Abstract
Context: Gastric bypass surgery (GBP) results in rapid weight loss, improvement of type 2 diabetes (T2DM), and increase in incretins levels. Diet-induced weight loss also improves T2DM and may increase incretin levels.
Objective: Our objective was to determine whether the magnitude of the change of the incretin levels and effect is greater after GBP compared with a low caloric diet, after equivalent weight loss.
Design and Methods: Obese women with T2DM studied before and 1 month after GBP (n = 9), or after a diet-induced equivalent weight loss (n = 10), were included in the study. Patients from both groups were matched for age, body weight, body mass index, diabetes duration and control, and amount of weight loss.
Setting: This outpatient study was conducted at the General Clinical Research Center.
Main Outcome Measures: Glucose, insulin, proinsulin, glucagon, gastric inhibitory peptide (GIP), and glucagon-like peptide (GLP)-1 levels were measured after 50-g oral glucose. The incretin effect was measured as the difference in insulin levels in response to oral and to an isoglycemic iv glucose load.
Results: At baseline, none of the outcome variables (fasting and stimulated values) were different between the GBP and diet groups. Total GLP-1 levels after oral glucose markedly increased six times (peak:17 ± 6 to 112 ± 54 pmol/liter; P < 0.001), and the incretin effect increased five times (9.4 ± 27.5 to 44.8 ± 12.7%; P < 0.001) after GBP, but not after diet. Postprandial glucose levels (P = 0.001) decreased more after GBP.
Conclusions: These data suggest that early after GBP, the greater GLP-1 and GIP release and improvement of incretin effect are related not to weight loss but rather to the surgical procedure. This could be responsible for better diabetes outcome after GBP.
A comparative study of matched weight loss by gastric bypass surgery or diet in morbid obese patients with type 2 diabetes suggests that the mechanisms of diabetes resolution after gastric bypass surgery, in concert with weight loss, may be the marked increases in incretin glucagon-like peptide 1 and gastric inhibitory peptide levels and their effects on insulin secretion.
Together with the epidemic of obesity (1), the number of weight loss surgeries has surged in the last decade (2). Roux-en-Y gastric bypass surgery (GBP) results in significant and prolonged weight loss with resolution of type 2 diabetes (T2DM) in 80% of cases (3). The mechanism by which T2DM improves rapidly after GBP, often before significant weight loss, has not yet been elucidated. The hormonal changes described after GBP suggest a possible endocrine effect of this surgery. We (4) and others (5,6,7,8,9,10) have shown that the meal- or glucose-stimulated incretin levels, which are blunted in T2DM, increase after GBP. In parallel with the increased levels of glucagon-like peptide (GLP)-1 and gastric inhibitory peptide (GIP), the incretin effect on insulin secretion, impaired in patients with T2DM, markedly increased to levels similar to that of matched controls without T2DM, 1 month after GBP (4).
The two main incretins, GIP and GLP-1 (11), are secreted by the endocrine cells of the intestinal mucosa (12) in response to food and are responsible for 50–60% of insulin secretion after meals (12,13). The incretin effect is impaired in patients with T2DM (14). GLP-1 levels are blunted (15), but the effect of administered GLP-1 on insulin secretion persists (16). GIP levels are usually normal in patients with T2DM, but the effect of administered GIP on insulin secretion is blunted (17), although can be restored under normal glycemic conditions (18). GIP and GLP-1 are rapidly degraded by the enzyme dipeptidyl peptidase-IV (DPPIV) (19). GLP-1 and GIP analogs and DPPIV inhibitors are in use or currently being developed as antidiabetic agents (20).
The markedly increased incretin levels and effect observed after GBP could be one of the key mediators of the antidiabetic effects of the surgery. However, weight loss occurs very rapidly after GBP, and it is unclear whether the early changes in incretin levels and effect are a result of the surgery or could be attributed to weight loss per se. The blunted postprandial response of GLP-1 observed in severely obese individuals (21) has improved after diet-induced weight loss (22). Other studies suggest that it is not the weight loss, but the surgical procedure, that is responsible for the improvement of glucose tolerance. Ileal transposition, which increases GLP-1 levels, results in improved glucose control (23) independently of weight loss in rodent models (24) as well as in humans (25).
