Abstract
Background: Younger age at the onset of menopause and lower circulating levels of estrogen are risk factors for cardiovascular disease. Several studies have detected associations between variations in genes encoding estrogen receptors α (ESR1) and β (ESR2), and enzyme aromatase (CYP19A1), which regulates the estrogen to testosterone ratio, and cardiovascular phenotypes in the Framingham Heart Study. To explore potential mechanisms by which these gene variants may contribute to cardiovascular disease, we tested the hypothesis that the polymorphisms were associated with endogenous steroid hormone levels.
Methods: Multiple regression analysis was used to assess the relation between reported polymorphisms and total serum estradiol, testosterone, and dehydroepiandrosterone sulfate levels in 834 men and 687 women who attended the third and fourth Framingham Heart Study examination cycles.
Results: In men, significant associations were detected between CYP19A1 polymorphisms and estradiol and testosterone levels, and the estradiol to testosterone ratio (P ranges 0.0005–0.01). Specifically, carriers of common haplotype rs700518[G]-(TTTA)n [L]-rs726547[C] had higher estradiol levels (5% per copy; P = 0.0004), lower testosterone levels (17% per copy; P = 0.036), and a higher estradiol to testosterone ratio (24% per copy; P < 0.0001) compared with the rs700518[A]-(TTTA)n [S]-rs726547[C] carriers. In addition, postmenopausal carriers of the ESR2 (CA)n long allele and rs1256031 [C] allele had moderately higher estradiol levels (P ≤ 0.03). No significant associations with the ESR1 variants were detected.
Conclusions: Our findings suggest that variations in CYP19A1 correlate with steroid hormone levels in men. Knowledge that a specific carrier status may predispose to altered steroid hormone levels may lead to targeted intervention strategies to reduce health risks in genetically susceptible individuals.
In a cohort study of women and men, minor alleles of closely related aromatase gene polymorphisms are associated with higher serum estradiol, lower testosterone levels, and a higher estradiol/testosterone ratio in men.
Male sex and the age of onset of menopause in women are independent factors that significantly increase the risk of hypertension, ventricular hypertrophy, and cardiac events, suggesting an important role for sex hormones in the etiology of cardiovascular disease (CVD) (1). A recent study has reported a lower risk of CVD events in older men with higher serum estradiol levels (2). Longer lifetime exposure to ovarian estrogens has protected against ischemic stroke (3). Moreover, low levels of dehydroepiandrosterone sulfate (DHEAS), the most abundant steroid mainly produced in the human adrenal gland and converted into potent androgens and/or estrogens through a series of enzymatic reactions, have been related to a greater risk of CVD in some (4,5), but not in other, studies (2,6,7). However, a controversy exists regarding the CVD risk associated with serum testosterone concentrations (2,4,7).
Recently, genetic variations of the major proteins involved in steroid hormone conversion and receptor function have been described as significant contributors to cardiac disease susceptibility, attracting increased attention to genes implicated in estrogen metabolism. Specifically, numerous studies have found a significant association between polymorphisms in genes encoding estrogen receptors (ERs) α and β (ESR1 and ESR2, respectively), which are expressed in the cardiovascular system, and myocardial infarction (8,9), coronary artery disease (10,11,12), elevated blood pressure (13), altered lipoprotein levels (14,15), adiposity (16), and left ventricular mass (17,18,19). In addition, variation in the gene encoding aromatase cytochrome 450 (CYP19A1), the enzyme that catalyzes the conversions of testosterone to estradiol and defines the estradiol to testosterone ratio, has been associated with blood pressure (13) and abdominal obesity (20). However, despite these and other studies, the molecular mechanisms that explain the relationship of estrogen-related genes with CVD risk are unclear.
Therefore, given moderate heritability ranging around 57–63% for testosterone (21,22), 29–74% for DHEAS (23,24), and about 25% for estradiol (22), and significant associations reported of single nucleotide polymorphisms (SNPs) in ESR1, ESR2, and CYP19A1 with cardiovascular phenotypes in the Framingham Heart Study (FHS) (9,13,15,16,18,19), we hypothesized that these relationships are mediated through circulating steroid hormone levels. We tested whether polymorphisms in these genes correlate with serum DHEAS, testosterone, and estradiol levels, and the estradiol to testosterone ratio in the FHS participants. This information may help shed light on the pathophysiology of CVD and its risk factors.
Subjects and Methods
Study sample
Participants in this study included unrelated individuals from the FHS’s Offspring Cohort Study described in detail elsewhere (25). They attended periodic clinical examination 3 (1984–1987) and/or 4 (1987–1990), underwent medical and menopausal history, physical examination, and blood collection, including measurement of sex hormone levels, whereas a DNA sample and consent for genetic analysis were obtained at a later examination cycle (1996–1997). Subjects were excluded from the study if they were receiving hormone replacement therapy (n = 84) or had one ovary surgically removed (n = 45). The total sample included 1521 participants, of them 687 women. Women were considered postmenopausal if their periods stopped for 1 yr or more at examination, or both of their ovaries were surgically removed, and premenopausal otherwise, as defined by detailed self-report. For women whose menopausal status changed between the two examination cycles, only postmenopausal levels were used. Time since menopause was calculated by subtracting the reported age at onset of menopause from the chronological age at each examination.
