Abstract
The tight junction (TJ) proteins claudin-3 and claudin-4 have been reported to be differentially expressed in uterine serous papillary carcinoma (USPC), a rare form of endometrial cancer characterized by a particularly high recurrence rate and poor prognosis. Preclinical experiments suggest that increased expression of both TJ proteins may in part mediate the biologically aggressive phenotype of USPC. Our aim was to determine claudin-3 and claudin-4 expression in a large cohort of surgically staged patients with USPC and clear cell endometrial cancer (n=137), and to compare the expression pattern and prognostic relevance of both claudins with that seen in patients with endometrioid endometrial cancer (n=150). The rate of claudin-3 and claudin-4 expression was significantly higher in USPC and clear cell endometrial cancer compared to endometrioid endometrial cancer (claudin-3: 78% and 61% versus 38%, p <.0001; claudin-4: 56% and 44% versus 9%, p <.0001). Furthermore, expression of both tight junction proteins was significantly associated with poor clinical outcome (claudin-3, DFS: Risk ratio (RR) 1.70, p=.0087, OS RR 1.62, p=.0247; claudin-4, DFS RR 2.66, p<0.0001, and OS RR 2.50, p<0.0001). However, claudin-3 and claudin-4 expression did not maintain prognostic independence in multivariate analyses, as their expression was tightly associated with more advanced disease stages (p <.0001 for both), and higher nuclear grade (p <.0001 for both). These clinical observations confirm the hypothesis based on preclinical evidence that increased expression of claudin-3 and claudin-4 may contribute to the aggressive phenotype of endometrial cancer of serous papillary or clear cell histology and suggest their potential utility as diagnostic biomarkers and possible targets for therapeutic intervention.
Keywords: Uterine serous papillary endometrial cancer, Claudin-3, Claudin-4, lapatinib, endometrium
Introduction
The acquisition of a cancerous phenotype by epithelial cells involves the disruption of intercellular adhesions that are maintained by adherens junctions (AJs) and tight junctions (TJs). The claudins comprise a multigene family of membrane proteins, which play a major role in TJ formation. The reorganization of the E-cadherin/b-catenin complex in AJs during cell transformation is widely recognized. In contrast, the implications of TJs in this process are less well understood. Claudin-3 and claudin-4 have been reported to be expressed at high levels in multiple cancers, such as ovarian (Hough 2000), breast (Soini 2004), prostate (Long 2001), and pancreatic (Michl 2003) cancers. Both claudin-3 and claudin-4 have also recently been reported to be highly differentially expressed in uterine serous papillary carcinoma (USPC), an aggressive form of endometrial cancer characterized by a high recurrence rate and a poor prognosis (Santin 2005).
Recent preclinical experiments suggest that claudin-3 and claudin-4 may be implicated in tumor invasion and metastasis (Agarwal 2005). Engineered expression of claudin-3 and claudin-4 in cultured human ovarian surface epithelial (HOSE) cells rendered a more invasive cell phenotype, while blocking claudin-3 and claudin-4 expression with small interfering RNA actually reduced invasiveness (Agarwal 2005). Moreover, both TJ proteins are currently being investigated as potential therapeutic targets. Claudin-3 and claudin-4 are receptors for the clostridium perfringens enterotoxin (CPE) (Katahira 1997). CPE is a peptide of 35 kDa, which, upon binding to its receptors, causes cytolysis through its effects on membrane permeability. Recent studies demonstrate that low doses of CPE induce a classic apoptotic pathway involving mitochondrial membrane depolarization, cytochrom release, and caspase 3/7 activation (McClane 2004). High expression of claudin-3 and claudin-4 in USPC may thus represent a unique opportunity for innovative therapy using CPE.
Earlier studies demonstrated that claudin-3 and claudin-4 mRNA expression was frequently up-regulated in primary USPC cultures compared to normal endometrioid endometrial cancer cell cultures (Santin 2005, Santin 2007). Increased expression of both TJ molecules was confirmed at the protein level by IHC on formalin-fixed tumor tissue from which the primary cultures were obtained (Santin 2005). The number of samples in these studies, however, were limited and associations with patient and disease characteristics or clinical outcome were not investigated. Currently it is unclear how specific the expression of claudin-3 or claudin-4 is for USPC as a recent study revealed a high rate of claudin-3 and claudin-4 expression also in endometrioid endometrial cancer (Pan 2007).
