Abstract
Context: Age at menarche (AgeM) is earlier in African-American (AA) than in European-American (EA) girls. Neither the physiological cause nor the health implications of this difference are known.
Objective: We tested the hypotheses that higher insulin among AA vs. EA precipitates an earlier elevation of estradiol (E2), an associated earlier AgeM, and greater gain in body fat.
Setting: The study was conducted at a university research laboratory and General Clinical Research Center.
Participants: Subjects were 137 girls (57 AA and 80 EA) aged 7–15 yr.
Design: The study had a longitudinal design. Annual evaluations were conducted for body composition by dual-energy X-ray absorptiometry, acute insulin response to glucose (AIRg) by iv glucose tolerance test, and reproductive-endocrine profile.
Main Outcome Measures: Multiple linear regression modeling and mixed model analyses were used to identify independent predictors of AgeM and E2 concentration at menarche.
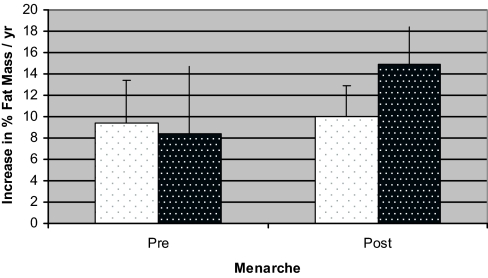
Results: AgeM was significantly earlier in AA vs. EA (10.8 vs. 11.6 yr). Neither E2 nor insulin was a significant independent predictor of AgeM. AIRg was a significant predictor of E2 concentration. AA had higher E2 than EA (P < 0.01), and girls with higher AIRg had higher E2. Total fat increased with age in both EA and AA. However, among EA, the increase in fat mass was similar both before and after menarche (9.4%/yr before vs. 10.0%/yr after), whereas among AA, fat deposition nearly doubled after menarche (8.4%/yr before vs. 14.9%/yr after).
Conclusion: Results did not support a direct cause-and-effect relationship between higher insulin, higher E2, and earlier AgeM in AA girls. However, the data suggested that higher insulin was associated with higher E2. Furthermore, reproductive maturation appeared to be associated with an acceleration of fat deposition among AA girls.
A polymorphism in the gene for the testosterone glucuronidation enzyme (UGT2B17) indicates the specificity and sensitivity of the testosterone doping test, such that 40% of the subjects without both alleles never reached the urinary testosterone/epitestosterone cut off ratio of 4.0.
Distinct racial differences in the time course of sexual maturation have been noted between African-American (AA) and European-American (EA) girls. Numerous studies have demonstrated AA mature at an earlier age, entering into puberty and experiencing menarche approximately a year before EA girls (1,2,3,4). For example, using data collected from National Health and Nutrition Examination Survey III (n = 1623), it has been reported that 52.7% of AA girls, compared with 23.2% of EA girls, had pubic hair by age 9 yr, whereas 35.1% of AA and only 11.8% of EA reported attaining menarche by age 11 yr (5). Similarly, the Pediatric Research in Office Setting group, a study of 17,077 children, reported mean age of breast development at 9 yr in AA, compared with 10 yr in EA, and mean onset of menarche at 12.2 yr in AA, compared with 12.9 yr in EA (6). The mechanism for these differences is not clear; however, age at menarche (AgeM) and the associated endocrine changes may affect a female’s health later in life.
One aspect of female health that may be affected by early menarche is obesity. Epidemiological and clinical research studies strongly suggest an association between menarche and adiposity. Numerous studies have indicated that early maturing girls demonstrate an increased body mass index (BMI), have higher body fat mass, are nearly twice as likely as average maturing girls to be overweight, and are more likely to be obese as adults (4,6,7,8). Although these observations suggest that the changes in the reproductive-endocrine milieu associated with menarche are involved in the regulation of body composition, a causal relationship between AgeM and adiposity has not been established.
