Abstract
Enterococcus faecalis LDR55, a human clinical isolate, is resistant to tetracycline (Tcr), erythromycin (Emr), and high levels (greater than 2,000 micrograms/ml) of spectinomycin (Spr) but not streptomycin. Filter matings between strain LDR55 and E. faecalis OG1-RF produced transconjugants with the following resistance phenotypes: Tcr Emr Spr, Tcr Emr, Tcr Spr, and Tcr only but never Emr or Spr only. The genetic determinant encoding resistance to spectinomycin was cloned in Streptococcus sanguis Challis from pDL55, a 26-kb plasmid harbored by a Tcr Spr transconjugant. Subcloning experiments yielded a 1.1-kb ClaI-NdeI fragment that encoded very high-level Spr in S. sanguis (10 mg/ml) and Escherichia coli (50 mg/ml). Cell extracts of cultures obtained from Spr strains expressed adenylating activity for spectinomycin but not for streptomycin, indicating that Spr was due to an AAD(9) activity. The nucleotide base sequence of the 1.1-kb ClaI-NdeI fragment contained a single 750-base open reading frame. The protein predicted from the open reading frame consisted of 250 amino acids and had a calculated size of approximately 28,000 daltons, similar to the size estimated from maxicell analysis (29,000 daltons). The deduced amino acid sequence of the streptococcal AAD(9) was compared with that of the AAD(9) encoded by staphylococcal transposon Tn554. The two proteins shared approximately 39% amino acid identity, which was expanded to 53% when conservative amino acid changes were included. When the streptococcal protein was compared with an AAD(3")(9) protein of E. coli, the degrees of identity were 27 and 47%, on the basis of actual amino acids and conservative replacements, respectively. The cloning and nucleotide base sequence analyses of the spectinomycin AAD(9) determinant from E. faecalis that results in high-level Spr when transferred to S. sanguis or E. coli are presented.
Full text
PDF


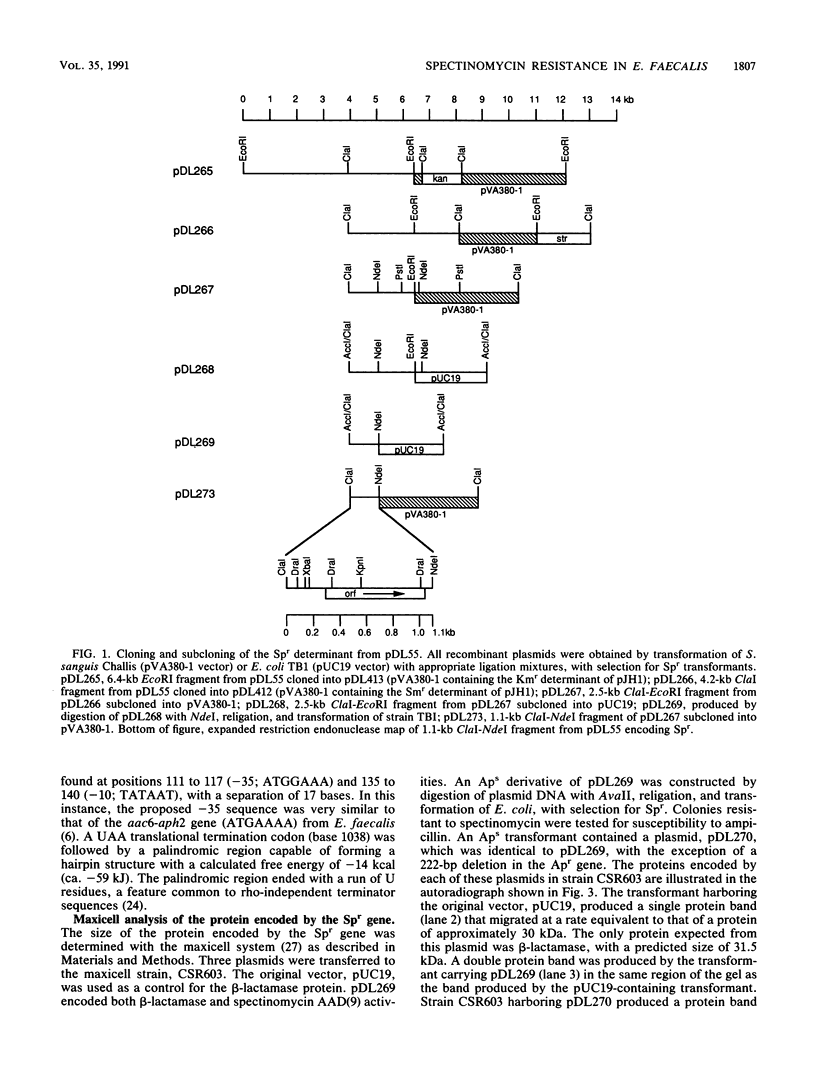
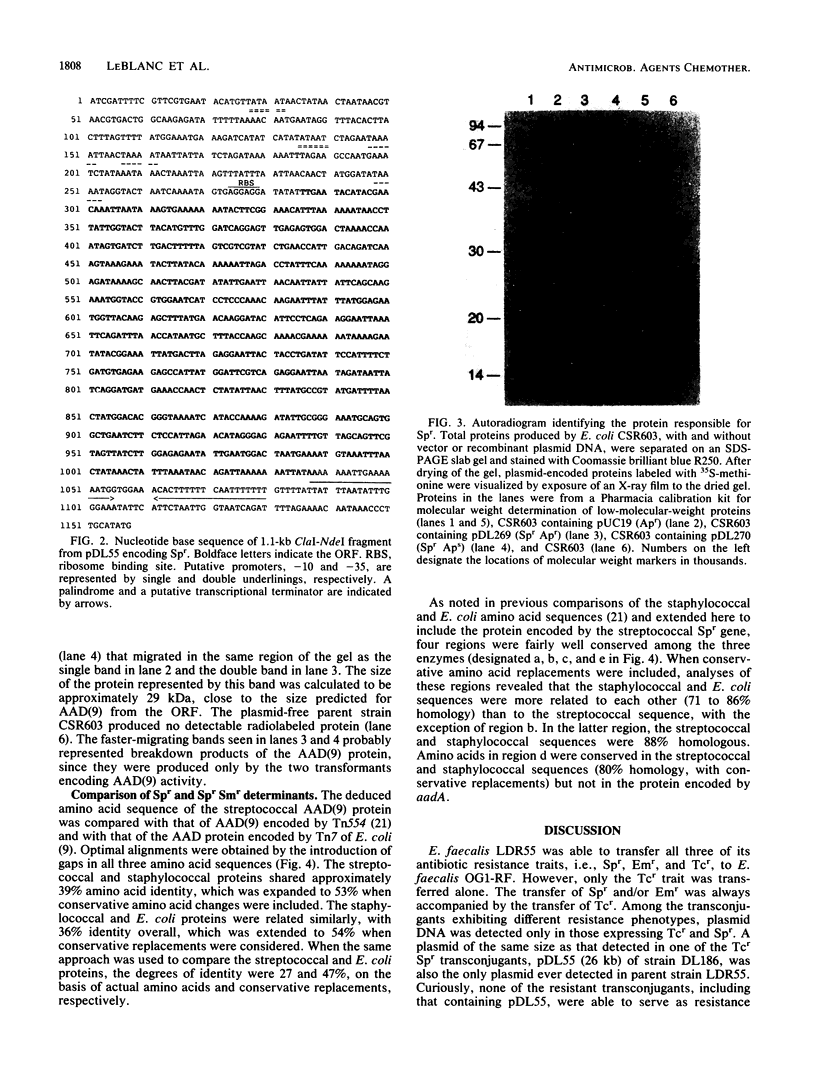


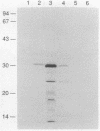
