Abstract
Context: Fasting is associated with suppressed insulin and augmented GH secretion. The involvement of each mechanism in the regulation of fuel mobilization during fasting is unknown.
Objective: To ascertain the role of GH in the regulation of the rates of lipolysis, proteolysis, and hepatic glucose production (HGP) during the physiological daily feed/fast cycle and after 2 d of complete fasting, we used a model of selective GH suppression by the administration of GHRH receptor antagonist (GHRH-A).
Design and Setting: We conducted an open label in-patient study in the General Clinical Research Center at the University of Michigan.
Participants: Six healthy, nonobese volunteers participated.
Main Outcome Measures: We assessed 24-h plasma GH concentration and rates of lipolysis, proteolysis, and HGP using stable isotope techniques after an overnight fast and after 2 d of fasting.
Results: GHRH-A suppressed plasma GH by about 65% during the fed state (P = 0.015) but did not alter the rates of lipolysis, proteolysis, or HGP. Fasting for 2 d suppressed plasma insulin concentration by about 80% and elevated plasma GH about 4-fold (both P < 0.01). This was accompanied by a doubling in the rate of lipolysis, an approximately 40% increase in proteolysis, and an approximately 30% decline in HGP (all P < 0.05). Preventing the fasting-induced increase in GH with GHRH-A largely abolished the increase in the rate of lipolysis. GHRH-A also augmented the fasting-induced reduction in HGP but did not alter proteolysis.
Conclusions: Endogenous GH plays a very limited metabolic role during the daily feed/fast cycle but is essential for the increased lipolytic rate found with more prolonged fasting.
Fasting-induced elevation of GH secretion is a major mechanism for the starvation-induced increase in the rate of lipolysis, indicating that ketosis of starvation cannot be explained exclusively by the absence of insulin.
Over 40 yr ago, Rabinowitz and Zierler (1) suggested that insulin is the main hormone controlling substrate metabolism during the fed state but that during fasting, when insulin secretion is suppressed, this function shifts to GH. During fasting, the adaptive responses aimed at increasing endogenous fuel mobilization are particularly important to maintain energy homeostasis. In general, endogenous fuels are mobilized through the processes of lipolysis, proteolysis, and hepatic glucose production (HGP) (i.e. gluconeogenesis and/or glycogenolysis). GH has been found to participate in the regulation of all of these processes (2,3,4) but the specific impact of GH on fuel mobilization during fasting is not well understood.
Experimental support for the role of GH on energy metabolism is limited because most of the studies assessing the metabolic effects of GH were performed in hypopituitary patients who were given exogenous GH (2,5,6,7). Ideally, the metabolic role of GH should be investigated in normal-healthy subjects who are made temporarily and selectively GH deficient. This experimental approach became feasible with the introduction of (Ac-Tyr1, d-Arg2) GHRH 1–29-amide, which is a selective GHRH receptor antagonist (GHRH-A) (8). We previously found that continuous infusion of this compound in healthy volunteers suppresses GH output in a dose-dependent manner, without affecting other pituitary hormone secretion (8,9,10). The primary goal of the present study was to determine the effects of GHRH-A-induced blockade of GH secretion on lipolysis, proteolysis, and HGP in healthy human volunteers after an overnight fast and after more prolonged fasting (∼2 d).
Subjects and Methods
Subjects
Six healthy lean subjects were studied (five men and one woman; age 26.6 ± 2.0 yr; body mass index 23.4 ± 2.5 kg/m2; all were Caucasian). No subject had a history or laboratory evidence of metabolic or cardiovascular disease. The female subject was examined during the follicular phase of her menstrual cycle. The study was approved by the Institutional Review Board of University of Michigan. Written informed consent was obtained from all participants.