Whether earlier AgeM among AA vs. EA girls is associated with ethnic differences body composition is not clear. However, ethnic differences in adiposity between AA and EA have been reported. In general, these differences are not present in prepubescent EA and AA girls but are apparent during early adulthood. It has been suggested that the racial disparity in adiposity likely develops in early adolescence and may be influenced by puberty. Kimm et al. (3) reported that the critical age for racial divergence in adiposity was age 12 yr, which also was the mean age of menarche in EA girls in that study (approximately a year after AA girls experienced menarche).
One of the factors that may affect both the timing of reproductive maturation and body composition, as well as ethnic differences in these measures, is insulin. In a study to ascertain whether insulin may be one of the factors related to the racial divergence in BMI and obesity, Klein et al. (9) observed that AA girls had higher baseline insulin, even after controlling for BMI, and that insulin increased more during puberty and decreased less after puberty among AA girls, compared with EA girls. Other investigators likewise have reported higher insulin among AA vs. EA children (10,11). It is possible that the differences in insulin response and action may stimulate the hypothalamic-pituitary-gonadal (HPG) axis (12,13) such that GnRH is stimulated earlier in AA girls.
The objectives of this study were to determine whether there were ethnic differences in insulin and reproductive hormone levels during the prepubertal and early pubertal years in a biracial cohort of girls from Birmingham, AL, and to determine whether insulin was associated with either the reproductive-endocrine axis or AgeM, as estimated using pubertal stage and concentrations of estradiol (E2) and FSH. We hypothesized that insulin would be positively associated with greater concentrations of reproductive hormones, and inversely associated with AgeM. Therefore, we hypothesized that AA, having higher insulin would have higher E2 and as a result, earlier AgeM. Furthermore, we hypothesized that AgeM would be inversely associated with body fat accumulation as girls traversed puberty. We presumed that with earlier AgeM in AA, more body fat would be accumulated throughout puberty.
Subjects and Methods
Experimental subjects
Subjects were the female participants of a longitudinal investigation on the influence of body composition on disease risk in children and adolescents. A total of 137 girls participated in the study; 57 were AA and 80 were EA. Average age at baseline was 8 yr. Average number of visits was four, commencing with 137 girls completing visit 1, 117 at visit 2, 94 at visit 3, 85 at visit 4, 67 at visit 5, 56 at visit 6, 39 at visit 7, 28 at visit 8, 6 at visit 9, and 10 at visit 2. There were no differences in evaluation number according to race.
Protocol
Subjects were recruited using advertisements at clinics and word of mouth and were excluded if they had prior illness, took medication known to affect body composition, or had a chronic medical condition (e.g. diabetes, asthma). Race/ethnicity was defined on the basis of self-ascribed ethnicity of children’s parents and grandparents. Subjects returned annually for metabolic testing. All testing was performed in the follicular phase of menstrual cycle after menarche was initiated. At each annual visit, girls were admitted to the General Clinical Research Center for an overnight evaluation. Anthropometric measurements, including assessment of sexual maturation, were obtained. After 2000 h, only water and/or noncaloric decaffeinated beverages were permitted until after morning testing. After the overnight fast, blood was collected for hormone analyses, and a tolbutamide-modified, frequently sampled intravenous glucose tolerance test was performed. Body composition was determined by dual x-ray absorptiometry (DXA). Before participating in the study, the girls and parents provided consent to the protocol, which was approved and followed regulations by the Institutional Review Board for Human Subjects at the University of Alabama at Birmingham.
Assessment of sexual maturation
Pubertal stage, according to the criteria of Marshall and Tanner (14) was assessed. With this method, pubertal status is assigned a value from 1 to 5, with 1 being prepubertal and 5 being postpubertal. Qualified pediatricians assessed pubertal stage in all girls.
