Abstract
β-catenin, the vertebrate homolog of the Drosophila Armadillo protein, has been shown to have dual cellular functions, as a component of both the cadherin-catenin cell adhesion complex and the Wnt signaling pathway. At Wnt signaling, β-catenin becomes stabilized in the cytoplasm and subsequently available for interaction with transcription factors of the lymphocyte enhancer factor-1/T-cell factor family, resulting in a nuclear localization of β-catenin. Although β-catenin does not bind DNA directly, its carboxyl- and amino-terminal regions exhibit a transactivating activity still not well understood molecularly. Here we report the identification of an interaction partner of β-catenin, a nuclear protein designated Pontin52. Pontin52 binds β-catenin in the region of Armadillo repeats 2–5 and, more importantly, also binds the TATA box binding protein. We provide evidence for an in vivo multiprotein complex composed of Pontin52, β-catenin, and lymphocyte enhancer factor-1/T-cell factor. Our results suggest involvement of Pontin52 in the nuclear function of β-catenin.
Keywords: transcription regulation
β-catenin originally was identified as a central component of the E-cadherin-catenin cell–cell adhesion complex, bridging the cytoplasmic domain of E-cadherin to α-catenin and thus connecting the adhesion complex to the actin cytoskeleton (1–3). β-catenin is also a member of the Armadillo-repeat protein family and like Armadillo plays an important role in the Wnt signaling pathway (reviewed in ref. 4).
In this pathway, a Wnt signal induces, via several intermediate steps from the Wnt receptor Frizzled over the cytoplasmic proteins Dishevelled and glycogen synthase kinase 3β, an increase in the cytoplasmic pool of β-catenin by inhibiting its ubiquitin-dependent degradation (5, 6). Stabilized cytoplasmic β-catenin thereby becomes available to form a bipartite complex with transcription factors of the lymphocyte enhancer factor-1/T-cell factor (LEF-1/TCF) family and accumulates in the nucleus (7–9).
The LEF-1/TCF transcription factors first were identified in lymphoid cells, but they exhibit a more complex expression pattern during embryonic development (10–12). They are characterized by their conserved high mobility group box DNA-binding domain, with members from different species sharing over 90% amino acid similarity. Alone, LEF-1/TCF transcription factors are able to bind their DNA target sequences but possess little or no transcription activation potential (9). In contrast, β-catenin has no DNA binding capacity but contains a transactivation domain in its carboxyl-terminal region (13). Both the DNA-binding domain of LEF-1/TCF and the transactivation domain of β-catenin are required for activation of target genes of Wnt/Wg signaling such as siamois, Twin, and Xnr3 in Xenopus or ultrabithorax (Ubx) in Drosophila (14–18).
Little is known about the molecular mechanism of β-catenin-dependent transcriptional activation. In particular, no direct interaction of β-catenin with components of the basic transcriptional machinery has been described so far. Here we report the identification of an interaction partner of β-catenin, Pontin52, a nuclear protein that may play a role in the nuclear function of β-catenin.
MATERIALS AND METHODS
Cell Lines, Reagents, and Antibodies.
All cells were grown in DMEM supplemented with 10% fetal calf serum in a 10% CO2 atmosphere at 37°C. Cells were transfected by a modified calcium phosphate precipitation method with 10 μg of plasmid DNA (19), using 2× Hepes instead of 2× N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES)-buffered saline.
Antibodies against three different parts of the Pontin52 sequence were produced in rabbits. Antibody anti-23 was generated against a synthetic peptide (amino acid positions 129–140) coupled to keyhole limpet hemocyanin. Antibodies anti-24 and anti-25 were raised against two recombinant Pontin52–TrpE fusion proteins, respectively including amino acids 195–334 or 341–456 of Pontin52. Anti-23 and anti-24 antibodies were affinity purified on recombinant glutathione S-transferase (GST)-Pontin52 coupled to Minileak beads (KemEnTec, Copenhagen, Denmark). mAbs against E-cadherin, β-catenin, glycogen synthase kinase 3β, p120ctn, and TATA box binding protein (TBP) were obtained from Transduction Laboratories (Lexington, KY), mAbs against the hemagglutinin (HA)-tag were from Boehringer Mannheim, and anti-myc (9E10) mAb was kindly supplied by Jörg Stappert (Max Planck Institute of Immunobiology, Freiburg).
