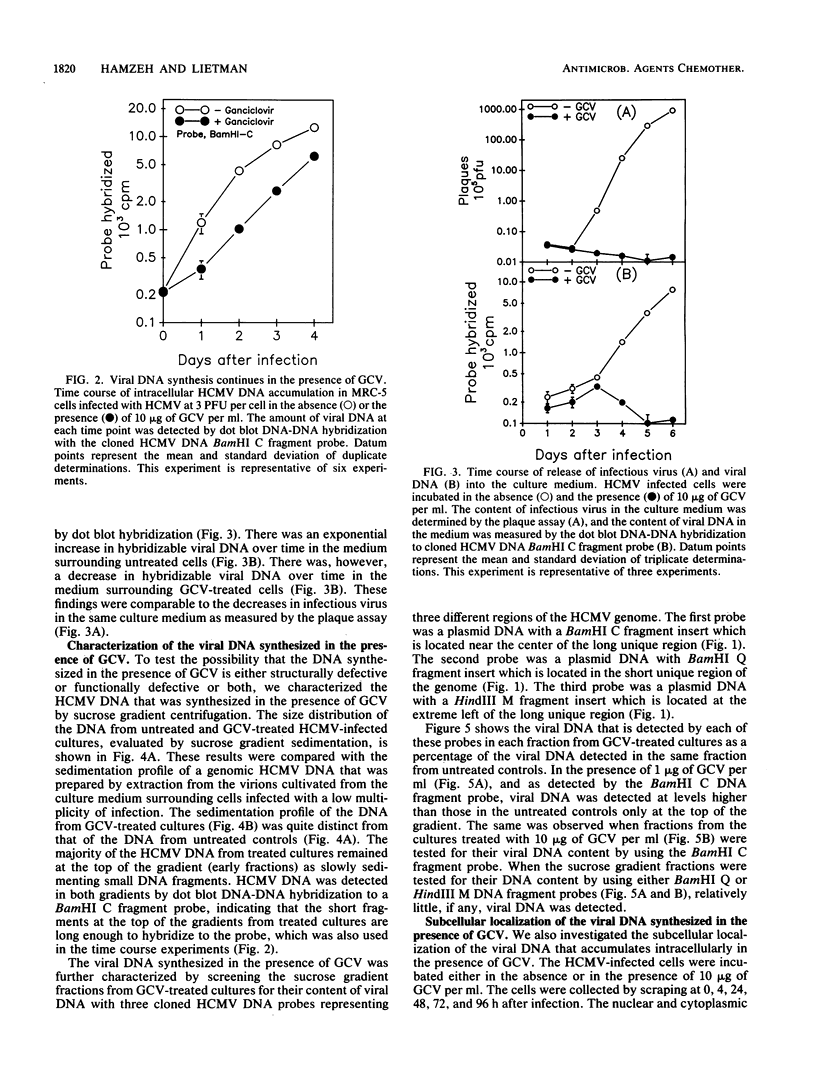
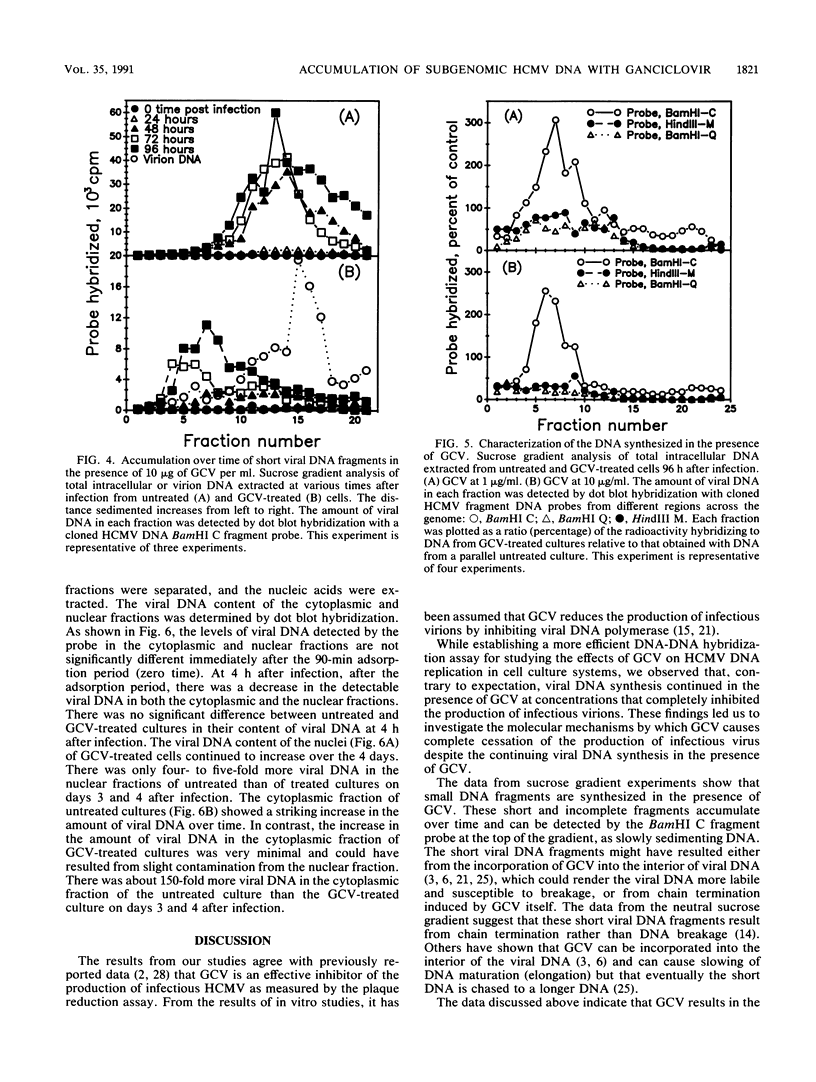
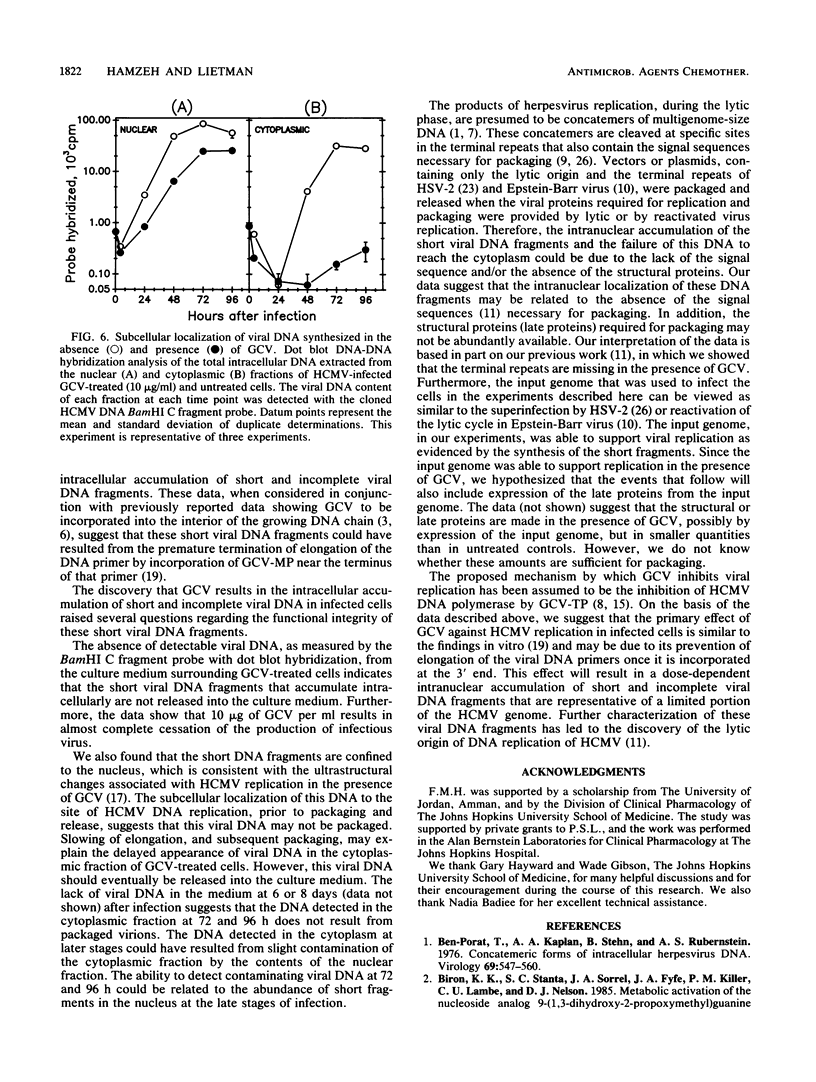
Abstract
In preparation for an attempt to elucidate some aspects of the interaction between ganciclovir and human cytomegalovirus (HCMV) DNA replication in cells infected with HCMV, we developed a dot blot DNA-DNA hybridization technique to quantify intracellular HCMV DNA replication. We studied the effect of ganciclovir on the time course of HCMV DNA replication in human fibroblasts. Ganciclovir resulted in complete cessation of the production of infectious virus, as detected by the plaque assay. However, viral DNA synthesis, as measured by dot blot DNA-DNA hybridization with cloned HCMV DNA BamHI C fragment probe, continued in the presence of ganciclovir at 10 times the 50% effective dose (i.e., 10 micrograms/ml). The continuation of viral DNA synthesis in ganciclovir-treated cultures leads to the intranuclear accumulation of short (subgenomic) HCMV DNA fragments. These DNA fragments are neither packaged nor released into the culture medium. Furthermore, the short DNA fragments were detected only by the BamHI C probe from the center of the unique long segment of the HCMV genome. The failure of the DNA probes from the termini of HCMV genome (BamHI-Q and HindIII-M) to detect the short DNA fragments and the intranuclear localization of these fragments suggest that these short fragments may lack the signal sequences necessary for packaging and release as infectious virions. These data strongly suggest that the anti-HCMV activity of ganciclovir is due mainly to the prevention of viral DNA chain elongation which results in the intranuclear accumulation of incomplete noninfectious viral DNA fragments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Kaplan A. S., Stehn B., Rubenstein A. S. Concatemeric forms of intracellular herpesvirus DNA. Virology. 