Assessment of body composition
Girls arrived at the Department of Nutrition Sciences in the fasted state. Body composition (total body fat mass and nonbone lean tissue mass) was measured by DXA using a Lunar DPX-L densitometer (Lunar Radiation Corp., Madison, WI). Subjects were scanned in light clothing, while lying flat on their backs with arms at their sides. DXA scans were performed and analyzed with pediatric software version 1.5e (Lunar Radiation Corp., Madison, WI). DXA has been found to be highly reliable for body composition assessment in children; in our laboratory the coefficient of variation (CV) for repeated measures of total body fat mass was 6.55%. Height was measured to the nearest centimeter using a stadiometer and weight was measured on an electronic scale while children wore light clothing.
Intravenous glucose tolerance testing
On the morning after the overnight fast, a topical anesthetic (Emla cream; AstraZeneca, Wilmington, DE) was applied to the antecubital space of both arms, and flexible iv catheters were placed in both arms. Baseline samples were collected for hormone analysis. At time zero, glucose (25% dextrose; 11.4 g/m2) was administered iv. Blood samples (2 ml) were collected at the following times relative to glucose administration at 0 min: −15, −5, −1, 2, 3, 4, 5, 6, 8, 10, 14, 19, 22, 25, 30, 40, 50, 70, 100, 140, and 180 min. Tolbutamide (125 mg/m2) was injected iv at 20 min. The acute insulin response to glucose (AIRg), an approximation of first-phase insulin secretion, was calculated as the incremental area under the curve for insulin during the first 10 min after glucose injection using trapezoidal methodology (15). Values for fasting insulin were obtained from the average of the two baseline values. Glucose and insulin values were entered into the MINMOD computer program for determination of insulin sensitivity as described elsewhere (16).
Biochemical analytes
All analyses were conducted in the core laboratory of the General Clinical Research Center and Clinical Nutrition Research Center at the University of Alabama at Birmingham.
Glucose was measured in 10 μl sera using an Ektachem DT system (Johnson & Johnson Clinical Diagnostics, New Brunswick NJ). The intraassay CV for this analysis was 0.61% and the mean interassay CV was 1.45%. Insulin was assayed in duplicate 200-μl aliquots with Coat-A-Count kits (Diagnostic Products, Los Angeles, CA). In our laboratory, this assay has a sensitivity of 11.4 pmol/liter (1.9 μIU/ml), a mean interassay CV of 5%, and a mean interassay CV of 6%. Commercial quality control sera of low, medium, and high insulin concentration (Lymphochek; Bio-Rad Laboratories, Inc., Anaheim, CA) were included in every assay to monitor variation over time. Serum E2 was measured in 200-μl aliquots using a double-antibody RIA (Diagnostic Products). Assay sensitivity is 15.42 pmol/liter, mean intraassay CV is 4.69%, and interassay CV is 6.0%. FSH and dehydroxyepiandrosterone sulfate (DHEA-S) were measured in 100-μl aliquots by immunoradiometric assay (kits acquired from Diagnostic Products). In the Core Laboratory, for DHEA-S the assay sensitivity is 7.0 μg/dl, the intra- and interassay CVs are 8.11 and 4.22%; for FSH the assay sensitivity is 0.05 mIU/ml and the intra- and interassay CVs are 3.47 and 7.59%, respectively.
Menarche
Age at menarche was calculated as the midpoint age between the age at the annual visit during which the subject first reported having a menstrual cycle and the age at the previous annual visit, as described elsewhere (5). In addition, girls who had E2 concentrations greater than 16 pg/ml and FSH concentrations greater than 3.4 IU/liter were classified as postmenarchal (5,13,17). Age at adrenarche, which occurs approximately 2 yr before menarche, also was estimated based on a surge in DHEA-S level greater than 13 pg/ml as described (13).
Statistics
Total fat mass, lean mass, fasting insulin, insulin sensitivity, and AIRg were not normally distributed and were log transformed before analyses. This transformation yielded a normal distribution, and these transformed variables were used in all subsequent analyses. Levels for all measures that were greater than 3 sd above or less than 3 sd below the mean were removed from analyses.