Weight and height were measured at each examination. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. Smoking status was defined by the self-reported number of cigarettes smoked per day in the year preceding the examination; alcohol consumption was recorded from the participant’s report in ounces per week. Physical activity, determined by questionnaire, was represented as the weighted sum of the proportion of a typical day spent sleeping and performing sedentary, slight, moderate, or heavy physical activities (26). The following weights were used: sleep/rest, 1.0; sedentary, 1.1; light activity, 1.5; moderate activity, 2.4; and heavy activity, 5.0.
All subjects gave written informed consent. The FHS protocol is approved by the Boston University Medical Center Institutional Review Board.
Hormone assays
Steroid hormone levels were measured in serum samples using RIAs (Diagnostic Products Corp., Los Angeles, CA) with interassay coefficients of variation of 11% for total testosterone, 4% for total estradiol, and 11% for DHEAS. Testosterone was measured in men only.
SNP genotyping
Participants’ genomic DNA was extracted from peripheral blood leukocytes using standard methods. Genotyping for the individual SNPs in ESR1 (rs2077647, rs2234693, rs9340799, and rs1801132), ESR2 (rs1256031 and rs1256059), and CYP19A1 (rs700518 and rs726547) was performed as described previously (13). ESR1 (TA)n, ESR1 (CA)n, ESR2 (CA)n, and CYP19A1 (TTTA)n repeat polymorphisms were genotyped using restriction fragment length analyses (Table 1 and supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Genotyping was blinded to participant characteristics.
Table 1.
Polymorphism characteristics
| Gene | dbSNP rs no. | Location | Nucleotide substitution | MAF | P value for HWE |
|---|---|---|---|---|---|
| ESR1 | (TA)n | Promoter | (TA)n | 0.50a | 0.76 |
| 6q25.1 | rs2077647 | Exon 1 (Ser10Ser) | T/C | 0.45 | 0.10 |
| rs2234693 | Intron 1 | T/C | 0.45 | 0.70 | |
| rs9340799 | Intron 1 | A/G | 0.36 | 0.83 | |
| rs1801132 | Exon 4 (Pro325Pro) | C/G | 0.23 | 0.08 | |
| (CA)n | Intron 5 | (CA)n | 0.36a | 0.48 | |
| ESR2 | rs1256059 | Intron 2 | C/T | 0.44 | 0.77 |
| 14q23.2 | (CA)n | Intron 5 | (CA)n | 0.21a | 0.67 |
| rs1256031 | Intron 7 | T/C | 0.48 | 0.58 | |
| CYP19A1 | rs700518b | Exon 3 (Val80Val) | A/G | 0.47 | 0.01 |
| 15q21.1 | (TTTA)n | Intron 4 | (TTTA)n | 0.48a | 0.99 |
| rs726547 | Intron 4 | C/T | 0.05 | 0.78 |
dBSNP, The Single Nucleotide Polymorphism database (http://www.ncbi.nlm.nih.gov/(SNP/); MAF, minor allele frequency.
Calculated using median number of repeats.
In HWE in postmenopausal women.
Statistical analysis
Observed genotype frequencies were compared with those expected under Hardy-Weinberg equilibrium (HWE) using a χ2 test. Given multiple alleles observed for the repeat polymorphisms and their bimodal distribution, the genotype carrier status for each variant was coded using the median number of repeat sequence base pairs as a cutoff. Specifically, genotype LL was assigned if both alleles contained at least the median number of base pairs [≥176 for ESR1 (TA)n; ≥ 277 for ESR1 (CA)n; ≥ 162 for ESR2 (CA)n, and ≥ 298 for CYP19A1 (TTTA)n]; SS was assigned if both alleles were “short” [<176 for ESR1 (TA)n; < 277 for ESR1 (CA)n; < 162 for ESR2 (CA)n, and < 298 for CYP19A1 (TTTA)n], and LS if one allele was “long,” and another one was “short.”
To address skewed distribution of steroid hormone levels, logarithmic transformation was applied before analysis, and all results are reported as geometrical means. To account for significant sex-specific differences in steroid hormone levels, each variable was analyzed separately by gender and menopausal status. Multivariate linear regression analyses were performed to assess genetic associations with circulating estradiol and testosterone levels in men and postmenopausal women, and with DHEAS levels in men and premenopausal and postmenopausal women. All analyses were adjusted for age, weight, smoking status, alcohol consumption, and a number of years after the onset of menopause (in postmenopausal women). For individuals with steroid levels measured at both examinations, mean levels across the two examinations were used. In secondary analyses, risk factors were compared between genotype groups using ANOVA.
Pairwise linkage disequilibrium (LD) was evaluated using Lewontin’s D’ (27). Haplotypes were inferred by the expectation-maximization-algorithm. To account for allelic interaction, haplotypes were used as predictors in the regression models along with the aforementioned covariates.
The nominal threshold for statistical significance of all analyses was set at 0.05 and was not adjusted for multiple testing. Greater credibility was given to association results if a consistent trend was observed for SNPs in LD. All analyses were performed using SAS/STAT and SAS/Genetics software version 9.1 (SAS Institute Inc., Cary, NC).
Results
Nongenetic predictors of steroid hormone levels
The characteristics of the 834 male and 687 female unrelated eligible participants in the FHS Offspring cohort are shown in Table 2. Premenopausal women had the highest circulating estradiol levels, whereas postmenopausal women had the lowest estradiol and DHEAS levels. In multivariable-adjusted regression analyses, older age was a significant predictor of lower steroid hormone levels, except estradiol in men. Self-reported weekly alcohol intake was negatively correlated with DHEAS levels in both sexes and with estradiol in men, whereas smoking was associated with lower testosterone levels in men and DHEAS in premenopausal and postmenopausal women. Body weight was a better predictor of steroid hormone levels than BMI, and was positively correlated with estradiol and testosterone concentrations in men, but not women. The physical activity score was not found to be associated with DHEAS, estradiol, testosterone levels, and the estradiol to testosterone ratio in either men or premenopausal or postmenopausal women.