Clear-cell endometrial cancer, like uterine papillary serous cancer, is also a rare subtype of endometrial cancer that is similarly characterized by a high recurrence rate and poor prognosis (Acharya 2005). Both of these non-endometrioid histological subtypes are classified as type II endometrial cancer for which hormonal risk factors have not been identified. In contrast type I endometrioid endometrial cancer has been associated with unopposed estrogen exposure and is often preceded by premalignant disease (Hecht 2006).
The primary objective of our Study was to determine the expression pattern of claudin-3, and claudin-4 in a large cohort of surgically staged patients with USPC or clear cell endometrial cancer (type II endometrial cancer) and compare the expression with that seen in endometrioid endometrial cancer. To better understand the clinical relevance of both claudin-3 and claudin-4 expression across these different histological subtypes we furthermore studied their association with other clinicopathologic variables as well as clinical outcome.
Material and Methods
Clinical cohorts and specimens
Upon approval from the Institutional Review Board at Mayo Clinic, we identified 137 patients from our data base that underwent surgery for type II endometrial cancer at Mayo Clinic between 5/1984 − 12/2004. Of these, 112 patients were diagnosed with USPC and 25 patients with clear cell endometrial cancer. Next, we randomly selected 150 patients who underwent surgery for endometrioid endometrial cancer during the same time period. Of the 287 patients included in this study, 279 patients (97%) had archived paraffin embedded tissue available for analysis of claudin-3 and claudin-4 expression. Tissue microarrays were created for each histological subtype. All patients had a hysterectomy and removal of existing adnexal structures performed and no other malignancy was diagnosed within 5 years before or after the diagnosis of endometrial cancer. Staging was defined according to the International Federation of Obstetricians and Gynecologists (FIGO) surgical staging system. For patients treated before 1988, stage was determined retrospectively on the basis of the surgical and pathologic assessments. The histological classification was according to the World Health Organization classification. Architectural grading was based on the degree of glandular differentiation in accordance with the FIGO guidelines. All surgical procedures were the responsibility of a gynecologic oncologist. Lymphadenectomy was performed in patients considered by the surgeon to be at risk for lymph node metastasis, according to the histologic grade of the tumor and the depth of myometrial invasion as determined by an intraoperative analysis of frozen tissue sections. Postoperative adjuvant radiotherapy consisted of external pelvic, paraaortic, or abdominal irradiation or vaginal brachytherapy or a combination of these.
TMA Construction and Immunohistochemistry
All hematoxylin- and eosin-stained slides of the tumors were reviewed by a gynecologic pathologist (G.A.K.) to confirm the original diagnosis of pure uterine serous papillary cancer, clear cell cancer, or endometrioid endometrial cancer. TMAs were constructed as described previously (Kallioniemi, 2001). Briefly, tissue cores (1.0 mm in diameter) were taken from spatially separate areas in a single donor block from each case using a tissue microarrayer (Beecher Instruments, Silver Spring, MD, USA). Cores were precisely arrayed into a recipient paraffin block at defined coordinates. The H&E-stained sections from donor and recipient paraffin blocks were used to confirm the area of tumor from which cores were retrieved. For the endometrioid endometrial cancer TMA one tissue core and for the USPC and clear cell TMA three tissue cores were arrayed per specimen. Claudin-3 and Claudin-4 Immunohistochemistry was performed by P.M. and has been described previously (Rangel 2003). Briefly, five-μm-thick sections were cut from the respective arrays, deparaffinized, and dehydrated. Immunohistochemical staining for claudin-3 and claudin-4 was performed using a streptavidin peroxidase procedure. Primary rabbit polyclonal antibodies against claudin-3 and claudin-4 were kindly provided by Drs. M. Furuse and S. Tsukita (Kyoto University, Kyoto, Japan). Antigen-bound primary antibody was detected using standard avidin-biotin immunoperoxidase complex (Dako Corp., Carpinteria, CA). Negative controls, in which the primary antibodies were absent, were processed in parallel, and no positive staining was observed. A cytotechnologist and imaging specialist in the Mayo Clinic Tissue Acquisition and Cellular/Molecular Analysis facility (D.R.) scanned each slide using a Slide Scanner (Bacus Laboratories, Inc.). The BLISS system is capable of digitally capturing images at 480 × 752 pixel resolution and at × 40 magnification. The entire slide is composed of multiple tiles to create a mosaic or composite picture. Cases were classified as follows regarding the intensity of protein expression (Negative: no or weak immunostaining present; positive: intense immunostaining present). We selected this dichotomized indicator variable as the primary analysis variable for claudin-3 and claudin-4 expression.
