Abstract
Epoetin-β is used to treat patients with metastatic cancer undergoing chemotherapy to alleviate the symptoms of anaemia, reduce the risk of blood transfusions and improve quality of life. This meta-analysis of 12 randomised, controlled studies evaluated the impact of epoetin-β on overall survival, tumour progression and thromboembolic events (TEEs). A total of 2297 patients were included in the analysis (epoetin-β, n=1244; control, n=1053; 65% solid and 35% nonmyeloid haematological malignancies). A prespecified subgroup analysis assessed the effects in patients with a baseline Hb⩽11 g dl−1, corresponding to current European Organisation for Research and Treatment of Cancer (EORTC) guidelines. No statistically significant effect on mortality was observed with epoetin-β vs control, both overall (hazard ratio (HR)=1.13; 95% CI: 0.87, 1.46; P=0.355) and in patients with baseline Hb⩽11 g dl−1 (HR=1.09; 95% CI: 0.80, 1.47; P=0.579). A trend for a beneficial effect on tumour progression was seen overall (HR=0.85; 95% CI: 0.72, 1.01; P=0.072) and in patients with an Hb⩽11 g dl−1 (HR=0.80; 95% CI: 0.65, 0.99; P=0.041). An increased frequency of TEEs was seen with epoetin-β vs control (7 vs 4% of patients); however, TEEs-related mortality was similar in both groups (1% each). The results of this meta-analysis indicate that when used within current EORTC treatment guidelines, epoetin-β has no negative impact on survival, tumour progression or TEEs-related mortality.
Keywords: anaemia, epoetin-β, survival
Anaemia is a common occurrence in patients with cancer, arising either as a result of the underlying malignant disease, as a consequence of myelosuppressive chemotherapy or radiotherapy, or a combination of both (Bokemeyer et al, 2005). Anaemia is associated with a multitude of symptoms that have a profound impact on a patient's condition and quality of life (QoL) (Ludwig et al, 2004). Furthermore, as an independent prognostic factor, anaemia has consistently been shown to be associated with adverse outcomes in patients with a variety of malignancies (Caro et al, 2001).
Erythropoiesis stimulating agents (ESAs) have been shown to increase Hb levels and reduce transfusion requirements in patients with cancer (Littlewood et al, 2001; Österborg et al, 2002; Vansteenkiste et al, 2002). In addition, treatment with ESAs has been shown to alleviate fatigue, one of the most distressing symptoms of anaemia, and result in an improvement in patients' QoL when compared with placebo or standard transfusion therapy (Littlewood et al, 2001; Crawford et al, 2002).
Preclinical data have suggested an enhanced tumour response and delayed tumour progression associated with ESA treatment (Mittelman et al, 2001; Thews et al, 2001; Stuben et al, 2003). Moreover, in clinical studies, a potential survival benefit has been shown in patients undergoing cancer therapy who received treatment with ESAs (Antonadou et al, 2001; Glaser et al, 2001; Littlewood et al, 2001). The results of the first meta-analysis of 19 randomised, controlled trials in 2865 cancer patients receiving ESAs, reported by the Cochrane Group, showed a trend towards increased survival in patients treated with ESAs (hazard ratio (HR)=0.81; 95% CI: 0.67, 0.99; P=0.04) and lent support to these findings (Bohlius et al, 2005).
In contrast to the above, two studies, one in patients with head and neck cancer (Henke et al, 2003) and the other in patients with breast cancer (Leyland-Jones et al, 2005) found higher rates of tumour progression and decreased survival, respectively, in patients receiving ESA treatment compared with placebo. Both studies had a number of methodologic limitations, including baseline imbalances and protocol violations, which confounded the interpretation (Leyland-Jones and Mahmud, 2004; Vaupel and Mayer, 2004). However, the results of an updated Cochrane meta-analysis by Bohlius et al (2006) of 57 trials including 9353 patients also found a shift of the HR for survival (HR=1.08; 95% CI: 0.99, 1.18; P=0.10) towards an increased risk for patients receiving various ESAs.
We previously reported the results of a meta-analysis of nine randomised, controlled studies of epoetin-β conducted in 1403 patients undergoing cancer therapy (Aapro et al, 2006). This meta-analysis did not demonstrate any evidence that treatment with epoetin-β impairs survival (HR=0.97; 95% CI: 0.69, 1.36; P=0.87) or promotes tumour progression (HR=0.78; 95% CI: 0.62, 0.99; P=0.042). However, one limitation of these findings was the short duration of follow-up (28 days), particularly for survival.
The present update to this previous meta-analysis reports the results from 12 randomised, controlled studies with epoetin-β in 2301 patients receiving anticancer therapy, including three recently completed trials with longer term follow-up in patients with head and neck cancer (Henke et al, 2003), patients with metastatic breast cancer (Aapro et al, 2008) and patients with cervical cancer (Strauss et al, 2008).
Materials and methods
This updated meta-analysis, using individual patient data, was designed to evaluate differences between epoetin-β and control (placebo or standard care) with regard to overall survival, disease progression and thromboembolic events (TEEs) during and up to 28 days after end of therapy with epoetin-β. Eligible studies included all randomised, controlled studies of epoetin-β conducted by the drug sponsor (F Hoffmann-La Roche or Boehringer Mannheim) in patients with cancer undergoing treatment (chemotherapy (seven studies), surgery (two studies), radiotherapy (two studies) or radio-chemotherapy (one study)). A subgroup analysis of four controlled studies with extended follow-up provides information on the effect of epoetin-β on long-term survival and malignancy progression. Individual study details are summarised in Table 1.