The goal of this study was to determine whether the magnitude of the change of the incretin levels and effect is greater after GBP compared with a low caloric diet, under conditions of short-term equivalent weight loss, in morbidly obese patients with T2DM. Specifically, we measured the changes in GLP-1 and GIP levels after oral glucose stimulation, and their incretin effect on insulin, in obese patients with T2DM before and 1 month after GBP, or after an equivalent diet-induced weight loss. A second goal was to determine whether an equivalent weight loss, achieved by GBP or by diet, would result in the same improvement of blood glucose levels in patients with T2DM.
Subjects and Methods
Subjects
Obese patients with body mass index (BMI) above or equal to 35 kg/m2, eligible candidates for GBP, younger than 60 yr of age, of both genders and all ethnic groups, with T2DM diagnosed for less than 5 yr, not on insulin, thiazolidinedione, exenatide, or DPPIV inhibitors, with an glycosylated hemoglobin (HbA1c) less than 8%, were invited to participate in the study. All participants signed an informed consent, approved by our institution, before enrolling in the study. One group of patients was studied before and 1 month after GBP (surgical group). A second group of patients, fulfilling the same recruitment criteria, was studied before and after a 10-kg diet-induced weight loss (diet group). In addition, patients in the diet group were matched for age, weight, BMI, T2DM duration and control (HbA1c) to patients from the surgical group.
Diet-induced weight loss and diabetes treatment
The diet consisted of a meal replacement plan of 1000 kcal/d. A 1-wk supply of meal replacement products (Robard Corp., Mt. Laurel, NJ), including high-protein shakes, bars, fruit drinks, and soups, was given to each patient during an individual weekly visit at the General Clinical Research Center. Fresh fruits and vegetables were allowed. Body weight was measured weekly and the diet adjusted when necessary. If no weight loss, or if weight gain occurred at two consecutive weekly visits, the patients were excluded from the study. Patients were kept on the 1000-kcal diet and in negative energy balance (active weight loss) while retested for incretin levels and effect after a 10-kg weight loss. Although there was no time limit, the expectation was that patients would lose 10 kg in 4–8 wk. During the weight loss, patients were asked to monitor blood glucose levels by finger stick and keep logs. Diabetes medications were adjusted by a nurse educator or a diabetologist to avoid hypoglycemia and to fulfill the American Diabetes Association standard of treatment, based on fasting and postprandial glucose levels. In most cases, patients on sulfonylureas had their medication decreased or discontinued to avoid hypoglycemia.
Roux-en-Y gastric bypass (GBP) protocol
All patients underwent a laparoscopic GBP. In brief, the jejunum was divided 30 cm from the ligament of Treitz and anastomosed to a 30-ml proximal gastric pouch. The jejunum was reanastomosed 150-cm distal to the gastrojejunostomy. All mesenteric defects were closed. The post-GBP diet recommendations included a daily intake of 600–800 kcal, 70 g protein, and 1.8 liter fluid. This was achieved, on an individual basis, with multiple small meals and snacks with various commercial protein supplements. The diet after GBP was monitored by food records but not directly supervised. The diet in the few days preceding the testing in surgical or diet patients before weight loss was not controlled for.
Incretin effect: insulin secretion after oral and isoglycemic iv glucose load (IsoG IVGT)
Subjects were studied for the oral glucose tolerance test (OGTT) and IsoG IVGT in the morning after a 12-h overnight fast, on 2 different days, separated by less than 5 d.
Three-hour OGTT
All patients underwent first a 3-h OGTT with 50 g glucose (noncarbonated, in a total volume of 200 ml). After iv insertion, at 0800 h, subjects received orally 50 g glucose. Blood samples, collected on chilled EDTA tubes with added aprotinin (500 kallikrein inhibitory U/ml blood) and DPPIV inhibitor (LINCO Research, Inc., St. Charles, MO) (10 μl/ml blood), were centrifuged at 4 C before storage at −70 C.