Constructs.
Two Pontin52 cDNA fragments were generated by PCR and ligated together to obtain the complete coding sequence for Pontin52. The first PCR product, obtained with oligonucleotides 5′-CCGGGATCCGCCATGAAGATTGAGGAGGTG-3′ and 5′-GTGCACATCCCCTTTTGG-3′, was digested with BamHI and EcoRI and cloned into the eukaryotic expression vector pCS2+. The second PCR product, generated with oligonucleotides 5′-AACAGTGGGGCCGTGAAG-3′ and 5′-CCTCGAGGATCCTCACTTCATGTACTTATC-3′, was cloned into the Smal site of pUC18. After EcoRI/XbaI digestion, the two PCR products were ligated together to generate plasmid pCS2+hp52. Digestion with BamHI generated a cDNA fragment encoding the full-length Pontin52 protein, which was cloned into pGEX4T1 (Pharmacia) for subsequent expression in Escherichia coli.
Human TBP cDNA was amplified by PCR from a HT29 cell solid-phase cDNA library with oligonucleotides 5′-ACGGAATTCGAGGATCCATGGATCAGAACAACAGCCTG-3′ and 5′-GGCGGATCCGCTCGAGGGCGTCGTCTTCCTGAATCCCTT-3′. The PCR product was digested with BamHI and cloned into the eukaryotic expression vector pCS2+ and the prokaryotic expression vector pQE60 (Qiagen).
pGEXβ-catenin1–284 was generated by PCR on pGEXβ-catenin as template with the primer pair pGEX5′-sequencing-primer and 5′-CGCGGATCCAGCAACCATTTTCTGCAGTCC-3′. The PCR product was digested with BamHI and cloned into pGEX4T1. pQE60β-catenin1–284 was obtained by the same strategy, but by using pQE60β-catenin as template. The plasmid pCGLEF-HA was obtained from R. Grosschedl (University of California, San Francisco).
Protein Purification and Sequencing.
Recombinant GST-β284, harboring amino acids 1–284 of β-catenin (ca. 5 mg), was covalently linked to Minileak beads (Kem-En-Tec). 35S methionine/35S cysteine metabolically labeled SW480 cells (1 × 107) or 1.5 × 109 SW480 cells were lysed in lysis buffer (10 mM imidazole, pH 6.8/100 mM NaCl/300 mM sucrose/2 mM MgCl2/10 mM EGTA/1 mM NaF/1 mM molybdate/1 mM vanadate/0.2% Triton X-100), and incubated for 4 h with GST-β284 coupled beads. After extensive washing with lysis buffer, bound proteins were eluted with 0.1 M glycine·HCl, pH 2.5.
Because the N terminus of Pontin52 was blocked for direct N-terminal sequencing, internal peptides were generated by tryptic digestion. Electrophoretically resolved Pontin52 (ca. 10 μg) was blotted onto poly(vinylidene difluoride) membrane, and the excised bands were digested with 5 μg of trypsin (sequencing grade, Boehringer Mannheim) as described (20). The resulting peptide mixture was separated by reversed-phase chromatography on a C2C18 column (Vydac, Hesperia, CA), and peptides in the peak fractions were directly sequenced on an automated gas-phase sequencer.
Affinity Precipitation and Immunological Procedures.
A recombinant GST-tagged Pontin52 was expressed in E. coli and affinity purified on GSH beads (Sigma); 2 μg of GST-Pontin52 was used for the affinity precipitation of β-catenin and TBP from cell lysates as described (21). Immunoprecipitations were carried out as described (22) except that the cell lysis buffer described above was used. For metabolic labeling, cells were grown 2 h in methionine- and cysteine-free DMEM and then cultured for 12 h in the presence of 50 μCi/ml of [35S] methionine/[35S] cysteine (3,000 Ci/mmol, Amersham). In vitro transcription and translation were performed according to the manufacturer’s descriptions (Promega).