For descriptive comparisons, subjects were grouped by both age (7–15 yr) and pubertal stage and stratified by race. Racial differences in reproductive hormones (E2, FSH), fasting insulin, AIRg, and percent fat were examined at each age and pubertal stage using independent t tests. Annual fat gain before and after menarche was compared using t tests. Girls with high vs. low AIRg (using median AIRg as a cutoff) were also compared using t tests.
Multiple linear regression analyses were conducted for the dependent variables AgeM and age at adrenarche. In each model, fasting insulin (or AIRg) and race were included as independent variables. All regression models included body composition measures as covariates. In addition, multiple linear regression models were constructed to determine independent predictors of E2. One model was analyzed according to age, whereas the other was analyzed according to pubertal stage. Race and insulin (either fasting insulin or AIRg) were independent variables. Both models were adjusted for lean body mass and total fat mass.
For longitudinal analyses, mixed-model analyses were used to account for intraperson correlations among repeated measures. Analyses were conducted for the dependent variables E2, FSH, AIRg, and total fat mass to identify independent contributions of age, race, and the age-by-race interaction. To identify predictors of E2 concentration as subjects traversed puberty, models were examined in which age and race were fixed variables, fasting insulin or AIRg and race were independent variables, and visit number and subject ID were used in the repeated statement.
Results and Discussion
Descriptive statistics
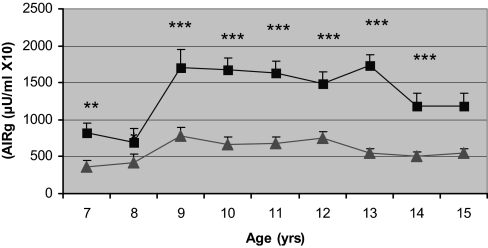
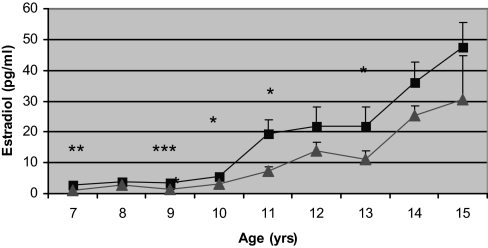
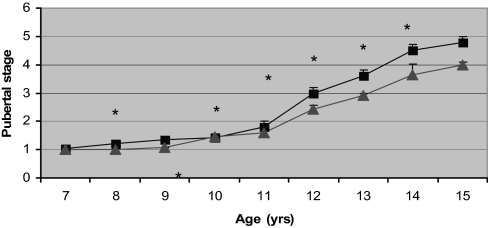
At baseline, AA had higher fasting insulin and AIRg than EA. AA experienced menarche and adrenarche nearly a year earlier than EA (Table 1). When stratified according to age, AA had significantly higher fasting insulin from age 8 to 10 yr and greater AIRg at all time points (Fig. 1). E2 was significantly higher among AA throughout most of the progression of puberty (Fig. 2). AA also had higher FSH concentrations as they entered and traversed puberty (P < 0.05 at ages 7, 9, and 11 yr). Pubertal stage was higher in AA than EA at all ages, with the difference being significant at age 9–14 yr (Fig. 3). When comparing E2 concentration within pubertal stages, E2 was higher in AA at each pubertal stage, albeit only significantly higher at Tanner stage I (P < 0.05). AA had significantly greater fat mass and percent fat at ages 9, 12, and 14 yr (P < 0.05). Among EA, the increase in fat mass was similar both before and after menarche (9.4% per year before vs. 10.0% per year after), whereas among AA, fat deposition nearly doubled after menarche (8.4% per year before vs. 14.9% per year after). Insulin sensitivity was significantly greater among EA vs. AA at ages 9, 11, 12, 13, and 14 yr. Before age 10 yr, there was no difference between ethnic groups in intraabdominal adipose tissue; however, EA girls aged 10–12 yr had greater intraabdominal adipose tissue than AA girls of the same age (P < 0.01, P < 0.001, P < 0.05, for ages 10, 11, and 12 yr, respectively). In both EA and AA, fat gain after menarche was distributed evenly between the upper and lower body, and there was no ethnic difference in how fat gain was distributed.