IsoG IVGT
The goal of the IsoG IVGT was to expose the pancreas to blood glucose levels matched to the ones obtained during the OGTT in the same subject. Glucose (sterile 20% dextrose solution in water) was infused iv over 3 h using a Gemini pump (Gemini, Inc., St. Louis, MI). A sample of blood was collected every 5 min, using a contralateral antecubital iv catheter, then transferred in a microcentrifuge tube without any additive and centrifuged at bedside for immediate measure of glucose levels. The glucose infusion rate was adjusted to match the glucose concentrations obtained for the same patient during the OGTT at each time point for 3 h. For insulin levels, blood samples were collected every 15 min for the first 90 min, then every 30 min until 180′. During the OGTT and IsoG IVGT, the arm used for blood sampling was kept warm with a heating pad.
Incretin effect
The difference in β-cell responses (insulin total area under the curve or INS AUC (0–180′) to the oral and isoglycemic iv glucose stimuli represents the action of the incretin factor expressed as the percentage of the physiological response to oral glucose, which is taken as the denominator (100%) (26). The formula is:
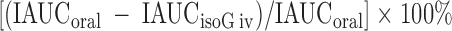 |
Assays
Total GLP-1, an indicator of GLP secretion, was measured by RIA (LINCO Research) after plasma ethanol extraction. The intraassay and interassay coefficients of variation (CVs) were 3–6.5% and 4.7–8.8%, respectively. This assay cross-reacts 100% with GLP-17–36, GLP-19–36, and GLP-17–37 but does not cross-react with glucagon (0.2%), GLP-2 (<0.01%), or exendin (<0.01%). Active GLP-1, an indicator of GLP potential action, was measured by ELISA (LINCO Research). The intraassay and interassay CVs were 3–7% and 7–8%, respectively. The assay cross-reacts 100% with GLP-17–36 and GLP-17–37 but does not cross-react with GLP-19–36, glucagon, or GLP-2. Total GIP was measured by ELISA. The assay cross-reacts 100% with GIP 1–42 and GIP 3–42 but does not cross-react with GLP-1, GLP-2, oxyntomodulin, or glucagon. The intraassay and interassay CVs were 3.0–8.8% and 1.8–6.1%, respectively. Plasma insulin, C peptide, proinsulin, and glucagon concentrations were measured by RIA (LINCO Research) with an intraassay CV of 3–8% and interassay CV of 5.5–9%. The glucagon assay cross-reacts 100% with glucagon but cross-reacts less than 0.1% with oxyntomodulin. Glucose concentration was measured at the bedside by the glucose oxidase method (Beckman glucose analyzer; Beckman Coulter, Inc., Fullerton, CA). All hormonal and metabolites assays were performed at the Hormone and Metabolite Core Laboratory of the New York Obesity Research Center.
Statistical analysis
Outcome variables were plasma glucose and plasma insulin, C peptide, glucagon, proinsulin, GLP-1, and GIP concentrations. Total AUCs 0–180′ for outcome variables were calculated using the trapezoidal method. ANOVA with repeated measures was used to detect glucose and hormonal changes over time during the OGTT within each condition, and for comparison before and after GBP and before and after diet, or between diet and surgical groups with T2DM. Paired t tests were used to compare data between before and after GBP or diet. Data are expressed as the mean ± sd, except in the figures where sems are used. Statistical significance was set at P < 0.05. Statistical analyses were performed with SPSS 14.0 (SPSS, Inc., Chicago, IL).
Results
Subject characteristics
Subject characteristics are shown in Table 1. Obese women with T2DM and normal liver enzymes, thyroid function tests, and blood pressure were studied before and 1 month after GBP (n = 9), and before and after an equivalent diet-induced weight loss (n = 10). Of the 12 women recruited in the diet group, two did not complete the weight loss due to pregnancy or breast cancer, and data from 10 diet completers are presented. Diabetes medications, sulfonylureas and/or metformin, were discontinued 3 d before being studied at baseline in all patients and were adjusted during the diet-induced weight loss to avoid hypoglycemia. Patients from the diet and surgical group were matched for age, body weight, BMI, diabetes duration and control (HbA1c) (Table 1). Before weight loss, neither fasting glucose (P = 0.874), proinsulin (P = 0.797), insulin (P = 0.629), C peptide (P = 0.589), glucagon (P = 0.363), GLP-1 (P = 0.832) and GIP (P = 0.414) and incretin effect (P = 0.245), nor stimulated variables during the OGTT were significantly different between the diet and GBP groups.
Table 1.