Statistical analysis
Associations between categorical variables were analyzed by the χ2 statistic. The prognostic significance of claudin-3, claudin-4, and other clinical/pathological variables was determined using a univariate Cox model and the log rank statistic. Prognostic independence was analyzed using a multivariate Cox regression model. Statistical calculations were performed using SPSS (SPSS, Inc., Chicago, IL) or SAS (SAS, Inc., Cary, NC) software. All of the tests of statistical significance were two-sided. Differences were considered statistically significant at P < 0.05.
Results
The current study is an observational study in which we compared the pattern of claudin-3 and claudin-4 expression between a consecutive series of 137 non-endometrioid endometrial cancers (112 USPC and 25 clear cell type histology) and a representative group of 150 endometrioid endometrial cancers. Tissue microarrays were constructed from tumor specimens for each histological subtype (Uterine serous papillary, clear cell, and endometrioid type histology) and claudin-3 and claudin-4 expression was assessed using immunohistochemistry. Uterine serous papillary carcinoma showed the highest rates of both claudin-3 and claudin-4 expression (78% and 56%, respectively; Table 1). Clear cell endometrial cancers showed lower but not significantly different rates of claudin-3 and claudin-4 expression (61% and 44%; P = .104 and P = .296, respectively, Table 1). In contrast, endometrioid endometrial cancers demonstrated significantly lower rates of claudin-3 and claudin-4 expression (38% and 9%; P ≤ .0001 and P ≤ .0001, respectively, Table 1), and there was a significant increase in claudin-4 expression from grade 1 to grade 3 endometrioid cancers (from 2% to 18%, P = 0.026, Table 2), but not for claudin-3 expression (40% and 39%, P = 0.963; Table 2).
Table 1.
Patient and disease characteristics of endometrial cancer patients with endometrioid, uterine serous papillary (USPC), and clear cell type histology.
| Endometrioid | Serous Papillary | Clear Cell | ||||
|---|---|---|---|---|---|---|
| No. | Valid % | No. | Valid % | No. | Valid % | |
| 150 | 112 | 25 | ||||
| Median Age (Range) | 68 (38 − 90) | 69 (47 − 93) | 66 (41 − 86) | |||
| Median follow up | 64 (0.3 − 199) | 20 (0.1 − 162) | 34 (0.2 − 180) | |||
| Surgery dates (Range) | 5/84 − 3/04 | 2/84 − 12/04 | 3/88 − 5/04 | |||
| Stage | ||||||
| I | 102 | (68) | 32 | (29) | 12 | (48) |
| II | 9 | (6) | 0 | (0) | 0 | (0) |
| III | 24 | (16) | 21 | (19) | 7 | (28) |
| IV | 15 | (10) | 59 | (53) | 6 | (24) |
| Grade | ||||||
| 1 | 52 | (35) | 1 | (1) | 2 | (8) |
| 2 | 58 | (39) | 1 | (1) | 3 | (12) |
| 3 | 40 | (27) | 93 | (98) | 20 | (80) |
| Claudin-3 | ||||||
| Positive | 53/138 | (38) | 69/89 | (78) | 14/23 | (61) |
| Claudin-4 | ||||||
| Positive | 12/140 | (9) | 49/88 | (56) | 10/23 | (44) |
Table 2.