Experimental protocol
All subjects were admitted to the University of Michigan hospital for two randomly assigned visits, 4 d each. Admissions differed only by the administration of 1) a continuous infusion of GHRH-A (10 μg/kg·h) or 2) normal saline (control) throughout the entire hospital visits. During both admissions, subjects were fed a weight-maintaining diet on the first day, and then they fasted for 59 h. GH was measured every 20 min for 24 h (Q20) from d 1–2 of the study (fed state) and d 3–4 of the study (fasting state). The response of GH to GHRH was assessed by measuring GH level every 20 min for 2 h after injection of GHRH (0.33 μg/kg) after an overnight fast, and again after 2 d of fasting. Both before the fast and after 2 d of fasting, we performed stable-isotope tracer infusion studies to assess rates of lipolysis [(d5)-glycerol rate of appearance in plasma (Ra)], proteolysis [(d3)-leucine Ra], and HGP [(d2)-glucose Ra], as described previously (11,12). We also measured plasma concentrations of glucose, insulin, cortisol, IGF-I, and IGF-binding protein (IGFBP)-3 at regular intervals throughout the study.
Analytical methods
Plasma GH concentrations were measured using immunochemiluminescent assay (Nichols Advantage, San Juan Capistrano, CA) with an assay sensitivity of 0.01 μg/liter. Plasma IGF-I, IGFBP-3, and cortisol concentrations were all measured by immunochemiluminescent assays (Nichols Advantage, San Juan Capistrano, CA). Insulin concentrations were measured by RIA (DSL, Webster, TX). Tracer enrichment analysis was performed as described by Wolfe (13) with slight modifications.
Calculations
Discrete parameters of GH pulsatility were calculated using CLUSTER analysis (14). Acute GH response to exogenous GHRH (ΔGH) was calculated as the difference between the GH concentration immediately before GHRH bolus and the maximal GH within 60 min afterward. Undetectable GH values were assigned a number halfway between zero and assay detectability. Because isotope enrichments for [d5]-glycerol, [d2]-glucose, and [d3]-leucine were measured during steady-state conditions, glycerol Ra, glucose Ra, and leucine Ra were all calculated using the steady-state Steele equation (15).
Statistical analysis
A two-way ANOVA (GHRH-A treatment × fasting condition) for repeated measures, with Tukey and t test post hoc analysis was used to test for significant differences in all of the major endpoints of this study. Statistical analyses were performed using SigmaStat for Windows (version 3.1; Systat Software, Inc., Point Richmond, CA) and Excel 2000 (Microsoft Corp., Redmond, WA). Statistical significance was defined as P < 0.05.
Results
Plasma hormone concentrations
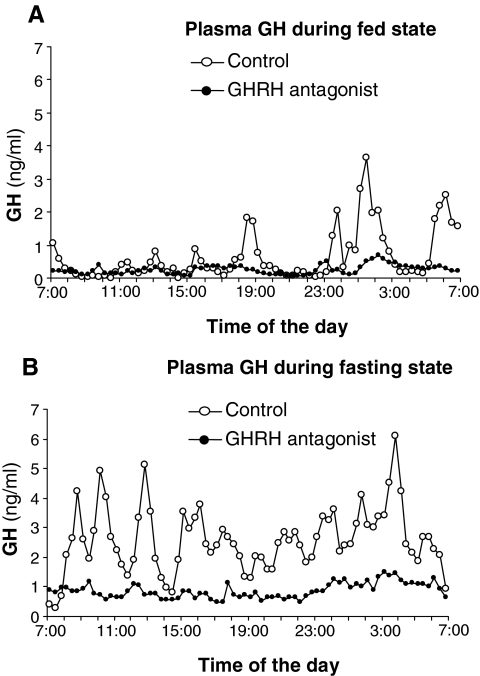