Table 1.
Descriptive statistics at baseline and ages of menarche and adrenarche for all children combined and by race
| EA (n = 80) | AA (n = 57) | Total (n = 137) | |
|---|---|---|---|
| Age (yr) | 8.1 ± 1.4 | 7.9 ± 1.9 | 8.0 ± 1.6 |
| Total fat mass (kg) | 9.6 ± 5.6 | 11.0 ± 7.1 | 10.2 ± 6.3 |
| Lean tissue mass (kg) | 20.0 ± 4.2 | 20.9 ± 5.8 | 20.4 ± 4.9 |
| BMI | 22.39 ± 1.0 | 21.04 ± 0.76 | 21.78 ± 0.64 |
| BMI z-score | 0.87 ± 0.18 | 0.84 ± 0.17 | 0.86 ± 0.12 |
| Height (cm) | 145.25 ± 2.2 | 146.40 ± 2.6 | 145.77 ± 1.7 |
| Fasting insulin (μIU/ml) | 10.5 ± 2.2a | 15.4 ± 9.0a | 12.4 ± 8.3 |
| SI (× 10−4 min−1/(μIU/ml) | 5.65 ± 1.4 | 3.42 ± 0.51 | 4.46 ± 0.75 |
| AIRg (μlU/ml× 10 min) | 732 ± 346a | 1639 ± 361a | 1216 ± 654 |
| E2 (pg/ml) | 2.1 ± 6.2 | 3.8 ± 4.0 | 2.8 ± 5.3 |
| Age at menarche (yr)b | 11.2a | 10.7a | 11.6 |
| Age at adrenarche (yr)b | 9.3a | 8.5a | 9.1 |
Means with different superscriptsare significantly different (P < 0.05).
Adjusted for age, race, body composition, E2, and AIRg.
Figure 1.
Comparison by age for AIRg in AA (▪) and EA (▴) girls. Multivariate linear regression modeling indicated significant age (P < 0.001) and race (P < 0.001) effects. Error bars, sem. **, P < 0.01; ***, P < 0.001.
Figure 2.
Comparison in serum E2 concentration by age in AA (▪) and EA (▴) girls. Multivariate linear regression modeling indicated significant age (P < 0.001) and race (P < 0.05) effects as well as age by race interaction (P < 0.001). Error bars, sem. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 3.
Racial differences in progression though puberty in AA (▪) and EA (▴) girls. Error bars, sem. *, P < 0.05.
In an effort to determine whether other factors may have contributed to the differences in insulin dynamics or hormonal response an estimate of socioeconomic status (SES; estimated by the Hollingshead 4 factor index), dietary intake (estimated by three 24 h recalls), and physical activity (assessed by self-reported questionnaire) were evaluated (data not shown). Although SES was lower among AA, SES was not found to be associated with insulin dynamics or hormone response in either group. Intake of macronutrients was not different between EA and AA and was not associated with outcome variables. In addition, AA reported greater amounts of daily physical activity than their EA counterparts, yet daily physical activity was not related to changes in insulin parameters or hormone levels.
When the sample was divided into groups based on high vs. low AIRg (using median AIRg = 800.3), higher AIRg was associated with higher E2 in both EA and AA girls. However, when stratified by race, AA girls had significantly higher E2 than EA in the high AIRg group, whereas in the low AIRg group there was not a difference in E2 concentration between EA and AA (Fig. 4).
Figure 4.
Comparison between increase in percent fat before and after menarche in AA (shaded bars) and EA (unshaded bars) girls.