Subject characteristics before and after weight loss, by either diet or GBP
| Before diet | After diet | Before GBP | After GBP | P value | |
|---|---|---|---|---|---|
| Age (yr) | 46.80 ± 7.24 | 44.56 ± 9.65 | |||
| Weight (kg) | 110.9 ± 10.2 | 101.1 ± 9.5a | 113.2 ± 15.5 | 103.2 ± 16.6a | 0.816 |
| BMI (kg/m2) | 43.3 ± 3.6 | 39.6 ± 3.5a | 43.3 ± 6.2 | 39.5 ± 6.5a | 0.939 |
| T2DM duration (months) | 22.2 ± 17.7 | 31.4 ± 26.4 | |||
| HbA1c (%) | 6.68 ± 0.88 | 6.51 ± 0.78 | |||
| Fasting glucose (mmol/liter) | 7.84 ± 1.09 | 6.34 ± 1.00a | 7.95 ± 1.74 | 6.42 ± 0.80b | 0.957 |
| 120′ glucose (mmol/liter) | 10.17 ± 2.40 | 9.62 ± 2.34 | 11.12 ± 1.64 | 7.22 ± 1.62a | 0.001 |
| Peak glucose (mmol/liter) | 12.83 ± 1.36 | 11.45 ± 3.04 | 14.24 ± 3.23 | 12.02 ± 1.38 | 0.514 |
| AUC glucose (mmol/liter−1·min−1) | 10.29 ± 1.54 | 9.13 ± 2.09b | 11.15 ± 1.80 | 8.51 ± 1.22a | 0.014 |
| Fasting insulin (pmol/liter) | 192 ± 108 | 110 ± 50a | 172 ± 69 | 127 ± 51b | 0.195 |
| Peak insulin (pmol/liter) | 545 ± 357 | 575 ± 522 | 492 ± 288 | 769 ± 335b | 0.286 |
| AUC insulin (pmol/liter−1·min−1) | 363 ± 237 | 350 ± 268a | 349 ± 188 | 341 ± 138 | 0.974 |
| Fasting C peptide (pmol/liter) | 4.09 ± 2.27 | 3.47 ± 2.35 | 4.04 ± 1.71 | 3.71 ± 1.01 | 0.656 |
| AUC C-peptide (pmol/liter−1·min−1) | 2.20 ± 1.01 | 2.19 ± 1.26 | 2.12 ± 0.77 | 2.48 ± 0.62 | 0.304 |
| Fasting glucagon (ng/liter) | 71.6 ± 10.6 | 58.5 ± 16.0b | 65.9 ± 15.0 | 67.0 ± 21.8 | 0.002 |
| AUC glucagon (ng/liter−1·min−1) | 58.1 ± 14.5 | 49.2 ± 12.4b | 58.4 ± 10.8 | 77.3 ± 17.5b | 0.002 |
| Peak glucagon (ng/liter) | 84.7 ± 24.2 | 68.5 ± 19.7b | 81.1 ± 14.8 | 96.9 ± 21.5b | 0.002 |
| Fasting proinsulin (pmol/liter) | 34.6 ± 26.1 | 16.3 ± 9.6b | 31.8 ± 17.0 | 19.2 ± 22.5a | 0.876 |
| Proinsulin/insulin | 0.18 ± 0.11 | 0.16 ± 0.11 | 0.19 ± 0.07 | 0.16 ± 0.19a | 0.082 |
| Fasting total GLP-1 (pmol/liter) | 6.18 ± 2.87 | 6.34 ± 4.36 | 6.52 ± 4.06 | 6.69 ± 3.28 | 0.998 |
| Peak total GLP-1 (pmol/liter) | 18.20 ± 16.33 | 9.80 ± 5.83 | 17.49 ± 6.02 | 112.5 ± 54.3a | 0.001 |
| AUC total GLP-1 (pmol/liter−1·min−1) | 8.20 ± 7.29 | 4.94 ± 1.96 | 7.55 ± 2.80 | 31.82 ± 8.10a | 0.000 |
| Fasting active GLP-1 (pmol/liter) | 6.04 ± 3.55 | 4.28 ± 0.90 | 7.91 ± 3.77 | 8.45 ± 4.41 | 0.216 |
| Peak active GLP-1 (pmol/liter) | 10.85 ± 9.73 | 5.27 ± 1.74 | 11.21 ± 3.94 | 24.13 ± 19.31 | 0.038 |
| AUC active GLP-1 (pmol/liter−1·min−1) | 6.43 ± 3.69 | 4.25 ± 0.96 | 7.38 ± 2.98 | 10.88 ± 4.94 | 0.029 |
| Fasting GIP (ng/liter) | 34.18 ± 11.41 | 33.84 ± 33.11 | 39.27 ± 15.05 | 40.54 ± 29.87 | 0.901 |
| Peak GIP (ng/liter) | 175 ± 60 | 208 ± 115 | 204 ± 56 | 316 ± 124a | 0.090 |
| AUC GIP (ng/liter−1·min−1) | 40.96 ± 12.71 | 54.00 ± 31.85 | 48.67 ± 11.35 | 51.56 ± 18.54 | 0.399 |
| HOMA-IR | 7.96 ± 4.17 | 4.04 ± 1.91a | 8.11 ± 3.61 | 5.16 ± 2.88b | 0.476 |
Data are expressed as mean ± sd. Fasting, peak, 120 min, and AUC (total AUC, 180′) values are obtained during the OGTT. The reported P value represents the difference between the changes occurring with either weight loss intervention. HOMA-IR, homeostasis model of assessment of insulin resistance.