Immunofluorescence.
Cells were grown on collagen-coated coverslips, washed three times with PBS, fixed in 3% paraformaldehyde at room temperature for 20 min, treated with 1 M glycine in PBS, pH 8.5, for 5 min, washed in PBS, and permeabilized with 0.5% Triton X-100 in PBS for 5 min at room temperature. Alternatively, cells were fixed with methanol for 4 min at −20°C. Cells were incubated with anti-Pontin52 antibodies (anti-25) at 1:100 dilution or anti-MYC antibodies (2 μg/ml each) at 37°C for 1 h, washed, and treated with fluorescent dye (dichlorotriazinyl aminofluorescein, CY3)-conjugated secondary antibodies (Sigma) (1:200) in PBS for 1 h at 37°C. For control experiments, anti-25 antibodies were preincubated with 10 μg of a recombinant GST-Pontin52 fusion protein for 1 h. For nuclear staining, cells were treated with Hoechst 33342 (1 μg/ml in PBS) for 5 min at room temperature. Finally, cells were mounted in 50% glycerol/50% PBS/100 mg/ml of 1,4-diazabicyclo[2.2.2.]octane and viewed under an Axioskop microscope (Zeiss).
Images were taken with a charge-coupled device C4880 digital camera (Hamamatsu Photonics, Hamamatsu City, Japan). Optical sections were taken at 0.2-μm intervals from the bottom to the top of the cell layers, and out-of-focus information was removed by using a deconvolution algorithm. Camera and microscope were controlled by the computer program openlab (Improvision, Coventry, U.K.).
RESULTS
Isolation and Identification of Pontin52.
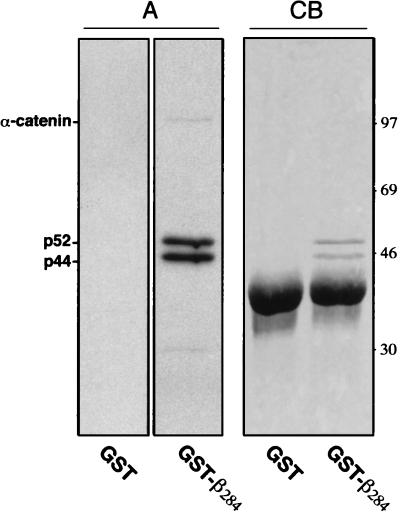
To identify new interaction proteins of β-catenin we looked for in vitro binding partners for the amino-terminal 284 aa of β-catenin expressed as a GST fusion protein (GST-β284). Three proteins from metabolically labeled human SW480 colon carcinoma cells, with relative molecular masses of 102, 52, and 44 kDa, were found to bind specifically to the GST-β284 fusion protein (Fig. 1, A). The 102-kDa protein, identified as α-catenin, was expected because GST-β284 harbors the binding site for α-catenin (amino acids 117–143) (23). The 52- and 44-kDa proteins were novel and also were detected as binding partners in other cell lines, including human AN3-CA and A431 carcinoma cells (not shown).
Figure 1.
Affinity binding experiments with recombinant GST-β-catenin284 (GST-β284). Cell lysates from 1 × 107 metabolically labeled and 1.5 × 109 nonlabeled SW480 cells were affinity precipitated with a recombinant GST-β284 fusion protein. Bound proteins were eluted, separated by SDS/PAGE and analyzed by autoradiography (A) and Coomassie blue staining (CB).