Rate of claudin-3 and claudin-4 expression (absent/weak vs. high) and disease characteristics including type of histology, FIGO stage, and grade.
| Patients | Claudin-3 Positive IHC | Claudin-4 Positive IHC | ||||
|---|---|---|---|---|---|---|
| No. | Valid % | No. | Valid % | No. | Valid % | |
| Histology* | ||||||
| Endometrioid grade 1 | 52 | (18) | 19/48 | (40) | 1/47 | (2) |
| Endometrioid grade 2 | 58 | (20) | 20/54 | (37) | 4/51 | (7) |
| Endometrioid grade 3 | 40 | (14) | 14/36 | (39) | 7/31 | (18) |
| Clear cell | 25 | (9) | 14/23 | (61) | 10/23 | (44) |
| Serous papillary | 112 | (39) | 69/89 | (78) | 49/88 | (56) |
| FIGO Stage** | ||||||
| I | 146 | (51) | 55/123 | (45) | 21/125 | (17) |
| II | 9 | (3) | 4/8 | (50) | 0/8 | (0) |
| III | 52 | (18) | 26/48 | (54) | 14/49 | (29) |
| IV | 80 | (28) | 51/71 | (72) | 36/69 | (52) |
| Grade*** | ||||||
| 1 | 55 | (20) | 20/50 | (40) | 2/50 | (4) |
| 2 | 62 | (23) | 23/58 | (40) | 5/59 | (9) |
| 3 | 153 | (57) | 81/128 | (63) | 56/128 | (44) |
Unknown data: grade (n = 17), claudin-3 (n = 37), claudin-4 (n = 36).
Chi square test: P < 0.001 for both Claudin-3 and Claudin-4.
Chi square test: P = 0.004 for Claudin-3, and P < 0.001 for Claudin-4.
Chi square test: P = 0.002 for Claudin-3, and P < 0.001 for Claudin-4.
When analyzing all 287 cases there was a significant increase in the rate of expression for both claudins from stage I to stage IV (for claudin-3 from 45% to 72%; P = 0.004, and for claudin-4 from 17% to 52%; P ≤ 0.001; Table 2). Similarly, there was also a significant increase in the rate of expression for both claudins from grade 1 to grade 3 endometrial cancer (for claudin-3 from 40% to 63%; P = 0.002 and for claudin-4 from 4% to 44%; P ≤ 0.001, Table 2).
Univariate analyses showed that a number of standard histopathological features were significantly associated with poor DFS and OS (Table 3, Figure 5). These features included high-grade (G3), non-endometrioid histology, and advanced stage (stage III and IV) for DFS and OS, and additionally age for OS (Table 3). High claudin-3 expression was associated with significantly worse DFS (Risk ratio (RR) 1.70, p=0.0087) and OS (RR 1.62, p=0.0247) when compared to absent or weak expression. Similarly, high claudin-4 expression was also associated with significantly worse DFS (RR 2.66, p<0.0001) and OS (RR 2.50, p<0.0001) when compared to absent or weak expression. Importantly, however, both claudin-3 and claudin-4 expression did not maintain independent prognostic relevance in multivariate analysis (Table 3). Only grade and tumor stage were selected as significant independent prognostic markers for DFS and grade, tumor stage, as well as age for OS (Table 3).
Table 3.
Univariate and multivariate analyses for all patients with all histological subtypes (N = 287) for disease-free and overall survival.