Table 1. Main features of randomised clinical trials of epoetin-β in patients with cancer.
| Study | Design and no. of patients (epoetin-β/control) | Diagnosis | Epoetin-β dosage and duration of therapy | Control | Cancer treatment |
|---|---|---|---|---|---|
| ten Bokkel Huinink et al (1998) (MF4249) | o, pg n=83/87 | Ovarian cancer, Hb<13 g dl−1 | 150 or 300 IU kg−1 3 × week × 6 months | Standard therapy | Chemotherapy |
| Österborg et al (1996) (MF4250) | o, pg n=95/49 | MM, NHL, CLL; transfusion-dependent, Hb<10 g dl−1 | 2000–10 000 IU day−1 titrated or 10 000 IU day−1 fixed dosage × 24 weeks | Standard therapy | Chemotherapy |
| Rau et al (1998) (MF4252) | db, pc and pg n=28/26 | Resectable rectal cancer, Hb⩾12.5 g dl−1 (men); ⩾12 g dl−1 (women) | 200 IU kg−1 daily × 11 days | Placebo | Surgery |
| Kettelhack et al (1998) (MF4253) | db, pc n=52/57 | Colorectal cancer suitable for hemicolectomy, Hb>8.5–13.5 g dl−1 | 20 000 IU day−1 × 10–15 days | Placebo | Surgery |
| Data on file (Study MF4266) | o, pg n=10/10 | AML | 10 000 IU day−1, then weekly or twice weekly × ⩽30 weeks | Standard therapy | Chemotherapy |
| Cazzola et al (1995) (MF4313) | o, pg n=117/29 | MM, NHL, CLL; transfusion-independent, Hb⩽11 g dl−1 | 1000, 2000, 5000 or 10 000 IU day−1 × 8 weeks | Standard therapy | Chemotherapy |
| Oberhoff et al (1998) (MF4421) | pg, n=114/104 | Solid organ tumours, Hb⩽11 g dl−1 | 5000 IU day−1 × 12–24 weeks | Standard therapy | Chemotherapy |
| Boogaerts et al (2003) (MF4321) | o, pg n=131/128 | Malignant disease, Hb⩽11 g dl−1 | 150 IU kg−1 3 × week adjusted for Hb response × 12 weeks | Standard therapy | Chemotherapy |
| Österborg et al (2002); Österborg et al (2005) (MF4467) | pc, db and pg n=170/173 | MM, NHL, CLL; transfusion-dependent and epo-deficient, Hb⩽10 g dl−1 | 150 IU kg−1 3 × week adjusted for Hb response × 16 weeks, 12-month study period* | Placebo | Chemotherapy |
| Henke et al (2003) (MF4449) | pc, db and pg n=171/180 | Head and neck cancer, Hb<13 g dl−1 (men), <12 g dl−1 (women) | 300 IU kg−1 3 × week, 6–8 weeks, 60-month study period | Placebo | Radiotherapy |
| Strauss et al (2008) (MO16375) | o, pg n=34/40 | Cervical cancer Stage FIGO IIB-IVA, Hb 9–13 g dl−1 | 150 IU kg−1 3 × week, 8–14 weeks, 6-month study period | Standard therapy | Radio-chemotherapy |
| Aapro et al (2008) (BA16756) | o, pg n=231/232 | Breast cancer, Hb <12.9 g dl−1 | 30 000 IU weekly × 24 weeks, 24-month study period | Standard therapy | Chemotherapy |
Abbreviations: AML=acute myeloid leukaemia; CLL=chronic lymphocytic leukaemia; db=double-blind; Hb=haemoglobin; MM=multiple myeloma; NHL=non-Hodgkin's lymphoma; o=open design; pc=placebo-controlled; pg=parallel group. Patients had anaemia unless stated otherwise, and standard therapy consisted of antitumour treatment plus blood transfusion as required.
*Information on disease progression not collected during the follow-up period of this study.
The primary objectives of the meta-analysis were to evaluate the effects of epoetin-β on overall survival, disease progression and TEEs in cancer patients in the overall data set, and for solid tumours and nonmyeloid haematological malignancies separately. Secondary predefined objectives included the evaluation of the potential impact of various Hb intervention levels on mortality and disease progression as well as evaluation of the latter outcomes in a predefined subgroup analysis including patients with long-term follow-up or those with an Hb level at baseline (intervention Hb)⩽11 g dl−1.
Most of the studies were originally designed to evaluate the efficacy of epoetin-β with respect to anaemia correction and thus there was no follow-up for survival or tumour progression beyond study treatment plus a standard 28-day period used to assess SAEs, including deaths and disease progression. Although tumour status was not prospectively assessed in many of the earlier trials with short-term follow-up, details of disease progression were routinely reported as adverse events. For the present meta-analysis, this information was analysed retrospectively by reviewers blinded to treatment assignment. Other studies were, however, designed to assess the effects of epoetin-β on survival and/or disease progression (Henke et al, 2003; Aapro et al, 2008) or Hb response to treatment (Strauss et al, 2008). Long-term follow-up information, up to 60 months, was available for overall survival in four studies (Henke et al, 2003; Österborg et al, 2005; Aapro et al, 2008; Strauss et al, 2008) and for tumour progression in three studies (Henke et al, 2003; Aapro et al, 2008; Strauss et al, 2008). All reported adverse events were also reviewed against a prespecified list of TEEs, the definition of which was consistently applied across all studies.