Multiple regression analyses
In multiple regression analyses, the only independent predictor of both age at menarche and age at adrenarche was race (P < 0.01, P < 0.01, respectively) (Table 2). E2 was predicted by both fasting insulin and AIRg (P < 0.05 for both).
Table 2.
Based on multiple linear regression modeling, race was identified as the only determinant of AgeM and age at adrenarche
| Total | EA | AA | |
|---|---|---|---|
| P | P | P | |
| Age at menarche | |||
| Total fat mass | 0.427 | 0.776 | 0.792 |
| E2 | 0.658 | 0.638 | 0.802 |
| AIRg | 0.633 | 0.984 | 0.554 |
| SI | 0.689 | 0.989 | 0.475 |
| Race | 0.008 | ||
| Age at adrenarche | |||
| Total fat mass | 0.548 | 0.596 | 0.931 |
| E2 | 0.263 | 0.148 | 0.694 |
| Insulin | 0.660 | 0.767 | 0.859 |
| DHEA-S | 0.350 | 0.328 | 0.931 |
| Race | 0.009 |
Values in bold represent significant determinants.
When E2 levels within each pubertal stage were analyzed according to race, fasting insulin was associated with E2 in Tanner stages I (P < 0.01) and II (P < 0.01) among AA but not EA. When AIRg was used in place of insulin, AIRg was not a significant predictor of E2 in EA or AA.
Longitudinal analyses
In mixed-model analysis, age and race were significantly associated with AIRg, such that AIRg increased with age in both AA and EA but to a significantly greater extent among AA (Fig. 1). E2 increased with age and was greater among AA vs. EA. A significant age-by-race interaction indicated that E2 concentration in AA increased at a greater rate in AA than EA (Fig. 2). Similar interactions were demonstrated for FSH (P < 0.001) (data not shown). Total fat increased with age in both EA and AA (P < 0.001) (data not shown).
In the mixed model with E2 as the dependent variable, fasting insulin and race were significant predictors. When AIRg was used instead of fasting insulin, both AIRg and race were significant predictors of E2. When data were analyzed within each race group, fasting insulin was significant among AA but not EA.
This study sought to determine whether higher insulin among AA vs. EA girls precipitates an earlier elevation of E2, an associated earlier AgeM, and greater gain in body fat. We found that higher AIRg was associated with greater E2. Fat deposition nearly doubled after menarche among AA, but not EA, girls. These results suggest that earlier age at menarche, higher E2, greater postchallenge insulin secretion, or a combination of these factors may enhance fat deposition in AA girls.
Several earlier studies have indicated that AA girls mature earlier than EA girls. Similarly, we observed that AA girls entered into menarche nearly a year earlier than EA girls (10.7 and 11.6 yr, respectively). Pubertal stage also was higher in AA relative to EA at each age. These observations suggest that AA girls may not only enter puberty sooner but also progress through puberty more quickly.
Earlier AgeM among AA may be due to their greater insulin. Insulin has been implicated as a causal factor in pubertal timing. Although increased insulin response, as a reflection of transient pubertal insulin resistance and is recognized as a characteristic of puberty, existing literature has suggested that girls who mature earlier have an exaggerated insulin response that is disproportionate to their insulin resistance (4). Ibanez et al. (18) demonstrated that both premature pubarche and premature adrenarche were associated with hyperinsulinemia in girls. Thus, an exaggerated insulin response may be causally related to earlier puberty.
Insulin may affect the timing of puberty via action on the HPG axis. The onset of menarche is characterized by changes in the HPG axis resulting in an increase in frequency and amplitude of the GnRH pulse generator and resultant surge in reproductive hormone secretion. The response to increased reproductive hormone production is activation of the ovary and initiation of menses. Although the exact mechanism remains unclear, it appears that insulin exhibits a stimulatory effect on ovarian cells resulting in an increased production of reproductive hormones and also may act on the pituitary to increase the sensitivity of gonadotropins to GnRH (19).
