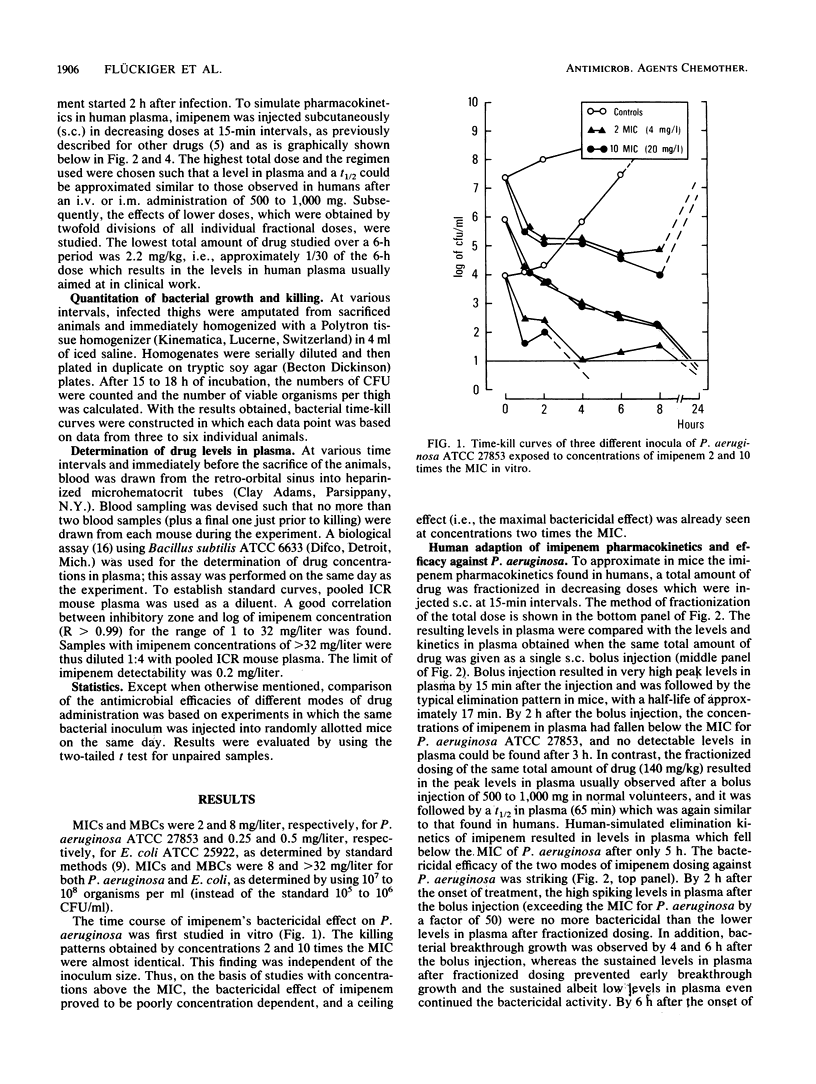
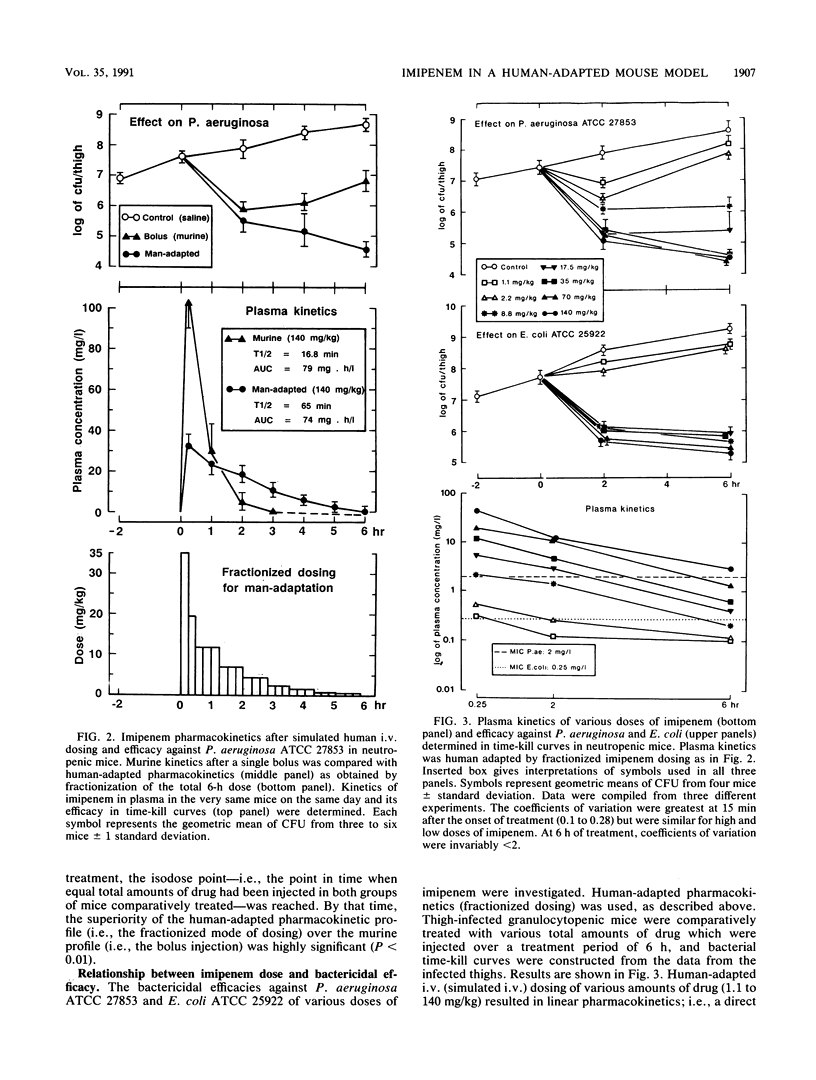
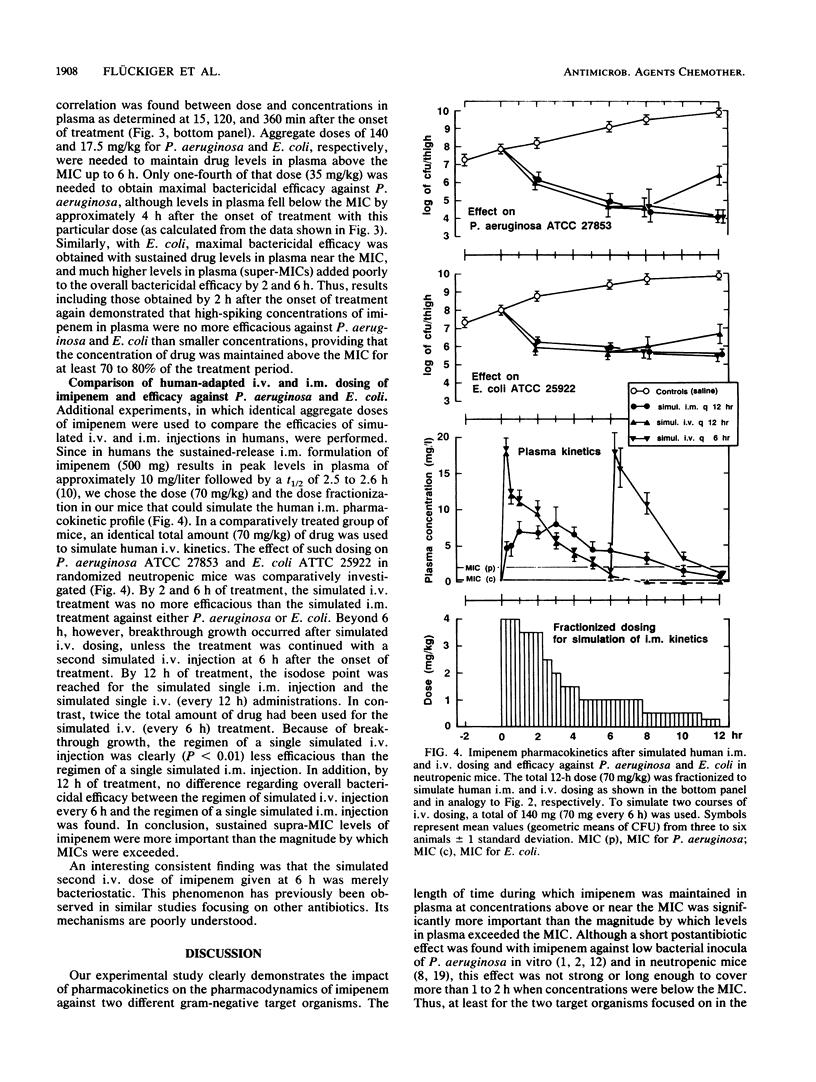
Abstract
The relationship between the pharmacokinetics and bactericidal activity of imipenem against Pseudomonas aeruginosa and Escherichia coli was investigated in a neutropenic mouse thigh infection model. To circumvent the problem of short elimination time in small animals, imipenem was administered in fractionized, decreasing doses such that the pharmacokinetic profiles as observed in humans after intravenous and intramuscular injections were approximated in mice. The human-simulated kinetic profile corresponding to an intramuscular injection of 500 mg at 12-h intervals proved to be as effective as the human-simulated profile of the same dose injected intravenously every 6 h. In contrast, the human-simulated profile corresponding to only one intravenous injection every 12 h resulted in bacterial breakthrough growth between 8 and 12 h after the onset of treatment. The results of our investigations confirm the hypothesis that the bactericidal effect of imipenem against P. aeruginosa and E. coli in vivo depends mainly on the time during which drug levels remain above the MIC rather than on the plasma peak/MIC ratio.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baquero F., Culebras E., Patrón C., Pérez-Díaz J. C., Medrano J. C., Vicente M. F. Postantibiotic effect of imipenem on gram-positive and gram-negative micro-organisms. J Antimicrob Chemother. 1986 Dec;18 (Suppl E):47–59. doi: 10.1093/jac/18.supplement_e.47. [DOI] [PubMed] [Google Scholar]