| DFS | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| |
Risk ratio |
(95% CI), |
p-value |
Risk ratio |
(95% CI), |
p-value |
| Age |
1.02 |
(1.00, 1.04), |
p=0.0929 |
NS |
|
|
| Stage 3−4 vs. 1−2 |
11.4 |
(6.92, 18.8), |
p<0.0001 |
6.82 |
(4.02, 11.6), |
p<0.0001 |
| Histology 1 |
1.0 |
|
|
|
|
|
| 4 |
4.26 |
(2.82, 6.44), |
p<0.0001 |
NS |
|
|
| 5 |
2.27 |
(1.18, 4.38), |
p=0.0145 |
NS |
|
|
| Grade 3 vs. 1−2 |
7.32 |
(4.15, 12.9), |
p<0.0001 |
3.66 |
(2.02, 6.65), |
p<0.0001 |
| Claudin3 pos. vs. neg. |
1.70 |
(1.14, 2.54), |
p=0.0087 |
NS |
|
|
| Claudin4 pos. vs. neg | 2.66 | (1.80, 3.95), | p<0.0001 | NS | ||
| OS | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| |
Risk ratio |
(95% CI), |
p-value |
Risk ratio |
(95% CI), |
p-value |
| Age |
1.03 |
(1.01, 1.05), |
p=0.0123 |
1.03 |
(1.01, 1.05), |
p=0.0068 |
| Stage 3−4 vs. 1−2 |
12.1 |
(6.98, 21.1), |
p<0.0001 |
7.33 |
(4.06, 13.2), |
p<0.0001 |
| Histology 1 |
1.00 |
|
|
|
|
|
| 4 |
4.26 |
(2.75, 6.61), |
p<0.0001 |
NS |
|
|
| 5 |
2.13 |
(1.04, 4.34), |
p=0.0386 |
NS |
|
|
| Grade 3 vs. 1−2 |
8.05 |
(4.27, 15.2), |
p<0.0001 |
3.58 |
(1.83, 7.02), |
p=0.0002 |
| Claudin3 pos. vs. neg. |
1.62 |
(1.06, 2.47), |
p=0.0247 |
NS |
|
|
| Claudin4 pos. vs. neg. | 2.50 | (1.64, 3.79), | p<0.0001 | NS | ||
NS: Not significant
Next, we studied the clinical significance of claudin-3 and claudin-4 expression more closely among the 137 patients with USPC or clear cell histology. Of the 112 patients diagnosed with USPC 80 (71%) presented with extra-uterine disease at the time of primary surgery (Stage FIGO III and IV, Table 1). Similarly, of the 25 patients diagnosed with clear cell cancer 13 (52%) presented with extra-uterine disease at the time of primary surgery. In this cohort of type II endometrial cancer both disease stage and type of histology were significant discriminators of clinical outcome. As expected, patients with FIGO stage III or IV disease had a significantly worse prognosis compared to those with earlier disease stages (FIGO III: DFS, RR 3.25, p=0.0045; OS, RR 3.19, p=0.0112; FIGO IV: DFS, RR 11.0, p<0.0001; OS, RR 10.4, p<0.0001, Table 5).
Patients with clear cell histology did have a significantly better DFS (RR 0.53, p=0.0431) and OS (RR 0.49, p=0.0362) when compared to USPC (Table 4 and Figure 3). Neither grade nor claudin-3 or claudin-4 expression was a significant prognostic factor for DFS or OS in the present cohort of 137 type II endometrial cancer patients (Table 4). In multivariate analysis stage remained an independent prognostic marker for DFS as well as age and stage for OS in this subset of type II endometrial cancer (Table 4).
Table 4.
Univariate and multivariate analyses for patients with type II endometrial cancer (uterine serous papillary and clear cell type histology (N = 137) for disease-free and overall survival.