1976 Feb;69(2):547–560. doi: 10.1016/0042-6822(76)90484-0. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Grill S. P., Dutschman G. E., Nakayama K., Bastow K. F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983 Oct 25;258(20):12460–12464. [PubMed] [Google Scholar]
- D'Aquila R. T., Hayward G. S., Summers W. C. Physical mapping of the human cytomegalovirus (HCMV) (Towne) DNA polymerase gene: DNA-mediated transfer of a genetic marker for an HCMV gene. Virology. 1989 Jul;171(1):312–316. doi: 10.1016/0042-6822(89)90546-1. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Chiou J. F., Cheng Y. C. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984 Feb 10;259(3):1566–1569. [PubMed] [Google Scholar]
- Freitas V. R., Smee D. F., Chernow M., Boehme R., Matthews T. R. Activity of 9-(1,3-dihydroxy-2-propoxymethyl)guanine compared with that of acyclovir against human, monkey, and rodent cytomegaloviruses. Antimicrob Agents Chemother. 1985 Aug;28(2):240–245. doi: 10.1128/aac.28.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann A., Shlomai J., Becker Y. Electron microscopy of herpes simplex virus DNA molecules isolated from infected cells by centrifugation in CsCl density gradients. J Gen Virol. 1977 Mar;34(3):507–522. doi: 10.1099/0022-1317-34-3-507. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Ludwig H., Buhk H. J. Specificity of cleavage in replicative-form DNA of bovine herpesvirus 1. J Virol. 1988 Apr;62(4):1355–1363. doi: 10.1128/jvi.62.4.1355-1363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S., Gibson W., Hayward G. S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990 Dec;64(12):6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble G. W., McCormick A. L., Pereira L., Mocarski E. S. A cytomegalovirus protein with properties of herpes simplex virus ICP8: partial purification of the polypeptide and map position of the gene. J Virol. 