Table 2.
Participant characteristics by gender and menopausal status
| Traits | Mean ± sd
|
||
|---|---|---|---|
| Men (n = 834) | Premenopausal women (n = 347) | Postmenopausal women (n = 340) | |
| Age (yr) | 50.6 ± 9.7 | 42.6 ± 5.6 | 57.1 ± 6.4 |
| BMI (kg/m2) | 27.6 ± 3.7 | 25.2 ± 5.4 | 26.5 ± 5.4 |
| Weight (lb) | 188.8 ± 28.7 | 148.2 ± 32.9 | 150.4 ± 31.8 |
| Smoking (%) | 26 | 29 | 26 |
| Alcohol consumption (oz/wk) | 4.2 ± 5.0 | 1.9 ± 3.0 | 1.9 ± 2.8 |
| Physical activity (h/d) | |||
| Sleep | 7.3 ± 1.1 | 7.3 ± 1.1 | 7.3 ± 1.1 |
| Sedentary activity | 6.2 ± 3.1 | 6.4 ± 3.1 | 6.4 ± 3.1 |
| Slight activity | 5.9 ± 2.6 | 5.8 ± 2.6 | 5.7 ± 2.6 |
| Moderate activity | 3.5 ± 2.4 | 3.3 ± 2.4 | 3.3 ± 2.3 |
| Heavy activity | 1.1 ± 1.6 | 1.2 ± 1.8 | 1.3 ± 1.8 |
| Median (25th-75th percentile) | |||
| Testosterone (ng/ml)a | 5.6 (4.6–6.5) | ||
| Estradiol (pg/ml)a | 28.4 (21.9–37.3) | 10.3 (3.0–10.3) | |
| DHEAS (μg/dl)a | 206 (136–327) | 154 (106–220) | 103 (70–155) |
| Years since menopause | 10.2 ± 7.2 | ||
Measurements were available from both examinations for men (n = 606 for testosterone, n = 564 for estradiol, and n = 600 for DHEAS), premenopausal women (n = 533 for DHEAS), and postmenopausal women (n = 5 for estradiol and n = 254 for DHEAS).
Values are raw examination measurements or averages over the two examinations.
CYP19A1 association analysis
The genotype frequencies conformed to those expected by HWE, except CYP19A1 rs700518, which was in HWE in postmenopausal women, but not in men and premenopausal women (P = 0.01). This polymorphism was in HWE in our previous study that tested a slightly different subset of the FHS unrelated individuals (13). Despite additional exclusion criteria applied to this study (e.g. availability of steroid hormone measurements, hormone replacement therapy, or surgical removal of one ovary), these factors are unlikely to select for or against this genetic variant, especially in men. Therefore, we assumed a random selection bias and included CYP19A1 rs700518 in analysis.
In men, significant associations were detected between CYP19A1 rs726547 and estradiol, testosterone, and the estradiol to testosterone ratio (P ranges between 0.01 and 0.0005) (Table 3). CYP19A1 (TTTA)n repeat polymorphism as well as CYP19A1 rs700518 were associated with estradiol concentrations (P = 0.02 and 0.005, respectively) and the estradiol to testosterone ratio (P = 0.01 and 0.006, respectively). Specifically, carriers of the minor alleles of CYP19A1 rs700518 [A], (TTTA)n [L], and rs726547 [T] alleles had higher estradiol, lower testosterone levels, and a higher estradiol to testosterone ratio than their noncarrier counterparts (Table 3).
Table 3.
