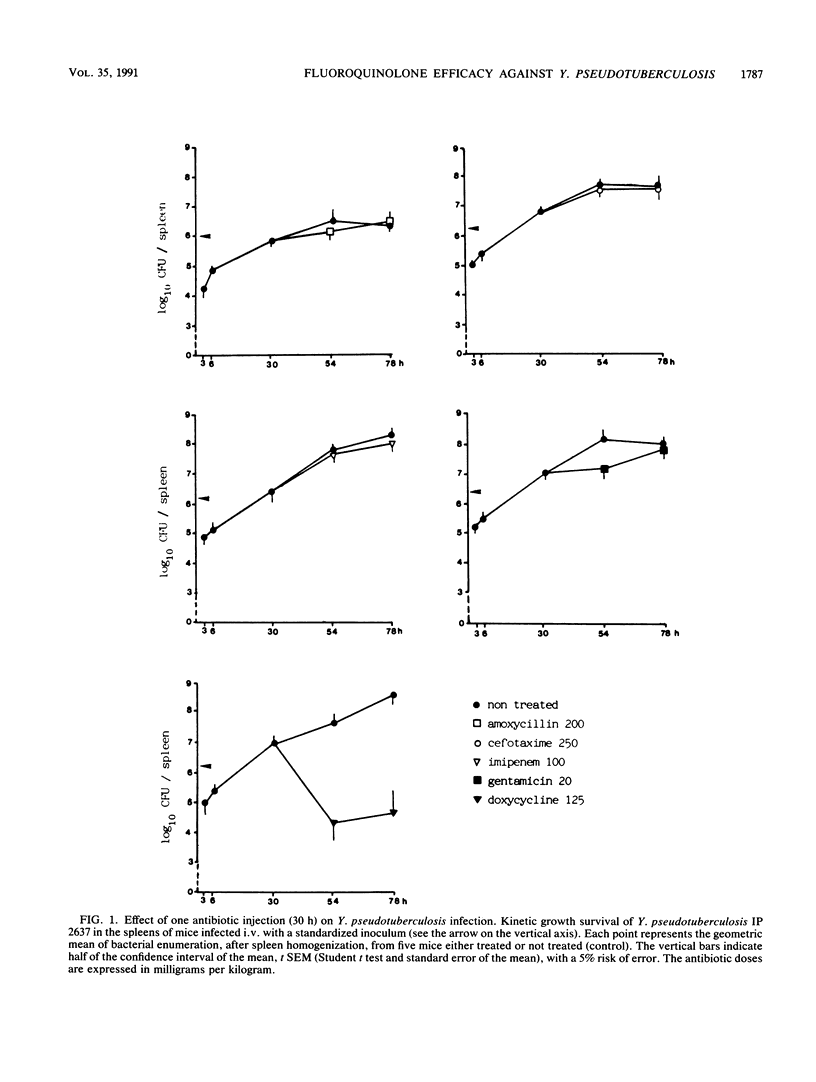
Abstract
The treatment of yersiniosis by beta-lactams is questionable considering the proven failure of newer beta-lactams for treating murine Yersinia enterocolitica infection. Another modality of experimental treatment was performed with a virulent strain of Y. pseudotuberculosis (nonproducer of beta-lactamase) highly susceptible (in terms of MICs) to amoxicillin, cefotaxime, ceftriaxone, imipenem, doxycycline, gentamicin, and ofloxacin. The in vivo comparative efficacy of these drugs was evaluated in a standardized and reproducible mouse model of systemic infection. Each single antibiotic was injected intravenously once, at 30 h after intravenous inoculation of the infective strain, and then repeatedly (at 30, 52, and 76 h postinfection). In vivo results were measured by counting the viable bacteria recovered from the whole spleens of mice sacrificed at selected times. Cefotaxime, even at high doses (250 mg/kg of body weight), was totally ineffective. Amoxicillin and imipenem at high doses (200 and 100 mg/kg, respectively) and ceftriaxone at usual doses (20 mg/kg) were active only in stopping bacterial proliferation to a more or less slight degree. Ceftriaxone was able to reduce viable counts in the spleen only at high doses (200 mg/kg), as were gentamicin (20 mg/kg) and doxycycline (125 mg/kg). Ofloxacin at the low dose of 5 mg/kg was demonstrated to be very effective by the very significant decrease observed in bacterial numbers from 10(6) to 10(3) CFU per spleen. The pharmacological parameters do not in themselves explain all the discrepancies between the in vitro and in vivo activities of beta-lactams on yersiniae. No emergence of beta-lactam-resistant organisms, which could explain the failure of beta-lactams, was detected. Thus, their use should be delayed in the therapy of human yersinosis until further investigations are carried out. The fluoroquinolone appeared more active and rapid than reference drugs in the treatment of murine yersinosis, which confirms initial clinical results.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. M., Mazigh D., Bercovier H., Mollaret H. H. Infection expérimentale de la souris par Yersinia enterocolitica (souche du chimiotype 4, du sérogroupe O:3, du lysotype VIII): devenir de l'inoculum chez des souris athymiques ou traitées par le cyclophosphamide. Ann Microbiol (Paris) 1978 Jul;129B(1):27–36. [PubMed] [Google Scholar]
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvergnat J. C., Lemozy J., Lokiec F., Pecking M. Diffusion lymphatique et ganglionnaire de la ceftriaxone. Incidence dans le traitement des fièvres typhoïdes. Pathol Biol (Paris) 1986 May;34(5):508–511. [PubMed] [Google Scholar]
- Beale A. S., Gisby J., Sutherland R. Efficacy of amoxycillin/clavulanic acid in experimental Bacteroides fragilis/Escherichia coli mixed infections. J Antimicrob Chemother. 1988 Apr;21(4):451–459. doi: 10.1093/jac/21.4.451. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- Bergeron M. G., Beauchamp D., Poirier A., Bastille A. Continuous vs. intermittent administration of antimicrobial agents: tissue penetration and efficacy in vivo. Rev Infect Dis. 1981 Jan-Feb;3(1):84–97. doi: 10.1093/clinids/3.1.84. [DOI] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Inoculum effect. Rev Infect Dis. 1989 May-Jun;11(3):361–368. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- Böcker R., Estler C. J., Maywald M., Weber D. Comparison of distribution of doxycycline in mice after oral and intravenous application measured by a high-performance liquid chromatographic method. Arzneimittelforschung. 1981;31(12):2116–2117. [PubMed] [Google Scholar]