P < 0.001, effect of weight loss within each group (diet, n = 10; or GBP, n = 9).
P < 0.05 effect of weight loss within each group (diet, n = 10; or GBP, n = 9).
Side effects
Although 50 g glucose drink was used rather than 75 g to minimize the risk of dumping syndrome after GBP, four patients experienced stomach cramping and discomfort, nausea, sweating, flushing, and palpitations 5–20 min into the OGTT. No severe adverse effects were observed. There was no adverse effect from the diet.
Effect of weight loss
All patients in the surgical group discontinued their diabetes medications the day of the surgery. In the diet group, diabetes medications were either discontinued (n = 2), or the dosage was decreased, with patients taking only low doses of metformin (n = 8) at the completion of the weight loss. The duration of weight loss was shorter for the GBP group (32.3 ± 13.1 d) compared with the diet group (55.0 ± 9.9 d; P = 0.001). Body weight, BMI, fasting glucose, insulin, C peptide, proinsulin, proinsulin to insulin ratio, and homeostasis model of assessment of insulin resistance decreased significantly and equally in the surgical and diet groups (Table 1). Fasting incretins did not change with either weight loss treatment.
Glucose AUC and glucose levels at 120′ were significantly lower after GBP compared with diet (P = 0.014 and P = 0.001, respectively) (Table 1). Although the changes of insulin with weight loss (fasting, AUC, peak response) were not different between GBP and diet, the pattern of secretion of insulin changed considerably after GBP (P = 0.001), with recovery of the early phase with a peak at 30 min and a return to baseline after 180 min (Fig. 1).
Figure 1.
Glucose, insulin, C peptide, glucagon, total and active GLP-1 and GIP levels during the OGTT in patients before (diamond) and after (square) GBP, and before (triangle) and after (circle) diet. Data are mean ± sem. *, P < 0.05 between groups after weight loss by GBP or diet.
Stimulated levels of incretins increased significantly after surgery by a factor of six (peak GLP-1: 17 ± 6 to 112 ± 54 pmol/liter; P < 0.001), 2.1 (peak GLP-1 active; P = 0.038), and 1.5 (peak GIP; P = 0.006). (Table 1 and Fig. 1). On the contrary, after diet, GLP-1 levels (total and active) tended to decrease (P, Not significant), and GIP did not change significantly. There was no change in GLP-1 or GIP levels during the IsoG IVGT (data not shown). A significant linear relationship was found between total GLP-1 release and insulin release during the OGTT, only in patients after GBP (r = 0.762; P = 0.028).
The glucose concentrations were well matched between the IsoG IVGT and OGTT in the surgical and diet groups before and after the weight loss intervention (Table 2). The insulin response was not greater after oral than iv glucose before GBP or before diet, with a resulting blunted incretin effect. The incretin effect increased significantly by a factor of 3.8 after GBP (+35.4 ± 22.7%; P = 0.009), but only minimally after diet (+7.15 ± 18.13%; P = 0.244).
Table 2.