- Bustamante C. I., Drusano G. L., Tatem B. A., Standiford H. C. Postantibiotic effect of imipenem on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Nov;26(5):678–682. doi: 10.1128/aac.26.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Brugger H. P., Feller C., Stritzko T., Stalder B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986 Jan;153(1):90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Feller C., Brugger H. P. Time course of the pharmacological response to beta-lactam antibiotics in vitro and in vivo. Eur J Clin Microbiol. 1984 Dec;3(6):592–597. doi: 10.1007/BF02013630. [DOI] [PubMed] [Google Scholar]
- Gerber A. U. Impact of the antibiotic dosage schedule on efficacy in experimental soft tissue infections. Scand J Infect Dis Suppl. 1990;74:147–154. [PubMed] [Google Scholar]
- Gerber A. U., Vastola A. P., Brandel J., Craig W. A. Selection of aminoglycoside-resistant variants of Pseudomonas aeruginosa in an in vivo model. J Infect Dis. 1982 Nov;146(5):691–697. doi: 10.1093/infdis/146.5.691. [DOI] [PubMed] [Google Scholar]
- Gudmundsson S., Vogelman B., Craig W. A. The in-vivo postantibiotic effect of imipenem and other new antimicrobials. J Antimicrob Chemother. 1986 Dec;18 (Suppl E):67–73. doi: 10.1093/jac/18.supplement_e.67. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Rogers J. D. Imipenem/cilastatin: evolution of the sustained-release intramuscular formulation. Chemotherapy. 1991;37 (Suppl 2):21–25. doi: 10.1159/000238915. [DOI] [PubMed] [Google Scholar]