We used large-scale preparations (1.5 × 109 SW480 cells) to purify the 52- and 44-kDa proteins in sufficient amounts for detection by Coomassie blue staining and amino acid sequence analysis of tryptic fragments (Fig. 1, CB). For the 52-kDa protein, sequences were obtained for three peptides (Fig. 2), and database searches revealed that all three peptides have strong matches with several expressed sequence tag (EST) cDNA clones of human and mouse origin. By using EST sequence information, specific oligonucleotides were designed as primers for reverse transcription–PCR, and from several overlapping clones isolated from a SW480 cell cDNA library the human full-length coding sequence was established. Nucleotide and amino acid sequence comparison revealed a high sequence homology of the 52-kDa protein to several known database sequences (Fig. 2). Among these were two inferred proteins from the yeast Saccharomyces cerevisiae genome with approximately 70% and 42% sequence identity. A similar high degree of homology was found with two inferred proteins of unknown function from the Caenorhabditis elegans genome (not shown). Of particular interest was the high homology to the recently described rat protein TIP49 (rTIP49), which was identified as a binding partner of the TBP, a component of the basic transcription machinery (24). This finding suggested that the 52-kDa protein also could bind to TBP and thus bridge β-catenin to TBP. The 52-kDa protein therefore was named Pontin52 (pons, bridge in Latin).
Figure 2.
Sequence-alignment of human Pontin52 with homologous proteins from rat (rTIP49) and S. cerevisiae (YDR190c). The boxed sequences contain WalkerA and WalkerB motifs responsible for ATP binding and ATP hydrolysis. Sequences derived from peptide sequencing analysis are underlined. Protein regions used for antibody production are indicated in filled boxes.
In Vivo Interaction of β-Catenin and Pontin52.
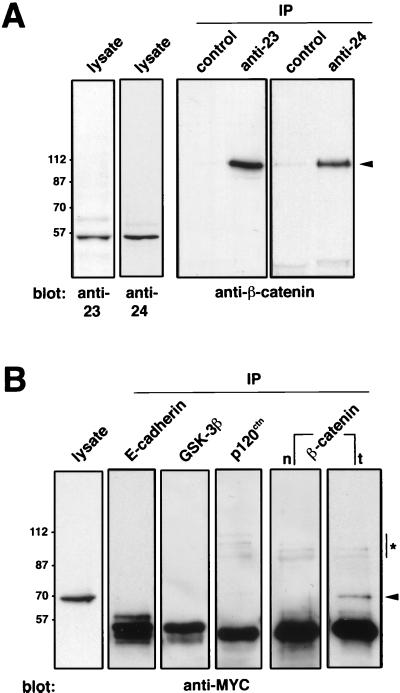
To identify Pontin52 unambiguously in subsequent biochemical analysis, three independent polyclonal antibodies were raised against different parts of the protein (Fig. 2). Each antibody specifically recognized Pontin52, as shown here for the anti-23 and anti-24 antibodies in immunoblots on SW480 cell lysates (Fig. 3A). Immunoprecipitation with Pontin52-specific anti-23 and anti-24 antibodies on cell lysates from SW480 cells and subsequent analysis of the obtained immunocomplex by Western blotting with anti-β-catenin antibodies demonstrated an association of endogenous β-catenin and Pontin52 in vivo (Fig. 3A).
Figure 3.
Association of Pontin52 and β-catenin in vivo. (A) Two independently raised anti-Pontin52 antibodies, anti-23 and anti-24, were used for immunoblots against cell lysates of SW480 cells. Immunoprecipitates (IP) collected from SW480 cell lysates with the same antibodies were subjected to Western blot analysis with anti-β-catenin antibodies, demonstrating an association of Pontin52 and β-catenin in living cells. Corresponding nonimmune sera were used in controls. Arrowhead indicates β-catenin. (B) For the reciprocal experiment, myc-tagged Pontin52 was transfected into SW480 cells, and the expression of Pontin52-MYC was monitored by immunoblotting with anti-myc antibodies (lysate). Transfected cells were immunoprecipitated with antibodies as indicated, and the immunoprecipitates were blotted and probed with anti-myc antibodies. Only immunoprecipitates collected with anti-β-catenin from transfected (t) cells contained Pontin52-MYC. Immunoprecipitations with anti-β-catenin from nontransfected SW480 cells (n) were included as controls. Arrowhead indicates Pontin52-MYC; ∗ indicates unreduced IgG heavy chains.