Statistical analyses
Overall survival and time to progression were analysed by Kaplan–Meier estimates, log-rank testing and Cox regression analysis. Thromboembolic events were summarised in terms of crude rates independent of onset. Time to TEEs was analysed as for survival and time to progression. Differences in duration of survival, time to tumour progression and time to TEEs (time between start of epoetin/control therapy or baseline visit and time of event) were tested using log-rank tests.
Two sets of analyses were performed. One analysis included data from all 12 studies. For these analyses, patients without events were censored at 4 weeks after the last entry in the drug administration record. A second analysis using only pooled data from the studies with long-term follow-up, in which all events were included in the analysis, was performed for overall survival (all four studies) and time to progression (three studies). In the study by Österborg et al (2005), patients were followed for survival but not for disease progression; therefore; this study was excluded from the time-to-progression analyses. Patients without an event were censored at the time of last follow-up or, if no follow-up information was available, 4 weeks after the last entry in the administration record.
A predefined subgroup analysis was performed using a subgroup of patients with a baseline Hb intervention level corresponding to the European Organisation for Research and Treatment of Cancer (EORTC) guidelines (i.e., ⩽11 g dl−1).
The primary analysis was based on a simple pooling strategy without further stratification. Analyses stratified by study were also performed.
Results
Analysis populations
A total of 2301 patients were enrolled in the 12 trials of whom 2297 (epoetin-β, n=1244; control, n=1053) were included in the analysis; four patients who received no treatment of any kind during the trials were excluded. All patients who received at least one dose of study medication were analysed according to the treatment received. Five patients randomised to control received epoetin-β and three patients in the epoetin group received no epoetin-β treatment.
Baseline characteristics and follow-up
Of the 2297 patients in the analysis, 35% had nonmyeloid haematological malignancies and 65% had solid tumours (Table 2). Most patients with solid tumours had primary malignancies of the breast, head and neck, colon/rectum and ovary. Among patients with nonmyeloid haematological malignancies, the most common were non-Hodgkin's lymphoma (56%) and multiple myeloma (41%). A slightly higher proportion of patients in the epoetin group had ovarian carcinoma as a result of the three arm design of the study by ten Bokkel Huinink et al (1998). No other clinically relevant differences between the groups were noted. Tumour stage at baseline was not consistently collected in the various studies as assessment of tumour progression was not a predefined study objective in most of the earlier studies. However, information on tumour stage (FIGO or TNM) was available for nearly 70% of patients with solid tumours. There were no relevant differences between the treatment arms with respect to tumour staging with the exception of FIGO Stage III, which was more common in the epoetin-β arm (16%) than in the control arm (8%) of patients with solid tumours in which this staging criteria was used.
Table 2. Baseline characteristics of pooled study populations.
| Parameter | Control (N=1053) | Epoetin-β (N=1244) |
|---|---|---|
| Gender (% male/female) | 37/63 | 38/62 |
| Race | ||
| n | 921 | 1069 |
| Caucasian | 882 (96%) | 1029 (96%) |
| Other | 39 (4%) | 40 (4%) |
| Mean age in years (range) | 58.8 (19–91) | 59.3 (20–87) |
| Mean weight in kg (range) | 67.7 (30.0–131.5) | 67.1 (35.0–118.0) |
| n | 1048 | 1235 |
| Mean height in cm (range) | 166.7 (140–198) | 166.4 (126–198) |
| n | 809 | 1012 |
| Tumour type, n (%) | ||
| Haematological | 331 (31.4) | 465 (37.4) |
| Acute myeloid leukaemia | 10 (3.0) | 10 (2.2) |
| Multiple myeloma | 125 (37.8) | 204 (43.9) |
| Non-Hodgkin's lymphoma | 195 (58.9) | 247 (53.1) |
| Hodgkin's lymphoma | 1 (<1) | 4 (<1) |
| Solid | 722 (68.6) | 779 (62.6) |
| Breast | 261 (36.2) | |
| Head/neck | 174 (24.1) | 261 (33.5) |
| Gynaecological | 133 (18.4) | 181 (23.2) |
| Gastrointestinal | 96 (13.3) | 186 (23.9) |
| Other | 58 (8.0) | 100 (12.8) |
| 51 (6.6) | ||
| Haemoglobin | ||
| N | 1050 | 1241 |
| Mean (range) | 10.6 (5.7–16.7) | 10.5 (4.2–17.1) |
| Median | 10.5 | 10.4 |
Data were collected from all 2297 patients unless otherwise stated.
Mean baseline Hb level was 10.6 g dl−1 in the control arm and 10.5 g dl−1 in the epoetin-β arm. Median initial weekly epoetin-β dose was 27 000 IU (range 0–90 000 IU). During treatment, mean maximum Hb level was 12.0 g dl−1 in the control arm and 13.4 g dl−1 in the epoetin-β arm. The mean baseline-adjusted Hb area under the curve was 0.07 g dl−1 with control compared with 1.24 g dl−1 with epoetin-β.
Duration of follow-up across the 12 studies was comparable in the control (median 3.8 months) and epoetin-β (median 3.9 months) treatment groups (patients without events from the four studies with long-term follow-up were censored 4 weeks after last entry in the administration record). In the four studies with long-term follow-up data, when all events were included, median follow-up was also comparable (29.8 months with control and 28.8 months with epoetin-β).