During the control trial (i.e. normal saline infusion), the mean 24-h GH increased from 1.1 ± 0.3 μg/liter in the fed state to 4.1 ± 0.9 μg/liter during prolonged fasting (P = 0.007). Infusion of GHRH-A reduced mean 24-h GH level during the fed state (0.41 ± 0.87 μg/liter, P = 0.015 vs. control) and blunted the fasting-induced increase in GH (1.36 ± 0.45 μg/liter, P = 0.010 vs. control) (Fig. 1, A and B). Confirming the effects of GHRH receptor blockade, we found that GHRH-A attenuated the magnitude of GH responses to GHRH from 3.7 ± 1.3 to 0.7 ± 0.3 μg/liter after overnight fast and from 6.6 ± 2.3 to 0.3 ± 0.3 μg/liter after 2 d of fasting (P < 0.0l for both). GHRH-A powerfully suppressed the amplitude of GH pulses both during fed and fasting states from 3.3 ± 1.4 to 0.8 ± 0.3 μg/liter after overnight fast and from 6.6 ± 1.4 to 1.2 ± 0.2 μg/liter after 2 d of fasting (P < 0.01 for both), but the pulse frequencies remained unaltered. Although plasma IGF-I concentration decreased nearly 30% after fasting during the control trial (from 211 ± 15 to 154 ± 29 μg/liter), this reduction did not reach statistical significance (P = 0.1). GHRH-A did not alter plasma IGF-I concentration after an overnight fast (205 ± 23 μg/liter, P = 0.78 vs. control), but after more prolonged fasting, GHRH-A administration reduced plasma IGF-I by half (101 ± 11 μg/liter, P = 0.004 vs. overnight fast). Plasma IGFBP-3 concentration did not change significantly with fasting during the control trial (from 1807 ± 114 to 1783 ± 285 mg/liter) or with the administration of GHRH-A (from 2050 ± 204 to 1530 ± 186 mg/liter). Mean 24-h plasma cortisol concentration increased from 5.79 ± 1.21 to 9.63 ± 1.55 μg/dl with prolonged fasting (P = 0.007), and GHRH-A infusion did not significantly alter this fasting-induced increase in plasma cortisol concentration. Fasting profoundly reduced plasma insulin concentration during both the control trial and with GHRH-A administration from 14.9 ± 2.2 to 3.1 ± 2.1 μU/ml during saline infusion and from 11.7 ± 1.8 to 0.65 ± 0.2 μU/ml (main effect for fasting P = 0.007), but plasma insulin concentrations were not significantly different between the treatments.
Figure 1.
Effects of GHRH-A on 24 h serum GH levels in the fed state (A) and during the last 24 h of a 2-d fast (B) . Only the mean data are shown, and the se bars are omitted for clarity.
Substrate kinetics
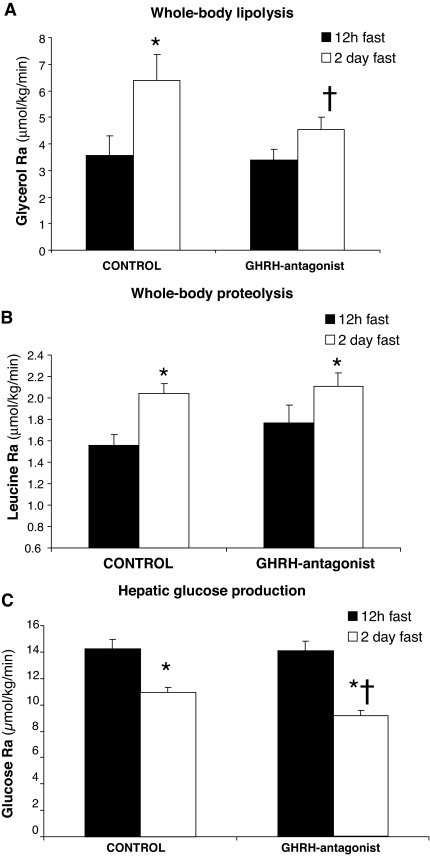
During the control trial, 2 d of fasting nearly doubled the lipolytic rate (glycerol Ra) from 3.56 ± 0.76 to 6.37 ± 0.98 μmol/kg·min compared with an overnight fast (P < 0.05) (Fig. 2A). Suppression of GH with GHRH-A during the fed day did not affect the rate of lipolysis after an overnight fast (3.39 ± 0.43 μmol/kg·min). However, infusion of GHRH-A prevented the fasting-induced rise in lipolytic rate (Fig. 2A). Similar to lipolytic rate, whole-body proteolysis (leucine Ra) increased significantly (P = 0.04) after 2 d of fasting during the control trial (Fig. 2B). However, in contrast to lipolytic rate, GHRH-A treatment did not alter the increase in proteolysis found with fasting (Fig. 2B). Suppression of GH secretion by GHRH-A also did not alter the rate of HGP after an overnight fast, as measured by glucose Ra (14.2 ± 0.8 vs. 14.0 ± 0.9 μmol/kg·min, P = 0.6). After 2 d of fasting, HGP was lower (P < 0.05) compared with an overnight fast during both the control trial (10.94 ± 0.41 μmol/kg·min) and with GHRH-A infusion (9.14 ± 0.40 μmol/kg·min) (Fig. 2C). After 2 d of fasting, HGP was slightly, yet significantly lower (P < 0.05) during GHRH-A administration compared with control (Fig. 2C).
