activity of aztreonam in low-birth-weight infants. Antimicrob Agents Chemother. 1987 Jan;31(1):81–83. doi: 10.1128/aac.31.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. G. Urinary enzymes, nephrotoxicity and renal disease. Toxicology. 1982;23(2-3):99–134. doi: 10.1016/0300-483x(82)90092-0. [DOI] [PubMed] [Google Scholar]
- Simon H. J., Yin E. J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970 Apr;19(4):573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling S., Hart C. A., Cooke R. W. Ceftazidime or gentamicin plus benzylpenicillin in neonates less than forty-eight hours old. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):353–356. doi: 10.1093/jac/12.suppl_a.353. [DOI] [PubMed] [Google Scholar]
- Stutman H. R., Marks M. I., Swabb E. A. Single-dose pharmacokinetics of aztreonam in pediatric patients. Antimicrob Agents Chemother. 1984 Aug;26(2):196–199. doi: 10.1128/aac.26.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swabb E. A. Review of the clinical pharmacology of the monobactam antibiotic aztreonam. Am J Med. 1985 Feb 8;78(2A):11–18. doi: 10.1016/0002-9343(85)90197-4. [DOI] [PubMed] [Google Scholar]
- Swabb E. A., Sugerman A. A., Platt T. B., Pilkiewicz F. G., Frantz M. Single-dose pharmacokinetics of the monobactam azthreonam (SQ 26,776) in healthy subjects. Antimicrob Agents Chemother. 1982 Jun;21(6):944–949. doi: 10.1128/aac.21.6.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]