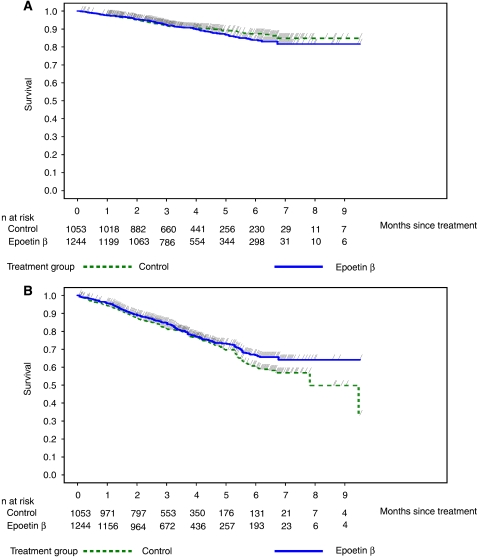
Effects on survival
The death rate in the control group was 0.29 deaths per patient-year and 0.33 in the epoetin-β group (Table 3). There was no statistically significant difference between patients receiving epoetin-β or control (standard treatment) in terms of overall survival in the pooled analysis of all 12 controlled studies (data collected up to 28 days after last dose) (HR=of 1.13; 95% CI: 0.87, 1.46; log-rank, P=0.355) (Figure 1A). Time-to-event analyses, however, suggested a numerically increased risk for mortality in the epoetin-β arm vs control with respective overall event rates of 10.9 and 9.4%. Comparable results were found in the pooled analysis of four studies with long-term follow-up. Mortality rates were 0.39 and 0.44 deaths per patient-year in the control and epoetin-β groups, respectively. Median survival was 20.6 months for control and 17.8 months for epoetin-β, with overall event rates of 60.5 and 64.5% and an HR of 1.13 (95% CI: 0.98, 1.31; log-rank, P=0.082) (Table 3). It is worth noting that the trend observed in this substudy analysis was due to the larger number of events associated with a longer follow-up.
Table 3. Kaplan–Meier and Cox regression analysis of survival and time-to-event data.
|
Control (N=1053)
|
Epoetin-β (N=1244)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient group | Total events | Mean patient-years of follow-up | Events per patient-year | Total events | Mean patient-years of follow-up | Events per patient-year | Hazard ratio (95% CI) | P-valuea |
| Pooled population of 12 controlled studies | ||||||||
| Overall survival | ||||||||
| Total | 99 | 0.32 | 0.29 | 136 | 0.33 | 0.33 | 1.13 (0.87–1.46) | 0.355 |
| Solid | 58 | 0.32 | 0.25 | 78 | 0.34 | 0.30 | 1.17 (0.83–1.64) | |
| Non-myeloid haematological | 41 | 0.34 | 0.37 | 58 | 0.32 | 0.39 | 1.04 (0.69–1.55) | |
| Time to progression | ||||||||
| Total | 254 | 0.28 | 0.86 | 268 | 0.29 | 0.74 | 0.85 (0.72–1.01) | 0.072 |
| Solid | 171 | 0.27 | 0.88 | 173 | 0.29 | 0.76 | 0.85 (0.68–1.05) | |
| Non-myeloid haematological | 82 | 0.31 | 0.81 | 93 | 0.29 | 0.69 | 0.84 (0.62–1.13) | |
| Time to thromboembolic event | ||||||||
| Total | 46 | 0.32 | 0.14 | 88 | 0.32 | 0.22 | 1.62 (1.13–2.31) | 0.008 |
| Solid | 29 | 0.32 | 0.13 | 61 | 0.33 | 0.24 | 1.92 (1.24–2.99) | |
| Non-myeloid haematological | 17 | 0.34 | 0.15 | 27 | 0.32 | 0.18 | 1.18 (0.64–2.16) | |
| Pooled population of studies with long-term follow-up | ||||||||
| Overall survival | ||||||||
| Total | 371 | 1.54 | 0.39 | 396 | 1.45 | 0.44 | 1.13 (0.98–1.31) | 0.082 |
| Solid | 262 | 1.61 | 0.37 | 286 | 1.49 | 0.43 | 1.17 (0.99–1.39) | |
| Non-myeloid haematological | 109 | 1.37 | 0.46 | 110 | 1.36 | 0.48 | 1.04 (0.80–1.36) | |
| Time to progression | ||||||||
| Totalb | 260 | 1.10 | 0.54 | 270 | 0.98 | 0.62 | 1.13 (0.95–1.34) | 0.165 |
Log-rank test P-value epoetin-β vs placebo/control.
All studies were in patients with solid tumours; ‘events’ refers to number of deaths for ‘overall survival’, number of malignant disease progressions for ‘time to progression’ and number of thromboembolic events for ‘time to thromboembolic event’.
Figure 1.
Kaplan–Meier curves of (A) overall survival and (B) time to progression in the pooled population of 12 controlled studies.
In both the analysis of the pooled population of 12 controlled studies (including events up to 28 days after end of treatment) and the analysis of four studies with long-term follow-up, the risk of death for patients with solid tumours (HR=1.17; 95% CI: 0.83, 1.64 and HR=1.17; 95% CI: 0.99, 1.39, respectively) was similar to that in the overall pooled population (HR=1.13; 95% CI: 0.87, 1.46 and HR=1.13; 95 % CI: 0.98, 1.31, respectively), however, it was lower for patients with nonmyeloid haematological malignancies (HR=1.04; 95% CI: 0.69, 1.55 and HR=1.04; 95% CI: 0.80, 1.36, respectively) (Table 3).