- Easmon C. S., Crane J. P., Blowers A. Effect of ciprofloxacin on intracellular organisms: in-vitro and in-vivo studies. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):43–48. doi: 10.1093/jac/18.supplement_d.43. [DOI] [PubMed] [Google Scholar]
- English A. R., Lynch J. E. Alpha-6-deoxyoxytetracycline. II. Activity in chemotherapeutic studies in the mouse. Proc Soc Exp Biol Med. 1967 Feb;124(2):586–591. doi: 10.3181/00379727-124-31798. [DOI] [PubMed] [Google Scholar]
- Frimodt-Møller N., Bentzon M. W., Thomsen V. F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986 Sep;154(3):511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L., Steinberg T. H. Interactions of antibiotics and phagocytes. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):1–11. doi: 10.1093/jac/12.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. The entry of antibiotics into human monocytes. J Antimicrob Chemother. 1989 May;23(5):681–689. doi: 10.1093/jac/23.5.681. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Uptake of antibiotics by human polymorphonuclear leukocyte cytoplasts. Antimicrob Agents Chemother. 1990 Jun;34(6):1189–1193. doi: 10.1128/aac.34.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N., Holman J. W. Entry of roxithromycin (RU 965), imipenem, cefotaxime, trimethoprim, and metronidazole into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Oct;31(10):1553–1557. doi: 10.1128/aac.31.10.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Gross U., Schmidt N., Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect Immun. 1986 Nov;54(2):561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein M. J., Jupeau A. M., Scavizzi M. R., Philippon A. M., Grimont P. A. In vitro susceptibilities of 126 clinical isolates of Yersinia enterocolitica to 21 beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 May;27(5):806–811. doi: 10.1128/aac.27.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Kahan J. S., Kahan F. M., Birnbaum J. MK0787 (N-formimidoyl thienamycin): evaluation of in vitro and in vivo activities. Antimicrob Agents Chemother. 1980 Jun;17(6):993–1000. doi: 10.1128/aac.17.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H., Angehrn P., Havas L. Autoradiographic evidence for penetration of 3H-ceftriaxone (Rocephin) into cells of spleen, liver and kidney of mice. Chemotherapy. 1986;32(2):102–112. doi: 10.1159/000238398. [DOI] [PubMed] [Google Scholar]
- Kállay Z., Trnovec T., Kettner M., Macicková T., Navarová J., Gregusková M. Kinetics of gentamicin accumulation in subcellular structures of the mouse kidney. J Pharm Pharmacol. 1982 Apr;34(4):276–277. doi: 10.1111/j.2042-7158.1982.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Lavoie G. Y., Bergeron M. G. Influence of four modes of administration on penetration of aztreonam, cefuroxime, and ampicillin into interstitial fluid and fibrin clots and on in vivo efficacy against Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Sep;28(3):404–412. doi: 10.1128/aac.28.