1987 Oct;61(10):3143–3151. doi: 10.1128/jvi.61.10.3143-3151.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini W. R., De Clercq E., Prusoff W. H. The relationship between incorporation of E-5-(2-Bromovinyl)-2'-deoxyuridine into herpes simplex virus type 1 DNA with virus infectivity and DNA integrity. J Biol Chem. 1983 Jan 25;258(2):792–795. [PubMed] [Google Scholar]
- Mar E. C., Chiou J. F., Cheng Y. C., Huang E. S. Inhibition of cellular DNA polymerase alpha and human cytomegalovirus-induced DNA polymerase by the triphosphates of 9-(2-hydroxyethoxymethyl)guanine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Virol. 1985 Mar;53(3):776–780. doi: 10.1128/jvi.53.3.776-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirt P. V., Shaw J. E., Elion G. B., Furman P. A. Identification of small DNA fragments synthesized in herpes simplex virus-infected cells in the presence of acyclovir. Antimicrob Agents Chemother. 1984 Apr;25(4):507–509. doi: 10.1128/aac.25.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobberley M. A., Ryder T. A., Hart H., Tyms A. S. Fine structure of cells infected with human cytomegalovirus after treatment with 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Gen Virol. 1987 Jun;68(Pt 6):1553–1562. doi: 10.1099/0022-1317-68-6-1553. [DOI] [PubMed] [Google Scholar]
- Prusoff W. H., Ward D. C. Nucleoside analogs with antiviral activity. Biochem Pharmacol. 1976 Jun 1;25(11):1233–1239. doi: 10.1016/0006-2952(76)90083-6. [DOI] [PubMed] [Google Scholar]