Mean glucose, insulin, and C peptide levels, AUC for glucose and insulin during the OGTT and Iso IVGT, before and after weight loss by either diet (n = 10) or GBP (n = 9)
| Before GBP | After GBP | Before diet | After diet | |||||
|---|---|---|---|---|---|---|---|---|
| Oral | IV | Oral | IV | Oral | IV | Oral | IV | |
| GAUC (mmol/liter·min) | 11.2 ± 1.8 | 11.4 ± 1.8 | 8.5 ± 1.2 | 9.8 ± 1.2a | 10.3 ± 1.5 | 10.1 ± 1.6 | 9.1 ± 2.1 | 8.9 ± 2.3 |
| Glucose (mmol/liter) | 10.7 ± 1.9 | 10.9 ± 1.8 | 8.5 ± 1.0 | 9.4 ± 0.9 | 9.8 ± 1.2 | 9.6 ± 1.3 | 8.4 ± 1.8 | 8.3 ± 1.9 |
| IAUC (pmol/liter·min) | 349 ± 188 | 296 ± 154 | 340 ± 145 | 183 ± 75a | 363 ± 237 | 250 ± 131 | 350 ± 268 | 220 ± 165 |
| Insulin (pmol/liter) | 315 ± 152 | 261 ± 127 | 347 ± 138 | 170 ± 62a | 330 ± 210 | 218 ± 113 | 302 ± 191 | 195 ± 130 |
| C peptide (pmol/liter) | 1.95 ± 0.7 | 1.97 ± 0.57 | 2.31 ± 0.54 | 1.58 ± 0.4a | 1.98 ± 0.92 | 1.62 ± 0.81 | 1.89 ± 1.05 | 1.55 ± 0.95 |
| Incretin effect (%) | 9.42 ± 27.46 | 44.84 ± 12.73b | 27.44 ± 14.909 | 34.59 ± 15.15 | ||||
Data are expressed as mean ± sd. The incretin effect was calculated by comparing the insulin response to oral and matched iv glucose load. GAUC, Glucose area under the curve 0−180; IAUC, insulin area under the curve 0−180.
Between oral and iv glucose challenges within each group (P < 0.05).
Between before and after weight loss by either GBP or diet (P = 0.002).
Glucagon levels were mildly suppressed during the OGTT from 90–180 min (P < 0.001), with no difference between the GBP and diet groups before intervention (P = 0.351). After diet, glucagon levels remained similarly suppressed during the OGTT. However, after GBP, there was a paradoxical increase of glucagon levels during the OGTT from 15–120 min (P = 0.001) (Fig. 1).
Discussion
The magnitude of the effect of GBP on T2DM resolution has thus far baffled scientists and clinicians. Many studies (5,6,8,10,20,27,28,29,30), including ours (4), have shown an increase of incretin levels after GBP. It is unclear whether the caloric restriction and/or rapid weight loss contributes to the change of the incretins levels because diet-induced weight loss has also been associated with an increase, although of lesser magnitude, of GLP-1 levels (22). In this study we compared the effect of an equivalent weight loss by GBP or by diet in two groups of matched morbidly obese patients with T2DM. The intensive diet intervention with meal replacements and weekly outpatient visits resulted in a weight loss equivalent to that lost by patients 1 month after the GBP. Although the well-matched surgical and diet groups lost the same amount of weight, their changes in incretin levels were strikingly different. As shown previously (4), GLP-1 response to oral glucose markedly increased 1 month after GBP. GLP-1 levels tended to decrease after diet intervention, although this decrease was not significant. This is contrary to the results by Verdich et al. (22), who showed an increase of GLP-1 levels, although of smaller magnitude, during a mixed meal after weight reduction in men. These differences between studies could be due to gender differences, the absence of T2DM, a greater weight loss (18.8 kg), and/or the use of a solid mixed meal as a stimulus in the study by Verdich et al. (22). Our data on increase GIP levels after GBP are consistent with data after jejunoileal bypass (JIB) (31) but contrary to another study showing a decrease of GIP levels in patients without T2DM 6 months after GBP (30). The time of testing after surgery, which varied between studies, could be an important variable because we have shown that the increase of GIP levels is transient and does not persist 6–12 months after GBP (32). In parallel with the increase in incretin levels, the incretin effect markedly increased after GBP, but not after diet. These results need to be interpreted with caution because the sample size was small with large individual variation.