| DFS | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| |
Risk ratio |
(95% CI), |
p-value |
Risk ratio |
(95% CI) |
p-value |
| Age |
1.02 |
(1.00, 1.05) |
p=0.0761 |
NS |
|
|
| Stage 1 | 1.0 | 1.0 | ||||
| 3 | 3.25 | (1.44, 7.33) | p=0.0045 | 3.25 | (1.44, 7.33) | p=0.0045 |
| 4 |
11.0 |
(5.45, 22.1) |
p<0.0001 |
11.0 |
(5.45, 22.1) |
p<0.0001 |
| Histology 5 vs. 4 |
0.53 |
(0.29, 0.98) |
p=0.0431 |
NS |
|
|
| Grade 3 vs. 1−2 |
3.94 |
(0.96, 16.1) |
p=0.0569 |
NS |
|
|
| Claudin3 pos. vs. neg. |
1.19 |
(0.71, 2.02) |
p=0.5122 |
NS |
|
|
| Claudin4 pos. vs. neg | 1.09 | (0.68, 1.75) | p=0.7163 | NS | ||
| OS | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| |
Risk ratio |
(95% CI), |
p-value |
Risk ratio |
(95% CI) |
p-value |
| Age |
1.04 |
(1.01, 1.06) |
p=0.0073 |
1.06 |
(1.03, 1.09) |
p<0.0001 |
| Stage 1 | 1.0 | 1.0 | ||||
| 3 | 3.19 | (1.30, 7.82) | p=0.0112 | 2.67 | (1.07, 6.63) | p=0.0349 |
| 4 |
10.4 |
(4.86, 22.1) |
p<0.0001 |
13.1 |
(5.96, 28.6) |
p<0.0001 |
| Histology 5 vs. 4 |
0.49 |
(0.25, 0.96) |
p=0.0362 |
NS |
|
|
| Grade 3 vs. 1−2 |
3.21 |
(0.78, 13.2) |
p=0.1061 |
NS |
|
|
| Claudin3 pos. vs. neg. |
0.94 |
(0.54, 1.61) |
p=0.8150 |
NS |
|
|
| Claudin4 pos. vs. neg. | 0.91 | (0.55, 1.49) | p=0.7010 | NS | ||
NS: Not significant
Figure 3.
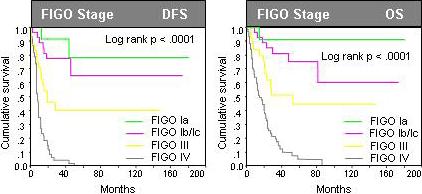
Prognostic relevance of disease stage in univariate analysis among 137 patients with type II endometrial cancer (Uterine serous papillary and clear cell endometrial cancer). The current study cohort did not include FIGO stage II endometrial cancer patients.
Discussion
Uterine serous papillary cancer (USPC) and endometrial clear cell cancer represent rare but highly aggressive variants of endometrial cancer. Extra-uterine disease is often found in these patients even in such without myometrial invasion (Goff 1994 and Bristow 2001). In the current study which involves a consecutive series of 137 patients with type II endometrial cancer two out of three patients diagnosed with USPC and every second diagnosed with clear cell cancer presented with extra-uterine disease at the time of primary surgery. These findings are consistent with earlier reports in which USPC and clear cell endometrial cancer have demonstrated distinct clinical characteristics with a high propensity of early extra-uterine spreading (Abeler 1991, Slomovitz 2003). Moreover, their clinical features are paralleled by genetic distinctions, in that type II cancers carry mutations of independent sets of genes compared to type I endometrial cancers. Although microsatellite instability and mutations in PTEN have been commonly associated with endometrioid carcinoma, these changes are rarely seen in USPC or clear cell cancer. In contrast, p53 mutations, which are not usually seen in endometrioid cancers, have been identified in most uterine papillary serous carcinomas and clear cell cancers (Hecht 2006). Here we show an additional significant molecular distinction between type I and type II endometrial cancer in that USPC and clear cell cancers demonstrated a significantly higher expression of two TJ proteins that are critical for maintaining permeability properties and cell polarity of epithelial cells. Epithelial cells display two particular phenotypic characteristics. First the formation of layers integrated by polygonal cells that are closely joined by TJs, and secondly an apical-basolateral polarization (Cereijido et al., 2004). During development and in cancer progression, a phenotype transition from epithelial to mesenchymal (EMT) takes place (Thiery 2006). In contrast to epithelial cells mesenchymal cells do not form organized cell layers, are not polarized, contact the neighboring cells only focally and are not associated with the basal lamina (Hay 1995). They display a spindle-like morphology and tend to be highly mobile (Hay 1995). In carcinomas, the initial step of metastasic dissemination includes the detachment of epithelial cells from such an cell integrated layer. Alterations in the expression of claudins are like to play an important role in this process.