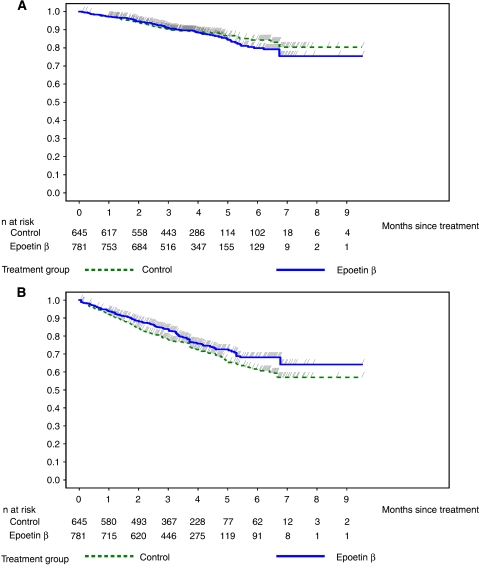
In the subgroup of patients with baseline Hb⩽11 g dl−1 from the pooled analysis of 12 controlled studies (N=1426) (i.e., in line with current EORTC guidelines), the overall event rate was comparable (11.5 and 12.5% for control and epoetin-β, respectively), and time-to-event analyses showed a HR of 1.09 (95% CI: 0.80, 1.47; log-rank, P=0.580) (Figure 2A). In the pooled population of four studies with long-term follow-up, the overall event rate was 68% for control and 70% for epoetin-β; median survival was 15.9 months in both treatment arms and the estimated HR=1.03 (95% CI: 0.85, 1.25; log-rank, P=0.750).
Figure 2.
Kaplan–Meier curves of (A) overall survival and (B) time to progression in patients with a baseline Hb⩽11 g dl−1 in the pooled population of 12 controlled studies.
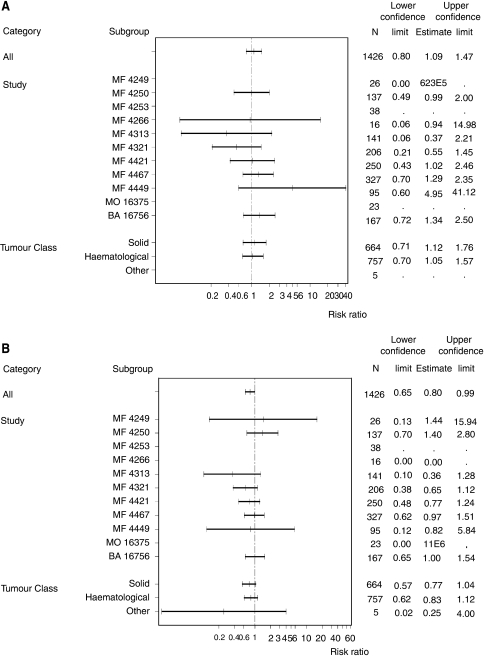
No significant differences in overall survival were seen between epoetin-β and control in patients with solid tumours or nonmyeloid haematological malignancies in the subgroup of patients with baseline Hb⩽11 g dl−1 (Figure 3A).
Figure 3.
Summary of hazard ratios for (A) overall survival and (B) time to progression in subgroup of patients with baseline Hb⩽11 g dl−1.
Effects on disease progression
No significant differences between the epoetin-β and control groups were seen in the number of patients with disease progression in the overall study population. The rates of disease progression were lower in patients receiving epoetin-β (0.74 events per patient-year) as compared to those in the control arm (0.86 events per patient-year)(Table 3). In the overall pooled population of the 12 controlled randomised studies, Kaplan–Meier analysis indicated a similar risk of progression, with a trend in favour of a reduced risk among patients treated with epoetin-β (HR=0.85; 95% CI: 0.72, 1.01; log-rank, P=0.072) (Figure 1B). In both subgroups of patients with solid or nonmyeloid haematological tumours, the HRs for disease progression were similar to that for the overall population (Table 3).
In the pooled analysis of the three studies where long-term disease progression follow-up was recorded, the rate of disease progression was higher (0.62 events per patient-year) compared with those in the control arm (0.54 events per patient-year). The overall event rate was 59% with control and 61% with epoetin-β, and the risk of progression was similar, with a trend for a higher risk of disease progression in patients receiving epoetin-β (HR=1.13; 95% CI: 0.95, 1.34; log-rank, P=0.165) (Table 3). Median time to progression was 11.2 months with control and 9.8 months with epoetin-β.
In the subgroup of patients with baseline Hb⩽11 g dl−1, in the pooled analysis of 12 controlled studies, the percentage of patients with disease progression was lower with epoetin-β (22.2%) than with control (27.0%). Similarly, in the pooled analysis of studies with long-term follow-up, a lower percentage of patients in the epoetin-β arm had disease progression (61.0%) compared with the control arm (69.4%). Time-to-event analyses showed a reduced risk of progression for patients with a baseline Hb⩽11 g dl−1 receiving epoetin-β in the pooled analysis of 12 controlled studies (HR=0.80; 95% CI: 0.65, 0.99; log-rank, P=0.041) (Figure 2B) and a trend towards a reduced risk with epoetin-β in the pooled analysis of three studies with long-term follow-up (HR=0.85; 95% CI: 0.64, 1.13; log-rank, P=0.267).
No significant differences in time to progression were seen between epoetin-β and control in patients with solid tumours or nonmyeloid haematological malignancies in the subgroup of patients with baseline Hb⩽11 g dl−1 (Figure 3B).