For a reciprocal binding experiment Pontin52 was fused to six myc-epitope tags to increase the molecular weight of the fusion protein, which was necessary because endogenous Pontin52 migrates at a similar position in SDS/PAGE to that of the antibody heavy chain. On transfection Pontin52-MYC was well expressed in SW480 cells as monitored by immunoblot with anti-myc antibodies (Fig. 3B, lysate). More importantly, immunoprecipitations with anti-β-catenin revealed an association of Pontin52-MYC and β-catenin in SW480 cells transfected with Pontin52-MYC but not in the untransfected cells used as a control [Fig. 3B, compare β-catenin immunoprecipitations of nontransfected (n) and transfected (t) cells]. Several additional controls were included in these experiments. For example, when cell lysates from SW480 cells transfected with Pontin52-MYC were immunoprecipitated with antibodies against E-cadherin, glycogen synthase kinase 3β, or p120ctn, no Pontin52-MYC was coimmunoprecipitated (Fig. 3B). The association of Pontin52 and β-catenin also was observed when both components were overexpressed in Neuro2A cells (not shown).
Direct Binding of Pontin52 to β-Catenin and TBP.
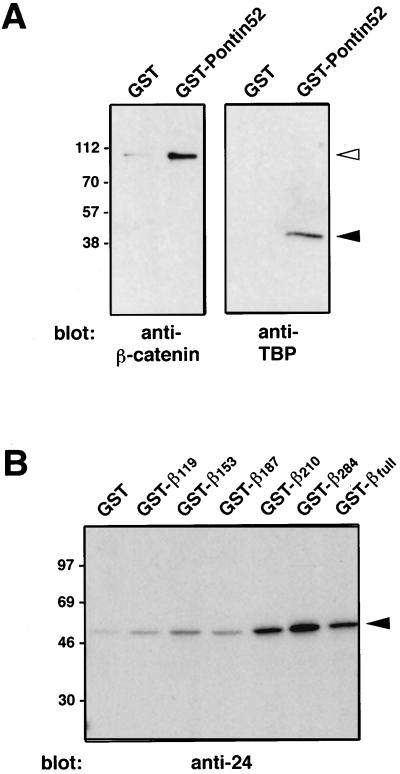
The results reported so far did not exclude the possibility of an indirect association between β-catenin and Pontin52 mediated by another cellular component. To test for a direct interaction of the two proteins in vitro, binding of recombinant GST-Pontin52 to His6-tagged β-catenin was investigated. The results clearly demonstrated a direct interaction of the two proteins (Fig. 4A, Left).
Figure 4.
Pontin52 interacts directly with β-catenin and TBP. (A) A direct interaction of Pontin52 with β-catenin and TBP was demonstrated by affinity precipitation of recombinant His6-tagged β-catenin or His6-tagged TBP with the recombinant GST-Pontin52 fusion protein. The precipitate was analyzed by SDS/PAGE and Western blotting with anti-β-catenin antibodies (Left) or anti-TBP antibodies (Right). Filled arrowhead indicates His6-tagged TBP; open arrowhead indicates His6-tagged β-catenin. (B) To map the binding site(s) of Pontin52 in β-catenin several recombinant GST-β-catenin fusion proteins were used to affinity precipitate in vitro-translated Pontin52. The precipitates were analyzed by autoradiography. Arrowhead indicates Pontin52.
The binding site in β-catenin for Pontin52 was mapped more precisely by testing for binding of a series of carboxyl-terminal-truncated, GST-tagged β-catenin fusion proteins with in vitro-translated Pontin52. The results indicate that the region of amino acids 187–284 of β-catenin, corresponding roughly to Armadillo-repeat motifs 2–5 of the protein, harbors a binding site for Pontin52, although weak binding also was observed in the amino-terminal region of β-catenin (Fig. 4B).