The greater insulin response among AA before the onset of puberty may accelerate hormonal changes. Gower and colleagues (20,21,22), as well as others, have noted that both prepubertal and pubertal AA are more insulin resistant and have a higher acute insulin response to glucose than age- and BMI-matched EA children. Thus, we tested the hypothesis in this study that earlier AgeM among AA girls would be associated with their higher insulin.
Our data did not provide direct support for the role of insulin or insulin sensitivity in the earlier AgeM of AA vs. EA girls. In addition, the insulin sensitivity index (SI) was not associated with any of the outcomes of interest, nor did it modulate the association with the outcomes of interest and AIRg.
In multiple linear regression analyses, insulin was not independently related to AgeM. However, in our subjects, AA had significantly greater AIRg, E2, and FSH levels than age-matched EA, and AIRg was positively associated with E2. Furthermore, when the sample was divided according to high vs. low AIRg, AA had greater E2 than EA in the high AIRg group. This observation suggests that high postchallenge insulin concentrations may have a greater effect on E2 synthesis among AA vs. EA. Therefore, although our data do not support a direct causal relationship between insulin level and AgeM, the association of higher insulin with higher E2 may indirectly contribute to earlier AgeM in AA.
In conjunction with accelerating the onset of puberty and advancing AgeM, relatively high insulin among girls may simultaneously accelerate fat deposition. Insulin participates in the regulation of lipid metabolism by both stimulating free fatty acid uptake into adipocytes, and inhibiting triglyceride lipolysis and fatty acid mobilization. Whether relatively high endogenous insulin promotes accelerated or preferential accretion of adipose tissue has not been established (23). However, the ability of relatively high insulin to stimulate the reproductive-endocrine axis could lead to a synergistic effect of insulin and E2 (24) on fat deposition. This effect may be further accelerated among girls with earlier AgeM.
It has been reported that earlier AgeM is associated with greater fat deposition. For example, in the Fels Longitudinal Study (17), earlier maturing girls were heavier and had greater adiposity than average- or late-maturing girls. However, the inverse association between BMI and AgeM is apparent only in EA girls (4,25); among AA, AgeM was independent of adiposity (6,25). This observation supports the hypothesis that adiposity per se does not accelerate AgeM. Rather, we propose that both adiposity and early AgeM have the common antecedent of relatively high circulating insulin.
In our subjects, AA but not EA girls showed an accelerated rate of fat deposition after menarche. In contrast, there was no difference between AA and EA in percent fat gained per year leading up to menarche. These results suggest that AA girls are uniquely sensitive to the endocrine changes associated with the onset of menarche, such that these changes have a profound effect on energy balance. Whether the greater increase in E2 and insulin during the pubertal transition among AA vs. EA girls is causally related to their accelerated fat gain is not clear and warrants further study.
In conclusion, results suggested that the higher insulin response in AA vs. EA girls was associated with their higher E2. Furthermore, reproductive maturation appeared to be associated with an acceleration of fat deposition among AA girls. Potential cause-and-effect associations among E2, insulin, and fat xcdeposition deserve further study. Studies are needed to determine whether diets that lower postprandial insulin secretion can mitigate maturation-related changes in fat accrual among AA girls and identify the optimal diet for healthy maturation in this population.
Footnotes
This work was supported by National Institute of Child Health and Human Development Grants R29 HD 32668 and R01 HD/HL 33064 (to M.I.G.), Clinical Nutrition Research Center Grant P30-DK56336, and General Clinical Research Center Grant M01-RR-00032.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 18, 2008
For editorial see page 2472
Abbreviations: AA, African-American; AgeM, age at menarche; AIRg, acute insulin response to glucose; BMI, body mass index; CV, coefficient of variation; DHEA-S, dehydroxyepiandrosterone sulfate; DXA, dual x-ray absorptiometry; E2, estradiol; EA, European-American; HPG, hypothalamic-pituitary-gonadal; SES, socioeconomic status; SI, insulin sensitivity index.