The data from this study suggest that the effect of GBP on the incretins is likely not weight loss related. However, the mechanism by which the incretins increase after GBP remains unclear. Whether it is the rapid and direct stimulation of the L cells of the distal ileum, referred to as the hindgut mechanism, or the bypass of the duodenum (the foregut hypothesis) is still unclear. Elegant studies in rodents support the foregut hypothesis. In these studies in Goto-Kakizaki type 2 diabetic rats, the improvement of glucose tolerance after surgical exclusion of the duodenum, but not after gastrojejunostomy, is independent of calorie restriction or weight loss (33). The hindgut hypothesis is based on the results of experimental ileal transposition, a surgical procedure that improves diabetes in rodents (23) independently of weight loss (24). The foregut/hindgut hypothesis has not been tested in humans. Recent data demonstrate that ileal transposition with sleeve gastrectomy can improve diabetes, even after minimal weight loss, in patient with BMI less than 35 kg/m2 (34). Our experiment was designed to address the effect of weight loss on incretins and did not allow us to separate the effect of duodenal bypass vs. rapid stimulation of the distal gut on incretin stimulation after GBP. Gastric emptying (GE) and intestinal transit time have increased after GBP (27,35), but not after diet (22). The release of GIP and GLP-1 is related to the rate of carbohydrate entry into the small intestine (36). Faster small intestinal glucose delivery increases plasma GIP and GLP-1 levels (37). The rapid delivery of nutrient after GBP could represent a mechanism by which incretins are markedly released after the surgery. We did not measure GE or intestinal transit time in our study. Therefore, we cannot exclude the possibility that, in the diet group, the glucose solution was absorbed entirely in the duodenum and did not reach the lower part of the ileum to exert its stimulating effect directly on the L cells to release GLP-1. However, recent data showed that the enteroendocrine K and L cells, which secrete GIP and GLP-1, respectively, are distributed all along the gut (38). Therefore, it is unlikely that the L cells would have had no contact with the glucose solution in the diet group.
We cannot exclude that gut adaptation played a role in the increased incretin response after GBP because hyperplasia of intestinal endocrine cells have been described 3 months after JIB (39). We have shown persistent increased GLP-1 levels 1 yr after GBP (32), and others have shown increased incretin levels 20 yr after JIB (31). Adaptative changes in gut motility have also been shown after a period of energy restriction and weight loss (40). We cannot exclude a role of calorie restriction per se, independently of weight loss, in the improvement of incretins after GBP. The daily calorie intake of patients after GBP was 600–800 kcal (data not shown) compared with 1000 kcal in the diet group. Although the calorie restriction was not matched between the two groups from day to day, the overall calorie deficit and weight loss were identical.
Diet-induced (41,42,43,44,45,46) or surgical weight loss (3,47) improves T2DM control. In this study the effect of an equivalent weight loss on diabetes control was greater after GBP than after diet. Patients in the surgical group had a better clinical outcome and did not require diabetes medications after the weight loss. In addition, postprandial glucose levels were lower after GBP compared with diet. In the fed state, GE (36,37), glucose absorption (48), and the release of incretins (12) are key determinants of postprandial glucose levels. In patients with T2DM, many components of the gut physiology are impaired, such as GE (40,49), and incretin release and effect (14), resulting in postprandial hyperglycemia, a predictor of cardiovascular complications and mortality (50). Our data show that a diet-equivalent weight loss does not lower postprandial glucose levels to the same extent as GBP in patients with T2DM. This may indicate that the marked increase of incretins associated with GBP, and not with the diet, could be responsible for the better postprandial glucose control. The administration of the synthetic exendin-4, a compound that binds to the GLP-1 receptor and exerts similar effects as the native GLP-1 (51), or of vildagliptin, an inhibitor of DPPIV, the enzyme that rapidly inactivates the endogenous incretins (52,53), has been shown to reduce postprandial glucose levels and improve diabetes control.