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calderwood S. B., Wennersten C., Moellering R. C., Jr Resistance to antibiotic synergism in Streptococcus faecalis: further studies with amikacin and with a new amikacin derivative, 4'-deoxy, 6'-N-methylamikacin. Antimicrob Agents Chemother. 1981 Apr;19(4):549–555. doi: 10.1128/aac.19.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hollingshead S., Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid. 1985 Jan;13(1):17–30. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J. M., Lee L. N., LeBlanc D. J. Molecular and genetic characterization of lactose-metabolic genes of Streptococcus cremoris. J Bacteriol. 1986 Sep;167(3):855–862. doi: 10.1128/jb.167.3.855-862.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Inamine J. M., Lee L. N. Broad geographical distribution of homologous erythromycin, kanamycin, and streptomycin resistance determinants among group D streptococci of human and animal origin. Antimicrob Agents Chemother. 1986 Apr;29(4):549–555. doi: 10.1128/aac.29.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N. Characterization of two tetracycline resistance determinants in Streptococcus faecalis JH1. J Bacteriol. 1982 May;150(2):835–843. doi: 10.1128/jb.150.2.835-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Virgili S. S., Scott C. L. Extrachromosomal gene systems in Streptococcus mutans. Adv Exp Med Biol. 1978;107:859–868. doi: 10.1007/978-1-4684-3369-2_96. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3") (9). Mol Gen Genet. 1985;200(1):33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M. Simple, inexpensive procedure for the disruption of bacteria. Appl Microbiol. 1974 Jul;28(1):66–69. doi: 10.1128/am.28.1.66-69.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins L. D., Lee L. N., LeBlanc D. J. Evidence for a disseminated erythromycin resistance determinant mediated by Tn917-like sequences among group D streptococci isolated from pigs, chickens, and humans. Antimicrob Agents Chemother. 1985 Apr;27(4):439–444. doi: 10.1128/aac.27.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]