As indicated above, Pontin52 is highly homologous to rTIP49, which was identified as an interaction partner of the TBP, although no direct interaction of these two proteins could be demonstrated (24). Therefore, similar in vitro reconstitution experiments were performed with recombinant GST-Pontin52 and His6-tagged TBP, and again a direct interaction between Pontin52 and TBP was found (Fig. 4A, Right). In analogous experiments, recombinant LEF-1 did not interact with Pontin52 (not shown).
Nuclear Localization of Pontin52.
Pontin52 is predominantly localized in the nucleus, as visualized by immunostaining of endogenous Pontin52 in COS cells (Fig. 5A). At higher magnification, Pontin52 is seen distributed over the nucleus in small dot-like structures, but with no staining of nucleoli. The nuclear localization also was observed with anti-23 and anti-24 antibodies (not shown), and the staining was eliminated when antibodies were preincubated with a recombinant GST-Pontin52 fusion protein (Fig. 5C). Nuclear localization of Pontin52 also was seen in human SW480 and HCT116 cells when using the same antibodies or when a myc-tagged Pontin52 was transiently expressed in COS cells and the cells were stained with anti-myc antibodies (not shown).
Figure 5.
Pontin52 is a nuclear protein. Indirect immunofluorescence tests with the Pontin52-specific anti-25 antibody were performed on COS cells (A and A′). Pontin52 was found predominantly localized in the nucleus in dot-like structures. The dotted structures throughout the cytoplasm represent unspecific staining, because they also can be seen with the nonimmune sera (B and B′) or with anti-25 preincubated with recombinant GST-Pontin52 fusion protein (C and C′). (A′, B′, and C′) Hoechst 33342 staining of the same nuclei. (Bar: 20 μm.)
Pontin52 appears to be rather ubiquitously expressed as monitored by immunoblot analysis of cell lysates from various human and mouse cell lines (Fig. 6A) and by Northern blot analysis of RNA from different human tissues (Fig. 6B). The notion that Pontin52 might be a constitutive cellular component is further supported by the fact that the expressed sequence tag clones were isolated from cDNA libraries of various human and mouse tissues, including several that were not tested here, such as liver, testis, breast, spleen, and bone marrow.
Figure 6.
Pontin52 exhibits a widespread expression pattern. (A) Western blot analysis of cell lysates from human and mouse cell lines with Pontin52-specific antibody (anti-24). An equal number of cells for each cell line was taken for comparing the relative amount of Pontin52 protein. Arrowhead indicates Pontin52. (B) Northern blot analysis on human tissues probed with a 0.69-kb BglI–EcoRV fragment of the Pontin52 cDNA. A REAL blot was purchased from Invitrogen, which as indicated by the manufacturer had been loaded with 2 μg of total RNA per lane. A band expected for the size of the Pontin52 mRNA was detected in each of the tissues. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Triple Complex Formation with LEF-1.
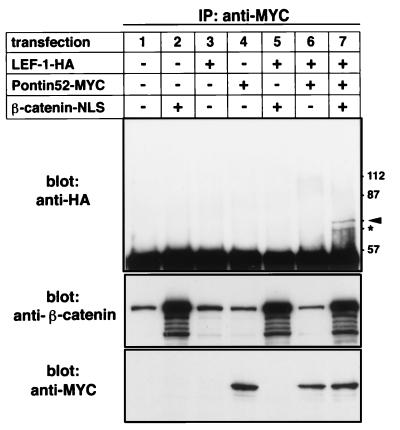
The results presented so far demonstrate that Pontin52 is a nuclear protein and can bind directly to β-catenin and TBP. To obtain further information about the protein–protein interactions of nuclear β-catenin, Neuro2A cells were transiently transfected with cDNAs coding for myc-tagged Pontin52, HA-tagged LEF-1, and β-catenin-nuclear localization signal. Cell lysates were immunoprecipitated with anti-myc antibodies, and the immunoprecipitates were subjected to immunoblot analysis with anti-HA antibodies. A complex composed of LEF-1, β-catenin, and Pontin52 could be detected when all three proteins were expressed in combination, demonstrating that Pontin52 is associated with the LEF-1-β-catenin complex (Fig. 7). No direct interaction of Pontin52 with LEF-1 in the absence of β-catenin was observed in these experiments, in agreement with the in vitro binding studies using recombinant proteins mentioned above. The faster migrating band recognized by the anti-HA antibody (Fig. 7, ∗) is likely a degradation product of LEF-1, because both the larger and smaller proteins were recognized by two independent anti-LEF-1 antibodies in parallel experiments (not shown). Attempts to demonstrate an even higher order complex composed of LEF-1, β-catenin, Pontin52, and TBP by including TBP cDNA in this kind of experiment have so far been inconclusive. But the triple complex formation by LEF-1, β-catenin, and Pontin52 provides good evidence that Pontin52 bridges the LEF-1-β-catenin complex to TBP and thus to the basic transcriptional machinery.