Adjusted circulating serum hormone levels in men by estrogen-related genotypes
| Gene | SNP | Genotype | No. | Estradiol
|
Testosterone
|
Estradiol to testosterone ratio
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Median (25th-75th percentile) | P value | Median (25th-75th percentile) | P value | Median (25th-75th percentile) | P value | ||||
| CYP19A1 | rs700518 | G/G | 210 | 26.73 (25.76–28.14) | 0.005 | 5.51 (5.14–5.86) | 0.60 | 4.86 (4.47–5.26) | 0.006 |
| G/A | 305 | 27.44 (26.29–28.83) | 5.35 (5.05–5.73) | 5.06 (4.74–5.60) | |||||
| A/A | 161 | 30.35 (29.42–31.65) | 5.26 (4.99–5.65) | 5.61 (5.33–5.96) | |||||
| (TTTA)n | S/S | 190 | 26.32 (25.47–27.55) | 0.02 | 5.45 (5.18–5.82) | 0.51 | 4.78 (4.48–5.18) | 0.01 | |
| S/L | 331 | 27.79 (26.82–29.10) | 5.31 (5.04–5.63) | 5.19 (4.87–5.61) | |||||
| L/L | 167 | 29.55 (28.62–30.60) | 5.26 (5.01–5.67) | 5.47 (5.19–5.78) | |||||
| rs726547 | C/C | 713 | 27.22 (26.33–28.57) | 0.03 | 5.40 (5.12–5.78) | 0.01 | 5.01 (4.67–5.42) | 0.0005 | |
| C/T | 85 | 29.89 (28.75–31.75) | 5.14 (4.79–5.42) | 5.87 (5.44–6.36) | |||||
| ESR1 | (TA)n | L/L | 164 | 27.45 (26.69–28.71) | 0.74 | 5.41 (5.16–5.73) | 0.41 | 5.05 (4.73–5.36) | 0.75 |
| L/S | 338 | 28.34 (27.54–29.43) | 5.34 (5.05–5.70) | 5.25 (4.95–5.72) | |||||
| S/S | 164 | 27.53 (26.81–28.46) | 5.28 (4.99–5.60) | 5.09 (4.79–5.45) | |||||
| rs2077647 | T/T | 224 | 27.57 (26.74–28.55) | 0.41 | 5.26 (4.97–5.59) | 0.22 | 5.16 (4.84–5.52) | 0.21 | |
| T/C | 408 | 28.18 (27.31–29.49) | 5.38 (5.06–5.75) | 5.20 (4.88–5.65) | |||||
| C/C | 159 | 26.70 (25.75–27.98) | 5.46 (5.22–5.87) | 4.84 (4.52–5.22) | |||||
| rs2234693 | T/T | 246 | 27.80 (26.96–28.96) | 0.84 | 5.40 (5.08–5.76) | 0.54 | 5.07 (4.78–5.45) | 0.79 | |
| T/C | 400 | 27.50 (26.47–28.97) | 5.32 (5.07–5.65) | 5.15 (4.81–5.54) | |||||
| C/C | 160 | 27.18 (26.30–28.83) | 5.47 (5.22–5.95) | 4.97 (4.60–5.36) | |||||
| rs9340799 | A/A | 327 | 27.99 (27.07–29.16) | 0.81 | 5.32 (5.04–5.70) | 0.59 | 5.18 (4.89–5.52) | 0.69 | |
| A/G | 378 | 27.36 (26.40–28.90) | 5.43 (5.13–5.75) | 5.06 (4.72–5.48) | |||||
| G/G | 109 | 26.87 (26.19–28.50) | 5.44 (5.21–5.94) | 4.94 (4.51–5.32) | |||||
| rs1801132 | C/C | 468 | 27.47 (26.47–28.81) | 0.90 | 5.34 (5.07–5.68) | 0.87 | 5.13 (4.78–5.48) | 0.76 | |
| C/G | 269 | 27.58 (26.74–28.88) | 5.41 (5.12–5.80) | 5.08 (4.79–5.46) | |||||
| G/G | 45 | 27.85 (27.30–29.43) | 5.39 (5.15–5.80) | 5.05 (4.69–5.49) | |||||
| (CA)n | S/S | 242 | 27.34 (26.60–28.55) | 0.70 | 5.27 (4.94–5.59) | 0.33 | 5.15 (4.79–5.58) | 0.93 | |
| S/L | 297 | 28.26 (27.47–29.32) | 5.44 (5.19–5.84) | 5.11 (4.82–5.53) | |||||
| L/L | 79 | 28.01 (27.06–29.10) | 5.23 (4.98–5.63) | 5.27 (4.96–5.71) | |||||
| ESR2 | rs1256059 | C/C | 225 | 28.06 (27.28–29.43) | 0.56 | 5.32 (5.08–5.70) | 0.18 | 5.23 (4.94–5.64) | 0.20 |
| C/T | 402 | 27.45 (26.53–28.59) | 5.34 (5.02–5.66) | 5.09 (4.76–5.51) | |||||
| T/T | 168 | 27.01 (26.15–28.17) | 5.58 (5.31–6.01) | 4.86 (4.52–5.22) | |||||
| (CA)n | S/S | 515 | 27.22 (26.41–28.46) | 0.20 | 5.42 (5.10–5.76) | 0.49 | 5.01 (4.71–5.45) | 0.60 | |
| S/L | 274 | 28.32 (27.38–29.73) | 5.33 (5.08–5.69) | 5.23 (4.95–5.58) | |||||
| L/L | 27 | 24.97 (24.13–25.71) | 5.20 (4.95–5.58) | 4.80 (4.55–5.02) | |||||
| rs1256031 | T/T | 213 | 28.05 (27.24–29.59) | 0.73 | 5.31 (5.07–5.69) | 0.09 | 5.19 (4.89–5.56) | 0.33 | |
| T/C | 403 | 27.37 (26.39–28.53) | 5.33 (5.02–5.65) | 5.13 (4.79–5.52) | |||||
| C/C | 194 | 27.43 (26.53–28.65) | 5.61 (5.32–6.05) | 4.90 (4.54–5.30) | |||||
Adjusted for age, weight, smoking, and alcohol consumption.
CYP19A1 haplotype analysis
CYP19A1 rs700518, (TTTA)n, and rs726547 are in strong LD (pairwise D’ ranges between 0.85 and 1.00), which resulted in three common haplotypes (frequency of > 5%): H2, rs700518 [G]- (TTTA)n [L]- rs726547[C] with the frequency of 45.3%; H7, rs700518 [A]- (TTTA)n [S]- rs726547[T] with the frequency of 5.1%; and H8, rs700518 [A]- (TTTA)n [S]- rs726547[C] with the frequency of 44.6%. Carriers of H2 had higher estradiol levels (1.16 pg/ml, or 5%, per each copy of haplotype; P = 0.0004), lower testosterone levels (0.94 pg/ml, or 17%, per each copy of haplotype; P = 0.036), and a higher estradiol to testosterone ratio (1.21, or 24%, per copy; P < 0.0001) compared with the H8 carriers.