Originally, gene expression studies using oligonucleotide microarrays of primary USPC cell cultures demonstrated that claudin-3 and claudin-4 were among the highest up regulated transcripts in USPC when compared to normal endometrial epithelial cells (Santin 2005). Here we confirm and extend the differential expression of claudin-3 and claudin-4 expression in USPC using IHC in a large cohort of surgically staged endometrial cancer patients of diverse histology. A closer review of the original gene expression data, however, demonstrates that both claudin-3 and claudin-4 were selected from a group of 529 genes showing >5-fold change in differential expression between USPC and normal endometrial epithelial cells (Santin 2005). High differential expression in serous papillary cells compared to normal endometrial epithelial cells, however, per se does not necessarily mean both genes are pathophysiologically important for tumor formation or progression of this aggressive endometrial cancer subtype as both genes may not be casually implicated in the disease process. However, the present clinical findings confirm recent preclinical experiments which suggest that alterations in claudin-3 and claudin-4 expression may be contributing to tumor formation and progression. Engineered expression of claudin-3 and claudin-4 in human ovarian surface epithelial (HOSE) cells increased cell invasion and motility as measured by Boyden chamber assays and wound-healing experiments (Agarwal 2005). Conversely, small interfering RNA (siRNA)–mediated knockdown of claudin-3 and claudin-4 expression in ovarian cancer cell lines reduced invasion (Agarwal 2005). Claudin expression also increased cell survival in HOSE cells but did not significantly affect cell proliferation (Agarwal 2005). Moreover, the claudin-expressing ovarian epithelial cells were found to have increased matrix metalloproteinase-2 (MMP-2) activity indicating that claudin-mediated increased invasion might be mediated through the activation of MMP proteins (Agarwal 2005). Additional functional studies will be necessary to further clarify the role that claudin-3 and claudin-4 may play in tumorigenesis and metastasis. Such studies are particularly important to help evaluate their role as therapeutic targets because the success of a pharmacological intervention may depend on the functional relevance of claudin-3 and claudin-4 expression for endometrial cancer development and progression.
The claudins are a group of over 20 proteins. As such, evidence is mounting that the expression and prognostic significance of individual claudin proteins is variable. Earlier studies have found low claudin-1 expression but high claudin-3 and claudin-4 expression in breast cancer (Tokes 2005). Claudin-7 has also been found to be down-regulated in invasive breast cancer (Kominsky 2003). A recent study in prostate cancer has shown that both high expression of claudin-3 and claudin-4 as well as low expression of claudin-1 and claudin-7 were associated with adverse histopathologic and clinical variables (Sheehan 2007)
Collectively it is the accepted idea that tumorigenesis is accompanied by a disruption and dysregulation of tight junctions, however, further research is necessary to better understand the biologic function of each member of the TJ protein family and help determine their clinical utility.
Differential expression of both claudin-3 and claudin-4 in type II endometrial cancer or other malignancies may be very useful for the development of toxin-directed therapies. Endometrial cancer cells expressing claudin-3 and claudin-4 have indeed been shown to be sensitive to CPE-mediated cytolysis (Santin 2007). Specificity for this approach was suggested as cancer cells lacking claudin-3 and claudin-4 were unaffected by CPE treatment (Long 2001). A recent study by Santin et al. demonstrated that In vivo, intratumoral injections of well-tolerated doses of CPE in large subcutaneous USPC xenografts led to tumor cell necrosis in treated animals. Furthermore sublethal intraperitoneal injections of CPE had a significant inhibitory effect on tumor progression, with extended survival of animals harboring chemotherapy-resistant intra-abdominal USPC carcinomatosis (Santin 2007). However, claudin-3 and/or claudin-4 are also expressed in several normal human tissues, including the gut, the lungs, and the kidneys (Morin 2005), and this expression pattern may represent a problem in the use of CPE for systemic cancer therapy.
In summary, the present study provides evidence that claudin-3 and claudin-4 expression is associated with USPC and clear cell type histology. The expression of both TJ proteins was tightly associated with more advanced disease stages, and higher nuclear grade leading to an adverse clinical outcome. These clinical observations confirm the hypothesis based on preclinical evidence that increased expression of claudin-3 and claudin-4 may contribute to the aggressive phenotype of endometrial cancer of serous papillary or clear cell histology and suggest their potential utility as diagnostic biomarkers and possible targets for therapeutic intervention.