Effects on thromboembolic events
Across the 12 studies in the pooled analysis, there is a statistically significantly shorter time to TEEs in the epoetin-β group compared with control (P=0.0075, log-rank test). Furthermore, a higher incidence of TEEs was seen with epoetin-β (7.1%) vs control (4.4%), largely due to reports of deep vein thrombosis (1.3 vs 0.4%), thrombophlebitis (0.6 vs 0.3%) and pulmonary embolism (1.2 vs 0.9%). However, there was no difference in the incidence of fatal TEEs between the treatment arms (1% each), the most common being pulmonary embolism.
The TEEs rate was higher in the epoetin-β group (0.22 events per patient-year) compared with the control (0.14 events per patient-year) with an overall HR for time to TEEs of 1.62 (95% CI: 1.13, 2.31; log-rank, P=0.008). The risk of TEEs in patients receiving epoetin-β was higher in the subgroup of patients with solid tumours (HR=1.92; 95% CI: 1.24, 2.99) than in those with nonmyeloid haematological malignancies (HR=1.18; 95% CI: 0.64, 2.16) (Table 3).
Sensitivity analyses
Results from analyses adjusting by study yielded results consistent with the primary analysis (data not shown).
Discussion
The results of the meta-analysis of 12 randomised controlled studies in patients with solid tumours or nonmyeloid haematological tumours (n=2297) treated with epoetin-β or control/placebo do not show any significant negative effect of epoetin-β on survival or tumour progression. Importantly, this updated meta-analysis also includes long-term follow-up data from more recent studies and largely confirms the results of the earlier meta-analysis of nine controlled studies (n=1413) (Aapro et al, 2006), which did not include the recently completed studies by Henke et al (2003); Aapro et al (2008) and Strauss et al (2008). Moreover, this update confirms the safety of epoetin-β in terms of overall survival and disease progression when used within current EORTC guidelines with respect to an intervention Hb level⩽11 g dl−1.
Overall survival
The results for overall survival are consistent with the findings from a recently updated meta-analysis of published, randomised clinical trials in patients with cancer receiving chemotherapy by the Cochrane Collaboration (Bohlius et al, 2006). A shift of the overall HR for mortality towards a more favourable outcome for patients in the control group compared to those receiving ESA treatment is different from the results of an earlier meta-analysis by the same group where a trend towards increased survival in patients treated with ESAs was shown (Bohlius et al, 2005). However, as in the present updated meta-analysis of controlled clinical trials with epoetin-β, the results of the updated meta-analysis seem to be driven by inclusion of data from studies, which allowed enrollment of patients with a baseline Hb up to and above 13 g dl−1. The outcomes reported in these trials have been either negative (Henke et al, 2003, Leyland-Jones et al, 2005) or neutral (Aapro et al, 2008; Strauss et al, 2008). An Hb initiation level above 11 g dl−1 is not in line with the current EORTC treatment recommendations (Bokemeyer et al, 2007). The subgroup analyses in our updated meta-analysis for epoetin-β are fully supportive of this conclusion.
To date, the results of two prospective, randomised studies suggesting that ESA treatment may have a negative impact on survival have been published in detail (Henke et al, 2003; Leyland-Jones et al, 2005). A negative impact of ESA treatment on survival is also suggested by three recent studies (Overgaard et al, 2007; Wright et al, 2007; Smith et al, 2008), two of which (Overgaard et al, 2007; Smith et al, 2008) have not yet been reported in full. The findings of Henke et al (2003) and Leyland-Jones et al (2005) should, however, be interpreted with caution as a number of limitations associated with the studies have been identified including baseline imbalances in prognostic factors, which favoured the placebo arm in both (Dunst 2004; Leyland-Jones and Mahmud, 2004; Vaupel and Mayer, 2004). Similar caution has to be exercised with respect to the interpretation of the other three studies mentioned above as they were either not designed to assess survival, have been terminated early, with a very limited sample size (Wright et al, 2007), have not been fully reported (Overgaard et al, 2007) or are in advanced patients not receiving chemotherapy (Smith et al, 2008) and do therefore not allow a conclusive interpretation. A common feature of these five studies is that all were conducted outside the currently approved indications and all were performed in predominantly mild or nonanaemic patients, with target Hb levels higher than those recommended by the EORTC guidelines (Bokemeyer et al, 2007), for the use of ESA therapy in cancer patients treated with chemotherapy.
Tumour progression
The results in this updated meta-analysis do not suggest an increased risk for disease progression in patients receiving epoetin-β treatment vs those receiving standard care, but show the risk to be similar, with a trend towards a reduction in risk favouring patients receiving epoetin-β. When this analysis was restricted to patients with Hb intervention levels of ⩽11 g dl−1 in line with recent EORTC guidelines (Bokemeyer et al, 2007), the results showed a statistically significantly lower risk for disease progression in patients receiving epoetin-β. A similar finding of a more favourable outcome with respect to tumour progression in patients treated with ESA vs control was recently reported by the Cochrane Collaboration (Bohlius et al, 2006), as well as in a systematic review of 46 ESA trials conducted for the National Institute of Clinical Excellence (NICE) (Wilson et al, 2007). Whether the obvious discrepancy between outcomes of disease progression favouring epoetin treatment and survival outcomes favouring control/placebo treatment may be caused by an underdiagnosis of fatal TEEs (see section below) must remain speculative.