Figure 2.
Effects of GHRH-A on rate of whole-body lipolysis (A) (i.e. glycerol Ra), rate of proteolysis (B) (i.e. leucine Ra), and HGP rate (i.e. glucose Ra) measured after an overnight (12-h) fast and again after 2 d of fasting. *, Significantly different from 12-h fast (P < 0.05); †, significantly different from 2-d control (saline) fast value (P < 0.05).
Discussion
This study describes the role of GH on the metabolic alterations in fuel handling during fasting through selective inhibition of GH output. As expected, fasting for about 2 d was associated with a significant increase in GH secretion, with nearly a doubling the prevailing cortisol concentrations and with a suppression of insulin secretion. These hormonal changes were accompanied by marked increases in the rate of lipolysis and proteolysis and with a reduction in hepatic glucose production. Although selective GH blockade did not influence any of these parameters after a physiological overnight fast, preventing the increase in GH found with 2 d of fasting suppressed the fasting-associated increase in the lipolytic rate. These results indicate that endogenous GH does not play an important role in the regulation of metabolic processes after a very short-term fast (i.e.; overnight fast) but assumes a major role as a stimulator of the lipolytic rate during prolonged fasting.
Although GH is recognized as lipolytic regulator, fasting-associated increase in lipolysis is commonly attributed to the suppression of insulin secretion (16,17,18,19). In contrast, our data show that suppression of insulin secretion even to nearly undetectable levels is not sufficient to accelerate the rate of lipolysis when GH secretion is low. Therefore, the large increase in lipolytic rate that occurs during a few days of fasting requires an augmentation of GH secretion.
The pattern of GH presentation to the peripheral tissues plays an independent and tissue-specific role in mediating the resultant metabolic effects (20). In our study, administration of GHRH-A suppressed plasma GH primarily at the expense of lowering GH pulse amplitude, effectively converting pulsatile GH profile into the one resembling continuous infusion. Although our findings cannot definitively determine whether the rate of lipolysis during fasting was affected because of alterations in the total GH output or because of changed GH mode of presentation to the fat tissue, previous work by Cersosimo et al. (21) demonstrate that only pulsatile GH administration in humans increased the rate of lipolysis.
Other hormones also have lipolytic properties (7,17,22,23). Importantly, we found that GHRH antagonist did not modify baseline or fasting-associated levels of cortisol. Fasting is known to increase catecholamine level, which in turn leads to increase of β-receptor-mediated lipolysis. However, adrenergic regulation of lipolysis is acute in nature and of very short duration, with rapid desensitization of the fat cell responsiveness to catecholamines (24,25). Therefore, the decrease in the rate of lipolysis observed in our study in fasted volunteers during GHRH-A administration is unlikely attributable to alterations in plasma cortisol or catecholamines concentrations.
Previous studies have found that exogenous GH administration during fasting increased the lipolytic rate in hypopituitary individuals (2). Interpretation of those results was complicated by the preexisting long-term GH deprivation of hypopituitary subjects, by alterations in their body composition, by the fixed and, inevitably, quasi-physiological replacement of other missing hormones (e.g. T4 and cortisol), and the uncertainty about the dose and the mode of administration of GH (2,6). In our study, the use of GHRH receptor blockade in nonobese subjects led to a selective decline in endogenous GH secretion without affecting other hormonal systems (8,9). Most importantly, GHRH-A did not modify the fasting-induced increase in cortisol (22), nor did it alter the reduction in insulin found with fasting. Therefore, this approach allows us to investigate the selective effects of endogenous GH deficiency on metabolic processes in the potentially cleanest physiological model.