- Kunin C. M. Dosage schedules of antimicrobial agents: a historical review. Rev Infect Dis. 1981 Jan-Feb;3(1):4–11. doi: 10.1093/clinids/3.1.4. [DOI] [PubMed] [Google Scholar]
- Manek N., Andrews J. M., Wise R. The postantibiotic effect of imipenem. J Antimicrob Chemother. 1986 Nov;18(5):641–641. doi: 10.1093/jac/18.5.641. [DOI] [PubMed] [Google Scholar]
- Mizen L., Woodnutt G. A critique of animal pharmacokinetics. J Antimicrob Chemother. 1988 Mar;21(3):273–278. doi: 10.1093/jac/21.3.273. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Current practices in antimicrobial dosing. Rev Infect Dis. 1981 Jan-Feb;3(1):12–18. doi: 10.1093/clinids/3.1.12. [DOI] [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I. A., van den Berg J. C., Michel M. F. Therapeutic efficacy of continuous versus intermittent administration of ceftazidime in an experimental Klebsiella pneumoniae pneumonia in rats. J Infect Dis. 1985 Aug;152(2):373–378. doi: 10.1093/infdis/152.2.373. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Reller L. B. Serum dilution test for bactericidal activity. I. Selection of a physiologic diluent. J Infect Dis. 1977 Aug;136(2):187–195. doi: 10.1093/infdis/136.2.187. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig W. A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988 Oct;158(4):831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Turnidge J., Leggett J., Craig W. A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988 Feb;157(2):287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]