ER gene association analysis
Postmenopausal women who carried the minor ESR2 (CA)n [L] and ESR2 rs1256031 [C] alleles, not in LD (D’= 0.005), had moderately higher estradiol levels (P = 0.02 and 0.03, respectively; Table 4). No significant associations of the steroid hormone phenotypes with the ESR1 SNPs were detected.
Table 4.
Adjusted estradiol levels in postmenopausal women by estrogen-related genotypes
| Gene | SNP | Genotype | No. | Estradiol
|
|
|---|---|---|---|---|---|
| Median (25th-75th percentile) | P value | ||||
| CYP19A1 | rs700518 | G/G | 72 | 8.70 (5.75–13.83) | 0.82 |
| G/A | 138 | 10.19 (5.42–16.42) | |||
| A/A | 72 | 9.88 (6.24–15.73) | |||
| (TTTA)n | S/S | 71 | 8.40 (5.36–13.94) | 0.74 | |
| S/L | 154 | 9.24 (5.73–14.72) | |||
| L/L | 64 | 10.15 (6.23–17.37) | |||
| rs726547 | C/C | 290 | 9.76 (6.04–14.98) | 0.84 | |
| C/T | 33 | 10.11 (6.60–13.95) | |||
| T/T | 1 | 13.20 (13.20–13.20) | |||
| ESR1 | (TA)n | L/L | 67 | 12.59 (7.09–21.07) | 0.56 |
| L/S | 149 | 9.26 (5.84–14.08) | |||
| S/S | 73 | 11.04 (6.96–15.86) | |||
| rs2077647 | T/T | 92 | 12.10 (7.77–18.11) | 0.26 | |
| T/C | 171 | 8.98 (5.79–14.95) | |||
| C/C | 59 | 8.61 (4.92–13.86) | |||
| rs2234693 | T/T | 97 | 11.61 (7.64–17.57) | 0.39 | |
| T/C | 172 | 9.00 (5.66–14.20) | |||
| C/C | 60 | 11.62 (6.76–17.38) | |||
| rs9340799 | A/A | 131 | 11.04 (7.13–16.55) | 0.62 | |
| A/G | 163 | 9.17 (5.73–14.10) | |||
| G/G | 37 | 11.28 (6.31–13.97) | |||
| rs1801132 | C/C | 194 | 9.08 (5.58–14.58) | 0.39 | |
| C/G | 101 | 12.29 (8.13–19.62) | |||
| G/G | 21 | 9.51 (5.76–11.93) | |||
| (CA)n | S/S | 126 | 9.16 (5.54–13.26) | 0.23 | |
| S/L | 115 | 10.96 (6.36–15.54) | |||
| L/L | 40 | 16.28 (10.33–27.41) | |||
| ESR2 | rs1256059 | C/C | 102 | 8.33 (5.21–12.12) | 0.22 |
| C/T | 163 | 8.78 (5.49–13.95) | |||
| T/T | 56 | 15.02 (10.84–24.61) | |||
| (CA)n | S/S | 213 | 10.70 (6.92–16.94) | 0.03 | |
| S/L | 107 | 7.54 (4.55–11.56) | |||
| L/L | 17 | 19.19 (13.06–21.01) | |||
| rs1256031 | T/T | 99 | 7.80 (4.92–10.88) | 0.02 | |
| T/C | 158 | 8.72 (5.63–14.27) | |||
| C/C | 74 | 15.73 (12.09–25.29) | |||
Adjusted for age, weight, smoking, and alcohol consumption.
Adjusted DHEAS means by genotype are shown in supplemental Tables 2 and 3.
Secondary analyses
Nongenetic predictors of steroid hormone levels by genotype are shown in supplemental Table 4. No consistent association of smoking status, alcohol consumption, and BMI with estrogen-related gene polymorphisms was found.
Discussion
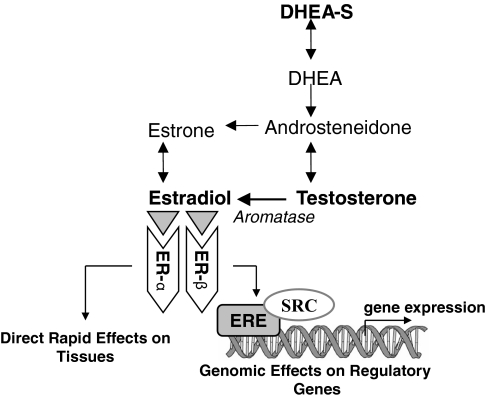
In this study we report association between variations in the aromatase gene (CYP19A1) and circulating estradiol and testosterone levels, as well as the estradiol to testosterone ratio, in men. It is well known that in postmenopausal women, when the ovaries cease to produce estrogens, and throughout life in men, estrogen is converted from steroid precursors through a series of enzymatic reactions. The enzyme aromatase is found in numerous tissues in the body where it catalyzes the conversion of testosterone into estrogens (Fig. 1). Experimental data have shown that disruption of Cyp19 was associated with the development of obesity, decrease in lean mass, hypercholesterolemia, hyperleptinemia, and insulin resistance (reviewed in Ref. 28).
Figure 1.
Schematic summary of the role of the genes involved in estrogen metabolism. DHEA, Dehydroepiandrosterone; ERE, estrogen response element; SRC, steroid receptor coactivators.