References
- Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, Sun SS 2003 Age at menarche and racial comparisons in U.S. girls. Pediatrics 111:110–113 [DOI] [PubMed] [Google Scholar]
- Kaplowitz P 2006 Pubertal development in girls: secular trends. Curr Opin Obstet Gynecol 18:487–491 [DOI] [PubMed] [Google Scholar]
- Kimm SY, Barton BA, Obarzanek E, McMahon RP, Sabry ZI, Waclawiw MA, Schreiber GB, Morrison JA, Similo S, Daniels SR 2001 Racial divergence in adiposity during adolescence: The NHLBI Growth and Health Study. Pediatrics 107:E34 [DOI] [PubMed] [Google Scholar]
- Slyper AH 2006 The pubertal timing controversy in the USA, and a review of possible causative factors for the advance in timing of onset of puberty. Clin Endocrinol (Oxf) 65:1–8 [DOI] [PubMed] [Google Scholar]
- Wu T, Mendola P, Buck GM 2002 Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics 110:752–757 [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME 2001 Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics 108:347–353 [DOI] [PubMed] [Google Scholar]
- Adair LS, Gordon-Larsen P 2001 Maturational timing and overweight prevalence in U.S. adolescent girls. Am J Public Health 91:642–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y 2002 Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 110:903–910 [DOI] [PubMed] [Google Scholar]
- Klein DJ, Aronson FL, Harlan WR, Barton BA, Schreiber GB, Cohen RM, Harlan LC, Morrison JA 2004 Obesity and the development of insulin resistance and impaired fasting glucose in black and white adolescent girls: a longitudinal study. Diabetes Care 27:378–383 [DOI] [PubMed] [Google Scholar]
- Arslanian S 1998 Insulin secretion and sensitivity in healthy African-American vs American white children. Clin Pediatr (Phila) 37:81–88 [DOI] [PubMed] [Google Scholar]
- Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI 2003 Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes 52:1047–1051 [DOI] [PubMed] [Google Scholar]
- Mayes JS, Watson GH 2004 Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 5:197–216 [DOI] [PubMed] [Google Scholar]
- Root AW 2000 Precocious puberty. Pediatr Rev 21:10–19 [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM 1969 Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe RM, Steil GM, Bergman RN 1998 Critical evaluation of the combined model approach for estimation of prehepatic insulin secretion. Am J Physiol 274:E172–E183 [DOI] [PubMed] [Google Scholar]
- Pacini G, Bergman RN 1986 MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 23:113–122 [DOI] [PubMed] [Google Scholar]
- Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM 2005 Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab 90:2718–2724 [DOI] [PubMed] [Google Scholar]
- Ibanez L, Potau N, Chacon P, Pascual C, Carrascosa A 1998 Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia 41:1057–1063 [DOI] [PubMed] [Google Scholar]
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC 1999 The insulin-related ovarian regulatory system in health and disease. Endocr Rev 20:535–582 [DOI] [PubMed] [Google Scholar]
- Goran MI, Gower BA 2001 Longitudinal study on pubertal insulin resistance. Diabetes 50:2444–2450 [DOI] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI 1998 Fat distribution and insulin response in prepubertal African American and white children. Am J Clin Nutr 67:821–827 [DOI] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Goran MI 1999 Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 48:1515–1521 [DOI] [PubMed] [Google Scholar]
- Johnson MS, Figueroa-Colon R, Huang TT, Dwyer JH, Goran MI 2001 Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. J Clin Endocrinol Metab 86:3182–3187 [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Rogol AD 1999 Hormonal changes during puberty and their relationship to fat distribution. Am J Hum Biol 11:209–224 [DOI] [PubMed] [Google Scholar]
- Anderson SE, Dallal GE, Must A 2003 Relative weight and race influence average age at menarche: results from two nationally representative surveys of U.S. girls studied 25 years apart. Pediatrics 111:844–850 [DOI] [PubMed] [Google Scholar]