Thromboembolic events
The present analysis showed a significantly increased TEEs rate with epoetin-β compared with control (7 vs 4%; P=0.008). These results are consistent with those reported in both meta-analyses of the Cochrane Collaboration (Bohlius et al, 2005, 2006). The risk of TEEs was shown to be higher in the subgroup of patients with solid tumours compared with those with nonmyeloid haematological malignancies. This may be driven mainly by the differences in TEEs risk in the underlying cancer population due to disease stage and activation of the coagulation system. Despite the well-known increased incidence of TEEs associated with epoetin-β treatment in the present analysis, importantly, the incidence of TEEs-related mortality was similar between the two treatment groups (1% in each group).
CONCLUSIONS
The results of this meta-analysis including all prospective, randomised studies conducted with epoetin-β in cancer patients showed no evidence for a significantly negative effect of epoetin-β treatment on survival in patients with metastatic cancer. Furthermore, there was no negative effect of epoetin-β on tumour progression. The risk of TEEs was consistent with the increased TEEs risk observed within the ESA class in general, with a higher incidence of TEEs in patients with solid tumours. Predefined subgroup analyses in patients with an initiation Hb level corresponding to the current EORTC treatment guidelines (i.e., Hb⩽11 g dl−1) confirm the safety of epoetin-β in the treatment of anaemia in patients with metastatic cancers receiving concurrent chemotherapy when used within its licensed indication.
References
- Aapro M, Coiffier B, Dunst J, Österborg A, Burger HU (2006) Effect of treatment with epoetin beta on short-term tumour progression and survival in anaemic patients with cancer: a meta-analysis. Br J Cancer 95: 1467–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aapro M, Leonard RC, Barnadas A, Marangolo M, Untch M, Malamos N, Mayordomo J, Reichert D, Pedrini JL, Ukarma L, Scherhag A, Burger H-U (2008) Effect of once weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy–results of the BRAVE study. J Clin Oncol 26: 592–598 [DOI] [PubMed] [Google Scholar]
- Antonadou D, Cardamakis E, Puglisi M (2001) Erythropoietin enhances radiation treatment efficacy in patients with pelvic malignancies: final results of a randomized phase III study. Eur J Cancer 37(Suppl 6): A530 [Google Scholar]
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, Engert A (2005) Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst 97: 489–498 [DOI] [PubMed] [Google Scholar]
- Bohlius J, Wilson J, Seidenfeld S, Piper M, Schwarzer G, Sandercock J, Trelle S, Weingart O, Bayliss S, Djulbegovic B, Bennet CL, Langensiepen S, Hyde C, Engert A (2006) Erythropoietin or darbepoetin for patients with cancer. Cochrane Database of Systematic Reviews, Issue 3. Art No.: CD003407, DOI: 10.1002/14651858.CD003407.pub4 [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Österborg A, Repetto L, Soubeyran P, European Organisation for Research and Treatment of Cancer (EORTC) Taskforce for the Elderly (2007) EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 43: 258–270 [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Oechsle K, Hartmann J-T (2005) Anaemia in cancer patients: pathophysiology, incidence and treatment. Eur J Clin Invest 35(Suppl. 3): 26–31 [DOI] [PubMed] [Google Scholar]
- Boogaerts M, Coiffier B, Kainz C, Epoetin Beta QOL Working Group (2003) Impact of epoetin beta on quality of life in patients with malignant disease. Br J Cancer 88: 988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JJ, Salas M, Ward A, Goss G (2001) Anemia as an independent prognostic factor for survival in patients with cancer: a systematic, quantitative review. Cancer 91: 2214–2221 [PubMed] [Google Scholar]
- Cazzola M, Messinger D, Battistel V, Bron D, Cimino R, Enller-Ziegier L, Essers U, Greil R, Grossi A, Jager G, Le Mevel A, Najman A, Silingardi V, Spriano M, van Hoof A, Ehmer B (1995) Recombinant human erythropoietin in the anaemia associated with multiple myeloma or non-Hodgkin's lymphoma: dose finding and identification of predictors of response. Blood 86: 4446–4453 [PubMed] [Google Scholar]
- Crawford J, Cella D, Cleeland CS, Cremieux PY, Demetri GD, Sarokhan BJ, Slavin MB, Glaspy JA (2002) Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 95: 888–895 [DOI] [PubMed] [Google Scholar]
- Dunst J (2004) Erythropoietin and radiotherapy: a dangerous combination ? Strahlenther Onkol 180: 133–135 [PubMed] [Google Scholar]
- Glaser CM, Millesi W, Kornek GV, Long S, Schull B, Watzinger F, Selzer E, Lavey RS (2001) Impact of hemoglobin level and use of recombinant erythropoietin on efficacy of preoperative chemoradiation therapy for squamous cell carcinoma of the oral cavity and oropharynx. Int J Radiat Oncol Biol Phys 50: 705–715 [DOI] [PubMed] [Google Scholar]
- Henke M, Laszig R, Rube C, Schafer U, Hasse KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, Frommhold H (2003) Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled, trial. Lancet 362: 1255–1260 [DOI] [PubMed] [Google Scholar]