In men, peripheral aromatization of testosterone to active estrogen accounts for at least 75% of estrogen production (29), and the hormone balancing is defined by the testosterone to estrogen ratio. Age-associated testosterone decline has been shown in relation to diabetes, metabolic syndrome, reduced body lean mass (reviewed in Ref. 30), and mortality in men (31).
This study shows that common polymorphisms in CYP19A1 are associated in a dose-response manner with higher circulating estradiol and lower testosterone levels, and, consequently, with a higher estradiol to testosterone ratio in men. The differences between homozygous carriers and noncarriers were 13% for estradiol levels, 5% for testosterone levels, and 17% for the estradiol to testosterone ratio. These differences are independent of genetic effects on BMI, smoking status, and alcohol consumption (supplemental Table 4). In men, lower testosterone levels and a reduced testosterone to estradiol ratio have been associated with coronary atherosclerosis (32), whereas testosterone insufficiency has been correlated with an increased risk of death over 20 yr, independent of risk factors and preexisting health conditions (33). Moreover, higher serum estradiol levels and an estradiol to testosterone ratio have been associated with a lower risk for CVD events in older men (2).
Our findings suggest that synonymous coding (rs700518) and intronic (rs726547) nucleotide substitutions and the (TTTA)n repeat polymorphism in CYP19A1 are linked to a gain-of-function variant or cause a splicing alteration that increases aromatase activity that results in the conversion of larger amounts of testosterone to active estrogen. In an earlier paper, a similar association between the “long” CYP19A1 (TTTA)n allele and circulating estradiol has been reported in elderly men (34).
Estrogen exerts its actions directly by interacting with nuclear or membrane ERs, α and β. ERs are transcription factors that, when activated by estrogens, bind to estrogen-response elements in the promoter regions of target genes regulating their expression (Fig. 1). Variation in ESR1 and ESR2 may cause an impaired binding and, consequently, altered expression of genes regulating steroid hormone biosynthesis resulting in decreased circulating hormone levels. Therefore, we hypothesized that variations in the ESR1 and ESR2 genes, which have been repeatedly implicated in numerous cardiovascular phenotypes, decrease circulating estradiol, testosterone, and DHEAS levels, which, in turn, increase cardiovascular risks. Although we tested this hypothesis in the same cohort in which significant sex-specific associations were detected between the ESR1 polymorphisms and higher risk of myocardial infarction (9), elevated blood pressure (13), altered lipoprotein particle size concentrations (14,15), lower waist circumference (16), and more prominent age-related changes in left ventricular structure (18), as well as between higher serum estradiol levels and lower risk of CVD events (2), no relation of variation in ESR1 with circulating steroid hormone levels was discovered. Nonetheless, a number of reports have shown significant associations between the two most studied variations in the first intron of ESR1, detected by digestion with restriction enzymes PvuII, rs2234693, and XbaI, rs9340799, and higher androstenedione, a precursor of testosterone (35), whereas conflicting results have been shown with serum levels of estradiol (36,37), both in postmenopausal Caucasian women.
In addition, we detected moderate associations between postmenopausal estradiol levels and ESR2 rs1256031 and ESR2 (CA)n. Carriers of minor alleles had higher circulating levels than their noncarrier counterparts. However, these findings were not supported by significant associations with another ESR2 SNP, rs1256059, which is in tight LD with ESR2 rs1256031; yet, an association similar in direction and magnitude, though not statistically significant (P = 0.22), was detected (Table 4). Even though, our data do not help explain our previously reported finding that, after the adjustment for menopausal status, hypertensive ESR2 rs1256031 [TC] female carriers had the largest left ventricular mass and wall thickness (19). Although we cannot completely exclude spurious associations common for this type of study, other reports have indicated significant relations of variation in ESR2 with circulating estrogen and androgen levels in postmenopausal women (37,38). Alternatively, we can speculate that the associations with the ESR1 and ESR2 SNPs, previously detected in the FHS, may be caused by hormone-independent effects of the receptors.
This study’s limitations include the fact that active sex steroids are also synthesized locally in peripheral tissues, providing target tissues with controls that adjust the formation and metabolism of sex steroids to local requirements (39). These steroids are not released into the circulation and are not detectable in blood. However, it has been shown that circulating hormone levels reflect local steroid concentrations because their availability in the circulation ensures precursors for local synthesis (40). In addition, no formal adjustment for the multiple testing was performed. A standard correction for multiple hypothesis testing relies on the assumption that all statistical comparisons are independent. In the case of CYP19A1, three polymorphisms were in strong LD with one another, and steroid hormone levels were correlated. Nevertheless, some of our individual SNP and haplotype associations would independently pass the Bonferroni correction for multiple testing per gene (α = 0.05/21 tests = 0.002 for CYP19A1), which represent a possible false-positive finding in one out of 2000 occurrences. The consistency and biological relevance of these associations should motivate further research to verify and extend these findings.
In summary, our findings suggest that associations between the ESR1 gene polymorphisms and the incidence of myocardial infarction, elevated blood pressure, altered lipoprotein levels, and left ventricular structure are unlikely to be mediated through circulating steroid hormone levels. Importantly, the correlation of common genetic variations in CYP19A1 with estradiol and testosterone concentrations and their ratio in men may have important health effects. Knowledge that a specific carrier status may predispose to altered steroid hormone levels, which can promote CVD risks, may lead to targeted intervention strategies to reduce health risks in genetically susceptible individuals.