Figure 7.
In vivo association of Pontin52 with the LEF-1-β-catenin complex. Several combinations of plasmids were transfected into N2A cells as indicated. Pontin52-myc was precipitated from cell lysates with myc-specific antibodies, and the immunoprecipitates were analyzed by Western blotting of HA-tagged LEF-1 with anti-HA specific antibodies. The expression of transfected β-catenin and Pontin52-MYC was monitored by Western blotting of cell lysates with anti-β-catenin and anti-myc antibodies. Arrowhead indicates LEF-1-HA; ∗ indicates degradation products of LEF-1-HA as monitored in parallel experiments with anti-LEF-1 antibodies (not shown).
DISCUSSION
We report here the identification of Pontin52 as an interacting partner of β-catenin, which also binds directly to the TBP. Pontin52 is a nuclear protein that is evolutionarily highly conserved even in lower eukaryotes (e.g., yeast) that do not have β-catenin. We show here expression of Pontin52 in several human tissues and cell lines, and it is represented by a large number of expressed sequence tag clones in mouse and human from a variety of different tissues. These data indicate that Pontin52 is rather ubiquitously expressed and may exhibit a constitutive cellular function. Such a view is supported by gene disruption of the Pontin52 homolog in yeast, which resulted in a lethal phenotype (unpublished observation). The interaction of Pontin52 with β-catenin could reflect a nuclear function of β-catenin that is independent of its interaction with LEF-1/TCF. It has been reported recently that β-catenin can be imported into the nucleus without being complexed with LEF-1/TCF proteins (25), which suggests that β-catenin may exhibit additional nuclear activities, as reflected in particular by its association with Pontin52. Alternatively, in terms of the current model of molecular interactions that transduce Wnt/Wg signaling, it is tempting to speculate that Pontin52 is involved in linking LEF-1/TCF-β-catenin complexes to the basic transcriptional machinery. Thus Pontin52 could function as a coactivator in regulating the expression of target genes on Wnt/Wg signaling. We show that a triple complex composed of LEF-1-β-catenin-Pontin52 can be formed in a cell, demonstrating that the binding sites of LEF-1 and Pontin52 in β-catenin are not mutually exclusive. Furthermore, a second potential transactivation domain recently was identified in the amino-terminal region of β-catenin (26), and as described here this amino-terminal transactivation domain is very close to or may even overlap with the binding site for Pontin52. Pursuing this attractive hypothesis, we have directly tested whether Pontin52 could enhance the transactivating activity of β-catenin in reporter assays, but this was only marginally the case (unpublished observation). More work is needed to elucidate the physiological role of Pontin52 for the nuclear function of β-catenin. Further hints for future experiments come from the structural features of Pontin52. It contains a P-loop motif responsible for ATP/GTP binding and exhibits multiple conserved regions, indicating that it is putatively an ATP-dependent DNA helicase. Thus, in the biological context studied here, i.e., Wg/Wnt-dependent formation of the LEF-1/TCF-β-catenin complex resulting in target gene expression, the role of Pontin52 might be to unwind nucleic acid duplexes, a step known to be essential for initiation of transcription. Members of the high mobility group-box transcription factor family, such as LEF-1 and TCFs, are known to bind with high affinity to four-way DNA junctions (27, 28). LEF-1 was reported to function as an architectural transcription factor by making a sharp bend in the DNA helix (29), whereas the additional binding of β-catenin to LEF-1 decreases the angle of the bent DNA (7). Thus, a possible role for Pontin52 in the LEF-1/TCF-β-catenin complex might be to modulate the specific DNA structure of target gene promoters.