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S. In-vitro and in-vivo antibacterial activity of imipenem against clinical isolates of bacteria. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):53–64. doi: 10.1093/jac/12.suppl_d.53. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Bryan L. E. Relationship between gentamicin susceptiblity criteria and therapeutic serum levels for Pseudomonas aeruginosa in mouse infection model. Antimicrob Agents Chemother. 1978 May;13(5):796–801. doi: 10.1128/aac.13.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner R., Weiss U., Brombacher U., Lanz P., Montavon M., Furlenmeier A., Angehrn P., Probst P. J. Ro 13-9904/001, a novel potent and long-acting parenteral cephalosporin. J Antibiot (Tokyo) 1980 Jul;33(7):783–786. doi: 10.7164/antibiotics.33.783. [DOI] [PubMed] [Google Scholar]
- Renneberg J., Walder M. A mouse model for simultaneous pharmacokinetic and efficacy studies of antibiotics at sites of infection. J Antimicrob Chemother. 1988 Jul;22(1):51–60. doi: 10.1093/jac/22.1.51. [DOI] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952 Oct;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavizzi M. R., Alonso J. M., Philippon A. M., Jupeau-Vessieres A. M., Guiyoule A. Failure of newer beta-lactam antibiotics for murine Yersinia enterocolitica infection. Antimicrob Agents Chemother. 1987 Apr;31(4):523–526. doi: 10.1128/aac.31.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner R. K., Marks M. I., Weber A., Pai C. H. Yersinia enterocolitica: comparative in vitro activities of seven new beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):140–141. doi: 10.1128/aac.22.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesnie J. C., Fritsch P. W., Griffin T. J., Heifetz C. L., Leopold E. T., Malta T. E., Shapiro M. A., Vincent P. W. Comparative chemotherapeutic activity of new fluorinated 4-quinolones and standard agents against a variety of bacteria in a mouse infection model. J Antimicrob Chemother. 1989 May;23(5):729–736. doi: 10.1093/jac/23.5.729. [DOI] [PubMed] [Google Scholar]
- Simonet M., Mazigh D., Berche P. Growth of Yersinia pseudotuberculosis in mouse spleen despite loss of a virulence plasmid of mol. wt 47 X 10(6). J Med Microbiol. 1984 Dec;18(3):371–375. doi: 10.1099/00222615-18-3-371. [DOI] [PubMed] [Google Scholar]
- Soriano F., Ponte C., Santamaría M., Jimenez-Arriero M. Relevance of the inoculum effect of antibiotics in the outcome of experimental infections caused by Escherichia coli. J Antimicrob Chemother. 1990 Apr;25(4):621–627. doi: 10.1093/jac/25.4.621. [DOI] [PubMed] [Google Scholar]
- Soriano F., Vega J. The susceptibility of Yersinia to eleven antimicrobials. J Antimicrob Chemother. 1982 Dec;10(6):543–547. doi: 10.1093/jac/10.6.543. [DOI] [PubMed] [Google Scholar]
- Tulkens P., Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978 Feb 15;27(4):415–424. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- Une T., Osada Y. Penetrability of ofloxacin into cultured epithelial cells and macrophages. Arzneimittelforschung. 1988 Sep;38(9):1265–1267. [PubMed] [Google Scholar]
- Van der Auwera P., Matsumoto T., Husson M. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother. 1988 Aug;22(2):185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]