Supplementary Material
Footnotes
This work was supported by the National Heart, Lung and Blood Institute’s project Grant HL077378 and training Grant HL069770 (to M.E.M.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 29, 2008
Abbreviations: BMI, Body mass index; CVD, cardiovascular disease; DHEAS, dehydroepiandrosterone sulfate; ER, estrogen receptor; ESR1, estrogen receptor α gene ESR2, estrogen receptor β gene; FHS, Framingham Heart Study; HWE, Hardy-Weinberg equilibrium; LD, linkage disequilibrium; SNP, single nucleotide polymorphism.
References
- Mendelsohn ME, Karas RH 1999 The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811 [DOI] [PubMed] [Google Scholar]
- Arnlov J, Pencina MJ, Amin S, Nam BH, Benjamin EJ, Murabito JM, Wang TJ, Knapp PE, D'Agostino Sr RB, Bhasin S, Vasan RS 2006 Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med 145:176–184 [DOI] [PubMed] [Google Scholar]
- de Lecinana MA, Egido JA, Fernandez C, Martinez-Vila E, Santos S, Morales A, Martinez E, Pareja A, varez-Sabin J, Casado I 2007 Risk of ischemic stroke and lifetime estrogen exposure. Neurology 68:33–38 [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Sprecher DL, Borecki IB, Rice T, Laskarzewski PM, Rao DC 1994 Evidence for an association between dehydroepiandrosterone sulfate and nonfatal, premature myocardial infarction in males. Circulation 89:89–93 [DOI] [PubMed] [Google Scholar]
- Feldman HA, Johannes CB, Araujo AB, Mohr BA, Longcope C, McKinlay JB 2001 Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: prospective results from the Massachusetts Male Aging Study. Am J Epidemiol 153:79–89 [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Bonora E, Schwarz S, Xu Q 2000 No association between dehydroepiandrosterone sulfate and development of atherosclerosis in a prospective population study (Bruneck Study). Arterioscler Thromb Vasc Biol 20:1094–1100 [DOI] [PubMed] [Google Scholar]
- Contoreggi CS, Blackman MR, Andres R, Muller DC, Lakatta EG, Fleg JL, Harman SM 1990 Plasma levels of estradiol, testosterone, and DHEAS do not predict risk of coronary artery disease in men. J Androl 11:460–470 [PubMed] [Google Scholar]
- Schuit SC, Oei HH, Witteman JC, Geurts van Kessel CH, van Meurs JB, Nijhuis RL, van Leeuwen JP, de Jong FH, Zillikens MC, Hofman A, Pols HA, Uitterlinden AG 2004 Estrogen receptor α gene polymorphisms and risk of myocardial infarction. JAMA 291:2969–2977 [DOI] [PubMed] [Google Scholar]
- Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, Mendelsohn ME, Housman DE, Levy D 2003 Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA 290:2263–2270 [DOI] [PubMed] [Google Scholar]
- Lu H, Higashikata T, Inazu A, Nohara A, Yu W, Shimizu M, Mabuchi H 2002 Association of estrogen receptor-α gene polymorphisms with coronary artery disease in patients with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 22:817–823 [DOI] [PubMed] [Google Scholar]
- Pollak A, Rokach A, Blumenfeld A, Rosen LJ, Resnik L, Dresner PR 2004 Association of oestrogen receptor α gene polymorphism with the angiographic extent of coronary artery disease. Eur Heart J 25:240–245 [DOI] [PubMed] [Google Scholar]
- Rokach A, Pollak A, Rosen L, Friedlander Y, Blumenfeld A, Reznik L, Dresner-Pollak R 2005 Estrogen receptor α gene polymorphisms are associated with the angiographic extent of coronary artery disease. J Clin Endocrinol Metab 90:6556–6560 [DOI] [PubMed] [Google Scholar]
- Peter I, Shearman AM, Zucker DR, Schmid CH, Demissie S, Cupples LA, Larson MG, Vasan RS, D'Agostino RB, Karas RH, Mendelsohn ME, Housman DE, Levy D 2005 Variation in estrogen-related genes and cross-sectional and longitudinal blood pressure in the Framingham Heart Study. J Hypertens 23:2193–2200 [DOI] [PubMed] [Google Scholar]
- Shearman AM, Demissie S, Cupples LA, Peter I, Schmid CH, Ordovas JM, Mendelsohn ME, Housman DE 2005 Tobacco smoking, estrogen receptor α gene variation and small low density lipoprotein level. Hum Mol Genet 14:2405–2413 [DOI] [PubMed] [Google Scholar]
- Demissie S, Cupples LA, Shearman AM, Gruenthal KM, Peter I, Schmid CH, Karas RH, Housman DE, Mendelsohn ME, Ordovas JM 2006 Estrogen receptor-α variants are associated with lipoprotein size distribution and particle levels in women: the Framingham Heart Study. Atherosclerosis 185:210–218 [DOI] [PubMed] [Google Scholar]
- Fox CS, Yang Q, Cupples LA, Guo CY, Atwood LD, Murabito JM, Levy D, Mendelsohn ME, Housman DE, Shearman AM 2005 Sex-specific association between estrogen receptor-α gene variation and measures of adiposity: the Framingham Heart Study. J Clin Endocrinol Metab 90:6257–6262 [DOI] [PubMed] [Google Scholar]
- Leibowitz D, Dresner-Pollak R, Dvir S, Rokach A, Reznik L, Pollak A 2006 Association of an estrogen receptor-α gene polymorphism with left ventricular mass. Blood Press 15:45–50 [DOI] [PubMed] [Google Scholar]