Our observation that suppressing GH secretion did not alter the rate of proteolysis differs from previous reports in patients with GH deficiency where muscle protein breakdown was 25% greater during fasting in participants without GH replacement (2,5,6,26). This difference in the outcomes could be explained by several factors, including the degree of GH suppression needed to achieve a protein-sparing effect in fasting and/or the methodology used to quantify proteolysis (e.g. stable isotope infusion in our study vs. urea excretion in earlier reports). Unlike proteolysis, our finding that GH suppression further decreased the already diminished HGP during fasting does agree with previous results in GH-deficient patients (27,28,29). Our data suggest that GH may not be important as a regulator of endogenous carbohydrate availability during the daily feed/fast cycles. However, during a more prolonged fast, when insulin is maximally suppressed, elevated GH levels maintain HGP to some degree, assuring provision of glucose to the central nervous system and other tissues requiring this fuel for survival.
Understanding the metabolic role of GH during fasting or a starvation state is very important to enhance our knowledge of physiological adaptations during complete starvation. But these findings are also relevant in evaluation of different catabolic disease states and could have implications for therapeutic interventions in starvation. In conclusion, we show here for the first time that fasting-induced elevation of endogenous GH secretion is a major and indispensable mechanism for the starvation-induced increase in the rate of lipolysis. The accepted dogma that the ketosis of starvation can be explained exclusively by the absence of insulin is obviously incomplete.
Acknowledgments
We thank Amy Kaufman and Al Hinko for their technical assistance and the subjects of this study for their participation.
Footnotes
This work was supported by the Department of Veterans Affairs Merit Review Program (A.B.), NIH Grant R0-1 DK071955 (J.H. and A.B.), a Genentech Fellowship (N.G.), and a Pfizer Fellowship (S.S.).
Disclosure Summary: The authors have nothing to declare.
First Published Online April 15, 2008
Abbreviations: GHRH-A, GHRH receptor antagonist; HGP, hepatic glucose production; IGFBP, IGF-binding protein.
References
- Rabinowitz D, Zierler KL 1963 A metabolic regulating device based on the actions of human growth hormone and of insulin singly and together on the human forearm. Nature 199:913–915 [DOI] [PubMed] [Google Scholar]
- Norrelund H, Djurhuus C, Jorgensen JO, Nielsen S 2003 Effects of growth hormone on urea, glucose and lipid metabolism and insulin sensitivity during fasting in GH-deficient patients. Am J Physiol 88:E3292–3298 [DOI] [PubMed] [Google Scholar]
- Norrelund H 2005 Consequences of growth hormone deficiency for intermediary metabolism and effects of replacement. Front Horm Res 33:103–120 [DOI] [PubMed] [Google Scholar]
- Jorgensen JOL, Pedersen SA, Thuesen L 1989 Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet 1:1221–1225 [DOI] [PubMed] [Google Scholar]
- Shi J, Sekhar RV, Ellis K 2003 Short- and long-term effects of growth hormone replacement on protein metabolism in GH-deficient adults. J Clin Endocrinol Metab 88:5827–5833 [DOI] [PubMed] [Google Scholar]
- Norrelund H, Moller N, Sreekumaran K 2001 Continuation of growth hormone (GH) substitution during fasting in GH-deficient patients decreases urea excretion and conserves protein synthesis. J Clin Endocrinol Metab 86:3120–3129 [DOI] [PubMed] [Google Scholar]
- Beauville M, Harant I, Crampes F 1992 Effect of long-term rhGH administration in GH-deficient adults on fat cell epinephrine response. Am J Physiol 263:E467–E472 [DOI] [PubMed] [Google Scholar]