- Reardon J. E. Herpes simplex virus type 1 and human DNA polymerase interactions with 2'-deoxyguanosine 5'-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J Biol Chem. 1989 Nov 15;264(32):19039–19044. [PubMed] [Google Scholar]
- Reardon J. E., Spector T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J Biol Chem. 1989 May 5;264(13):7405–7411. [PubMed] [Google Scholar]
- Reid R., Mar E. C., Huang E. S., Topal M. D. Insertion and extension of acyclic, dideoxy, and ara nucleotides by herpesviridae, human alpha and human beta polymerases. A unique inhibition mechanism for 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1988 Mar 15;263(8):3898–3904. [PubMed] [Google Scholar]
- Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985 Apr 1;34(7):1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982 Aug;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Furman P. A., Lubbers C. M., Elion G. B. Inhibition of cellular alpha and virally induced deoxyribonucleic acid polymerases by the triphosphate of acyclovir. Antimicrob Agents Chemother. 1980 Nov;18(5):741–745. doi: 10.1128/aac.18.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H., Lambe C. U., Furman P. A. Inhibition by ganciclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Antimicrob Agents Chemother. 1987 Jun;31(6):844–849. doi: 10.1128/aac.31.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Spector D. H. Terminal structure and heterogeneity in human cytomegalovirus strain AD169. J Virol. 1986 Sep;59(3):591–604. doi: 10.1128/jvi.59.3.591-604.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Tocci M. J., Livelli T. J., Perry H. C., Crumpacker C. S., Field A. K. Effects of the nucleoside analog 2'-nor-2'-deoxyguanosine on human cytomegalovirus replication. Antimicrob Agents Chemother. 1984 Feb;25(2):247–252. doi: 10.1128/aac.25.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]