- Kettelhack C, Hones C, Messinger D, Schlag PM (1998) Randomized multicenter trial of the influence of recombinant human erythropoietin on intraoperative and postoperative transfusion need in anaemic patients undergoing right hemicolectomy for carcinoma. Br J Surg 85: 63–67 [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B, Mahmud S (2004) Erythropoietin to treat anaemia in patients with head and neck cancer [letter]. Lancet 363: 80. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandi S, Makhson A, Roth A, Dodwell D, Basalga J, Biakhov M, Valuckas K, Voznui E, Lui X, Vercammen E (2005) Maintaining normal hemoglobin levels with epoetin alfa in mainly non-anaemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 23: 5960–5972 [DOI] [PubMed] [Google Scholar]
- Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapaport B, Epoetin Alfa Study Group (2001) Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol 19: 2865–2874 [DOI] [PubMed] [Google Scholar]
- Ludwig H, van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, Kosmidis P, Krzakowski M, Nortier J, Olmi P, Schneider M, Schrijvers D (2004) The European Cancer Anemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anemia in cancer patients. Eur J Cancer 40: 2293–2306 [DOI] [PubMed] [Google Scholar]
- Mittelman M, Neumann D, Peled A, Kanter P, Haren-Ghera N (2001) Erythropoietin induces tumour progression and antitumour immune responses in murine myeloma models. Proc Natl Acad Sci USA 98: 5181–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhoff C, Neri B, Amadori D, Petry KU, Gamucci T, Rebmann U, Nowrousian MR, Voigtmann R, Monfardini S, Armand JP, Herrman R, Netter-Pinon J, Tubiana-Mathiew N, Zwierzina H (1998) Recombinant human erythropoietin in the treatment of chemotherapy-induced anemia and prevention of transfusion requirement associated with solid tumors: a randomized, controlled study. Ann Oncol 9: 255–260 [DOI] [PubMed] [Google Scholar]
- Österborg A, Boogaerts MA, Cimino R, Essers U, Holowiecki J, Juliusson G, Jager G, Najmen A, Peest D (1996) Recombinant human erythropoietin in transfusion-dependent anaemic patients with multiple myeloma and non-Hodgkin's lymphoma–a randomized multicenter study. Blood 87: 2675–2682 [PubMed] [Google Scholar]
- Österborg A, Brandberg Y, Hedenus M (2005) Impact of epoetin-beta on survival of patients with lymphoproliferative malignancies: a long-term follow up of a large randomized study. Br J Haematol 129: 206–209 [DOI] [PubMed] [Google Scholar]
- Österborg A, Brandberg Y, Molostova V, Iosava G, Abdulkdyrov K, Hedenus M, Messinger D, Epoetin Beta Hematology Study Group (2002) Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 20: 2486–2494 [DOI] [PubMed] [Google Scholar]
- Overgaard J, Hoff C, Sand Hansen H, Specht L, Overgaard M, Grau C, Andersen E, Johansen J, Anderson L, Evensen J (2007) Randomized study of the importance of novel erythropoiesis stimulating protein (Aranesp®) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC) – the Danish Head and Neck Cancer Group DAHANCA 10. Eur J Cancer Supplements, 5(6): 7 [Google Scholar]
- Rau B, Schlag PM, Willeke F, Herforth C, Stephen P, Frake W (1998) Increased autologous blood donation in rectal cancer by recombinant human erythropoietin (rhEPO). Eur J Cancer 34: 992–998 [DOI] [PubMed] [Google Scholar]
- Smith Jr RE, Aapro MS, Ludwig H, Pintér T, Smakal M, Ciuleanu TE, Chen L, Lillie T, Glaspy JA (2008) Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 26(7): 1040–1050 [DOI] [PubMed] [Google Scholar]
- Strauss HG, Haensgen G, Dunst J, Hayward CR, Burger HU, Scherhag A, Koelbl H (2008) Effects of anemia correction with epoetin beta in patients receiving radiochemotherapy for advanced cervical cancer. Int J Gynecol Cancer 18(3): 515–524 [DOI] [PubMed] [Google Scholar]
- Stuben G, Pottgen C, Knuhmann K, Schmidt K, Stusche M, Thews O, Vaupel P (2003) Erythropoietin restores the anemia-induced reduction in radiosensitivity of experimental human tumors in nude mice. Int J radiat Oncol Biol Phy 55: 1358–1362 [DOI] [PubMed] [Google Scholar]
- ten Bokkel Huinink WW, de Swart CA, van Toorn DW, Morack G, Breed WP, Hillen HF, van der Haeven JJ, Reeds NS, Fairlamb DJ, Chan SY, Godfrey KA, Kristenson GB, van Tinteren H, Ehmer B (1998) Controlled, multicentre study of the influence of subcutaneous recombinant human erythropoietin on anaemia and transfusion dependency in patients with ovarian carcinoma treated with platinum-based chemotherapy. Med Oncol 15: 174–182 [DOI] [PubMed] [Google Scholar]
- Thews O, Kelleher DK, Vaupel P (2001) Erythropoietin restores the anemia-induced reduction in cyclophosphamide cytotoxicity in rat tumors. Cancer Res 61: 1358–1361 [PubMed] [Google Scholar]
- Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, Siena S, Gatkey J, Tomita D, Colowick AB, Musil J, Aranesp 980297 Study Group (2002) Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 94: 1211–1220 [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A (2004) Erythropoietin to treat anaemia in patients with head and neck cancer [letter]. Lancet 363: 992. [DOI] [PubMed] [Google Scholar]
- Wilson J, Yao GL, Raffery J, Bohlius J, Brunskill S, Sandercock J, Bayliss S, Moss P, Stanworth S, Hyde C (2007) A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess 11: 1–202 [DOI] [PubMed] [Google Scholar]
- Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L, Gagnon B, Okawara GS, Levine MN (2007) Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 25(9): 1021–1023 [DOI] [PubMed] [Google Scholar]