- Peter I, Huggins GS, Shearman AM, Pollak A, Schmid CH, Cupples LA, Demissie S, Patten RD, Karas RH, Housman DE, Mendelsohn ME, Vasan RS, Benjamin EJ 2007 Age-related changes in echocardiographic measurements: association with variation in the estrogen receptor-α gene. Hypertension 49:1000–1006 [DOI] [PubMed] [Google Scholar]
- Peter I, Shearman AM, Vasan RS, Zucker DR, Schmid CH, Demissie S, Cupples LA, Kuvin JT, Karas RH, Mendelsohn ME, Housman DE, Benjamin EJ 2005 Association of estrogen receptor β gene polymorphisms with left ventricular mass and wall thickness in women. Am J Hypertens 18:1388–1395 [DOI] [PubMed] [Google Scholar]
- Baghaei F, Rosmond R, Westberg L, Hellstrand M, Eriksson E, Holm G, Bjorntorp P 2003 The CYP19 gene and associations with androgens and abdominal obesity in premenopausal women. Obes Res 11:578–585 [DOI] [PubMed] [Google Scholar]
- Meikle AW, Bishop DT, Stringham JD, West DW 1986 Quantitating genetic and nongenetic factors that determine plasma sex steroid variation in normal male twins. Metabolism 35:1090–1095 [DOI] [PubMed] [Google Scholar]
- Ring HZ, Lessov CN, Reed T, Marcus R, Holloway L, Swan GE, Carmelli D 2005 Heritability of plasma sex hormones and hormone binding globulin in adult male twins. J Clin Endocrinol Metab 90:3653–3658 [DOI] [PubMed] [Google Scholar]
- Rice T, Sprecher DL, Borecki IB, Mitchell LE, Laskarzewski PM, Rao DC 1993 The Cincinnati Myocardial Infarction and Hormone Family Study: family resemblance for dehydroepiandrosterone sulfate in control and myocardial infarction families. Metabolism 42:1284–1290 [DOI] [PubMed] [Google Scholar]
- Nestler JE, Whitfield JB, Williams TY, Zhu G, Condon J, Kirk KM, Heath AC, Montgomery GW, Martin NG 2002 Genetics of serum dehydroepiandrosterone sulfate and its relationship to insulin in a population-based cohort of twin subjects. J Clin Endocrinol Metab 87:682–686 [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP 1979 An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110:281–290 [DOI] [PubMed] [Google Scholar]
- Sherman SE, D'Agostino RB, Silbershatz H, Kannel WB 1999 Comparison of past versus recent physical activity in the prevention of premature death and coronary artery disease. Am Heart J 138(5 Pt 1):900–907 [DOI] [PubMed] [Google Scholar]
- Lewontin RC 1964 The interaction of selection and linkage. II. Optimum models. Genetics 50:757–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Robertson KM, Jones ME, Simpson ER 2002 Effect of estrogen deficiency in the male: the ArKO mouse model. Mol Cell Endocrinol 193:7–12 [DOI] [PubMed] [Google Scholar]
- Hemsell DL, Grodin JM, Brenner PF, Siiteri PK, MacDonald PC 1974 Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. J Clin Endocrinol Metab 38:476–479 [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Dobs AS 2007 Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes 14:226–234 [DOI] [PubMed] [Google Scholar]
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR 2006 Low serum testosterone and mortality in male veterans. Arch Intern Med 166:1660–1665 [DOI] [PubMed] [Google Scholar]
- Dunajska K, Milewicz A, Jedrzejuk D, Szymczak J, Kuliczkowski W, Salomon P, Bialy D, Poczatek K, Nowicki P 2004 Plasma adiponectin concentration in relation to severity of coronary atherosclerosis and cardiovascular risk factors in middle-aged men. Endocrine 25:215–221 [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J 2008 Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, Mavilia C, Del MF, Gonnelli S, Lucani B, Gennari C, Brandi ML 2004 A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab 89:2803–2810 [DOI] [PubMed] [Google Scholar]
- Zofkova I, Zajickova K, Hill M 2002 The estrogen receptor α gene determines serum androstenedione levels in postmenopausal women. Steroids 67:815–819 [DOI] [PubMed] [Google Scholar]
- Schuit SC, de Jong FH, Stolk L, Koek WN, van Meurs JB, Schoofs MW, Zillikens MC, Hofman A, van Leeuwen JP, Pols HA, Uitterlinden AG 2005 Estrogen receptor α gene polymorphisms are associated with estradiol levels in postmenopausal women. Eur J Endocrinol 153:327–334 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Jannausch ML, McConnell DS, Kardia SR, Randolph Jr JF 2006 Endogenous estradiol and its association with estrogen receptor gene polymorphisms. Am J Med 119(Suppl 1):S16–S22 [DOI] [PubMed] [Google Scholar]
- Westberg L, Baghaei F, Rosmond R, Hellstrand M, Landen M, Jansson M, Holm G, Bjorntorp P, Eriksson E 2001 Polymorphisms of the androgen receptor gene and the estrogen receptor β gene are associated with androgen levels in women. J Clin Endocrinol Metab 86:2562–2568 [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Simard J 2001 DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 22:185–212 [DOI] [PubMed] [Google Scholar]
- Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Jones M, Davis S 2000 The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab 11:184–188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.