- Jaffe CA, De Mott Friberg R, Barkan AL 1993 Suppression of GH secretion by a selective GH-releasing hormone (GHRH) antagonist: direct evidence for the involvement of endogenous GHRH in generation of GH pulses. J Clin Invest 92:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel-Aulet M, Jaffe CA, De Mott-Friberg R, Barkan AL 1999 In vivo semiquantification of hypothalamic growth hormone-releasing Hormone (GHRH) output in humans: evidence for relative GHRH deficiency in aging. J Clin Endocrinol Metab 84:3490–3497 [DOI] [PubMed] [Google Scholar]
- Orrego JJ, Russel-Aulet M, Demott-Friberg R, Barkan AL 2001 Semiquantification of hypothalamic GH-releasing hormone output in women: evidence for sexual dimorphism in the mechanism of the somatopause. J Clin Endocrinol Metab 86:5485–5490 [DOI] [PubMed] [Google Scholar]
- Harber MP, Schenk S, Barkan AL, Horowitz JF 2005 Alterations in carbohydrate metabolism in response to short-term dietary carbohydrate restriction. Am J Physiol 289:E306–E312 [DOI] [PubMed] [Google Scholar]
- Harber MP, Schenk S, Barkan AL, Horowitz JF 2005 Effects of dietary carbohydrate restriction with high protein intake on protein metabolism and the somatotropic axis. J Clin Endocrionl Metab 90:5175–5181 [DOI] [PubMed] [Google Scholar]
- Wolfe RR 1992 Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley-Liss [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile and robust algorithm for endocrine pulse detection. Am J Physiol 250:E486–E493 [DOI] [PubMed] [Google Scholar]
- Steele R 1959 Use of C14-glucose to measure hepatic glucose production following an intravenous glucose load or after injection of insulin. Metab 8:512–519 [PubMed] [Google Scholar]
- Morin P, Bjorntorp P 1993 Endocrine-metabolic pattern and adipose tissue distribution. Horm Res 39(Suppl 3):81–85 [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Coppack SW 1999 Effects of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol 276:E278–E284 [DOI] [PubMed] [Google Scholar]
- Nair KS, Woolf PD, Welle SL, Mathews DE 1987 Leucine, glucose and energy metabolism after 3 days of fasting in healthy human subjects. Am J Clin Nutr 46:557–562 [DOI] [PubMed] [Google Scholar]
- Klein S, Peters EJ, Holland OB, Wolfe R 1989 Effects of short- and long-term β-adrenergic blockade on lipolysis during fasting in humans. Am J Physiol 257:E65–E73 [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins PB 2002 Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol 283:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- Cersosimo E, Danou F, Persson M, Miles JM 1996 Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am J Physiol 271:E123–E126 [DOI] [PubMed] [Google Scholar]
- Fraser DA, Thoen J, Selvaag AM, Djoseland O, Forre O, Kjeldsen-Kragh J 2001 A preliminary of circadian serum cortisol concentrations in response to a 72-h fast in rheumatoid arthritis patients not previously treated with corticosteroids. Clin Rheumatol 20:85–87 [DOI] [PubMed] [Google Scholar]
- Moller N, Norrelund H 2003 The role of growth hormone in the regulation of protein metabolism with particular reference to conditions of fasting. Horm Res 59:62–68 [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Bulow J, Frandsen E, Calbo H 1997 Desensitization of human adipose tissue to adrenaline stimulation studied by microdialysis. J Physiol 500:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nojiri H, Yoshizuka N, Takema Y 2007 Rapid desensitization of lipolysis in the visceral and subcutaneous adipocytes of rats. Lipid 42:307–314 [DOI] [PubMed] [Google Scholar]
- Salomon F, Cuneo R 1991 Growth hormone and protein metabolism. Horm Res 36(Suppl 1):41–43 [DOI] [PubMed] [Google Scholar]
- Bak JF, Moller N, Schmitz O 1991 Effects of growth hormone on fuel utilization and muscle glycogen synthase activity in normal humans. Am J Physiol 260:E736–E742 [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain PR, Smith D, De Fronzo RA 1982 The effects of growth hormone on glucose metabolism and insulin secretion in man. J Clin Endocrinol Metab 55:973–982 [DOI] [PubMed] [Google Scholar]
- Moller N, Butler PC, Antsiferov MA, Alberti KGMM 1989 Effects of growth hormone on insulin sensitivity and forearm metabolism in normal man. Diabetologia 32:105–110 [DOI] [PubMed] [Google Scholar]