Acknowledgments
We thank Dr. R. Deutzmann (University of Regensburg, Germany) for the peptide sequence analysis, Dr. Randy Cassada for critically reading the manuscript, and Rosemary Schneider for secretarial work. This work was supported by the Max-Planck Society and the German-Israeli Foundation.
ABBREVIATIONS
- LEF-1/TCF
lymphocyte enhancer factor-1/T-cell factor
- GST
glutathione S-transferase
- TBP
TATA box binding protein
- HA
hemagglutinin
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF099084).
References
- 1.Ozawa M, Baribault H, Kemler R. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. J Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 3.Rimm D L, Koslov E R, Kebriaei P, Cianci C D, Morrow J S. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadigan K M, Nusse R. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 5.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papkoff J, Rubinfeld B, Schryver B, Polakis P. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens J, von Kries J P, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Nature (London) 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 8.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann B G, Kemler R. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 9.Molenaar M, Van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 10.Travis A, Amsterdam A, Belanger C, Grosschedl R. Genes Dev. 1991;5:880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- 11.Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Development (Cambridge, UK) 1993;118:439–448. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- 12.Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Mol Cell Biol. 1998;18:1248–1256. doi: 10.1128/mcb.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 14.Carnac G, Kodjabachian L, Gurdon J B, Lemaire P. Development (Cambridge, UK) 1996;122:3055–3065. doi: 10.1242/dev.122.10.3055. [DOI] [PubMed] [Google Scholar]
- 15.Brannon M, Kimelman D. Dev Biol. 1996;180:344–347. doi: 10.1006/dbio.1996.0306. [DOI] [PubMed] [Google Scholar]
- 16.Yang-Snyder J, Miller J R, Brown J D, Lai C J, Moon R T. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 17.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S C, Grosschedl R, Bienz M. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 18.Laurent M N, Blitz I L, Hashimoto C, Rothbacher U, Cho K W. Development (Cambridge, UK) 1997;124:4905–4916. doi: 10.1242/dev.124.23.4905. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2725. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber O, Sumper M. EMBO J. 1994;13:4212–4222. doi: 10.1002/j.1460-2075.1994.tb06741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aberle H, Schwartz H, Hoschuetzky H, Kemler R. J Biol Chem. 1996;271:1520–1526. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- 22.Hoschuetzky H, Aberle H, Kemler R. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber O, Krohn M, Kemler R. J Cell Sci. 1997;110:1759–1765. doi: 10.1242/jcs.110.15.1759. [DOI] [PubMed] [Google Scholar]
- 24.Kanemaki M, Makino Y, Yoshida T, Kishimoto T, Koga A, Yamamoto K, Yamamoto M, Moncollin V, Egly J M, Muramatsu M, Tamura T. Biochem Biophys Res Commun. 1997;235:64–68. doi: 10.1006/bbrc.1997.6729. [DOI] [PubMed] [Google Scholar]
- 25.Fagotto F, Glueck U, Gumbiner B M. Curr Biol. 1998;8:181–195. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- 26.Hsu S C, Galceran J, Grosschedl R. Mol Cell Biol. 1998;18:4807–4818. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.P-ohler J R, Norman D G, Bramham J, Bianchi M E, Lilley D M. EMBO J. 1998;17:817–826. doi: 10.1093/emboj/17.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosschedl R, Giese K, Pagel J. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 29.Giese K, Kingsley C, Kirshner J R, Grosschedl R. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]