Figure 1.
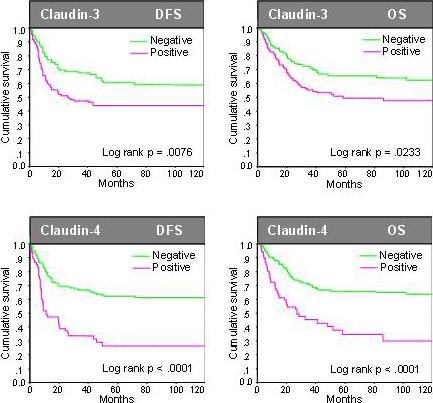
Prognostic relevance of claudin-3 and claudin-4 expression in univariate analysis among all 287 patients with endometrial cancer of diverse histology. Cases were classified as follows regarding the intensity of protein expression (Negative: no or weak immunostaining present; positive: intense immunostaining present). We selected this dichotomized indicator variable as the primary analysis variable for claudin-3 and claudin-4 expression.
Figure 2.
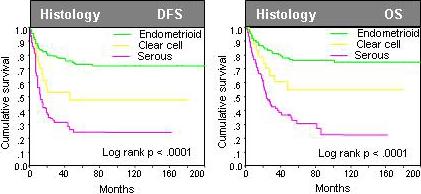
Prognostic relevance of the histology type in univariate analysis among all 287 patients with endometrial cancer of diverse histology.
References
- Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum. Mol. Genet. 2001;10:657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- Hough CD, Sherman-Baust CA, Pizer ES, et al. Largescale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–7. [PubMed] [Google Scholar]
- Rangel LBA, Agarwal R, D'Souza T, et al. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res. 2003;9:2567–75. [PubMed] [Google Scholar]
- Santin AD, Zhan F, Bellone S, et al. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer. 2004;112:14–25. doi: 10.1002/ijc.20408. [DOI] [PubMed] [Google Scholar]
- Agarwal R, D'Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65:7378–85. doi: 10.1158/0008-5472.CAN-05-1036. [DOI] [PubMed] [Google Scholar]
- Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. JBiol Chem. 1997;272:26652^8. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- McClane BA, Chakrabarti G. New insights into the cytotoxic mechanisms of Clostridium perfringens enterotoxin. Anaerobe. 2004;10(2):107–14. doi: 10.1016/j.anaerobe.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Soini Y. Claudins 2, 3, 4, and 5 in Paget's disease and breast carcinoma. Hum Pathol. 2004;35(12):1531–6. doi: 10.1016/j.humpath.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001;61(21):7878–81. [PubMed] [Google Scholar]
- Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Lohr M, Leder G, Iwamura T, Adler G, Gress TM. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63(19):6265–71. [PubMed] [Google Scholar]
- Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24(29):4783–91. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- Abeler VM, Kjorstad KE. Clear cell carcinoma of the endometrium: a histopathological and clinical study of 97 cases. Gynecol Oncol. 1991;40:207–17. doi: 10.1016/0090-8258(90)90279-t. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L. Cell adhesion, polarity, and epithelia in the dawn of metazoans. Physiol Rev. 2004;84:1229–62. doi: 10.1152/physrev.00001.2004. [DOI] [PubMed] [Google Scholar]
- Tokes AM, Kulka J, Paku S, Szik A, Paska C, Novak PK, Szilak L, Kiss A, Bogi K, Schaff Z. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res. 2005;7(2):R296–305. doi: 10.1186/bcr983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Karesen R, Axcrona U, Noren T, Sauer T. Expression pattern of adhesion molecules (E-cadherin, alpha-, beta-, gamma-catenin and claudin-7), their influence on survival in primary breast carcinoma, and their corresponding axillary lymph node metastasis. APMIS. 2007;5(1):52–65. doi: 10.1111/j.1600-0463.2007.apm_524.x. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Morin P. Claudin Proteins in Human Cancer: Promising New Targets for Diagnosis and Therapy. Cancer Res. 2005;65:21. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]