Patients with T2DM have typically hyperglucagonemia (54), which contributes to the postprandial hyperglycemia. It is puzzling to see that the decrease of postprandial glucose levels after GBP is associated with a paradoxical increase of glucagon levels during the OGTT. The increase in glucagon is seen despite a marked increase in GLP-1, a gut hormone that inhibits glucagon release (55). This increase of glucagon levels was previously shown after GBP (56) and ileal transposition in dogs (56). The source of this increase in glucagon is unclear. Although the commercial assay used in this study is specific for pancreatic glucagon, cross-reactivity with enteroglucagon or oxyntomodulin cannot be entirely excluded. Our study group was small and limited to women. Future studies will need to address gender differences in incretin levels and effect after bariatric surgery.
In summary, our data suggest that it is the surgical procedure per se, rather than weight loss, that stimulates incretin release and effect after GBP. The rapid and marked increase of GLP-1 levels after GBP plays an important role in insulin secretion, and could be a key determinant in the decrease of postprandial glycemia and the resolution of T2DM after GBP.
Acknowledgments
We thank our volunteer participants, Ping Zhou and Yim Dam for their technical help, and Mousumi Bose for editing this manuscript.
Footnotes
This work was supported by grants from the American Diabetes Association CR-7-05 CR-18, NIH R01-DK67561, GCRC 1 UL1 RR024156-02, ORC DK-26687, DERC DK-63068-05, Merck Investigator Initiated Studies Program.
Disclosure Statement: B.L. received grant support through the Merck Investigator Initiated Studies Program in 2007. J.T., J.M., H.T., J.R.E., A.C., B.K., B.B., N.K., H.L., K.Y., and B.O. have nothing to declare.
First Published Online April 22, 2008
Abbreviations: AUC, Area under the curve; BMI, body mass index; CV, coefficient of variation; DPPIV, dipeptidyl peptidase-IV; GBP, Gastric bypass surgery; GE, gastric emptying; GIP, gastric inhibitory peptide; GLP, glucagon-like peptide; HbA1c, glycosylated hemoglobin; IsoG, isoglycemic; IVGT, iv glucose test; JIB, jejunoileal bypass; OGTT, oral glucose tolerance test; T2DM, type 2 diabetes.
References
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM 2004 Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291:2847–2850 [DOI] [PubMed] [Google Scholar]
- Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I 2007 Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery 142:621–632 [DOI] [PubMed] [Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K 2004 Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B 2007 Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen KB, Christensen KC, Stokholm KH 1980 Gastric inhibitory polypeptide (GIP) release and incretin effect after oral glucose in obesity and after jejunoileal bypass. Scand J Gastroenterol 15:489–495 [DOI] [PubMed] [Google Scholar]
- Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ 1990 Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 211:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund E, Bogefors J, Skogar S, Gryback P, Jacobsson H, Holst JJ, Hellstrom PM 1999 GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol 277(3 Pt 2):R910–R916 [DOI] [PubMed] [Google Scholar]
- Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL 2004 Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg 70:1–4 [PubMed] [Google Scholar]
- Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, Delgado S, Casamitjana R, Vidal J 2006 Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 91:1735–1740 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR 2006 Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SR, Polak JM 1980 Gut hormones. Adv Clin Chem 21:177–244 [DOI] [PubMed] [Google Scholar]
- Perley MJ, Kipnis DM 1967 Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest 46:1954–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R 2004 Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 113:635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M, Stockmann F, Ebert R, Creutzfeldt W 1986 Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29:46–52 [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ 2001 Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723 [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Madsbad S, Holst JJ 1999 Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 22:1137–1143 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA 2006 Incretins and the development of type 2 diabetes. Curr Diab Rep 6:194–201 [DOI] [PubMed] [Google Scholar]
- Holst JJ 2007 The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- Flock G, Baggio LL, Longuet C, Drucker DJ 2007 Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes 56:3006–3013 [DOI] [PubMed] [Google Scholar]
- Green BD, Flatt PR 2007 Incretin hormone mimetics and analogues in diabetes therapeutics. Best Pract Res Clin Endocrinol Metab 21:497–516 [DOI] [PubMed] [Google Scholar]
- Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V 1996 Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38:916–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdich C, Toubro S, Buemann B, Lysgard MJ, Juul HJ, Astrup A 2001 The role of postprandial releases of insulin and incretin hormones in meal-induced satiety–effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25:1206–1214 [DOI] [PubMed] [Google Scholar]
- Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ 2005 Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 288:E447–E453 [DOI] [PubMed] [Google Scholar]
- Patriti A, Facchiano E, Annetti C, Aisa MC, Galli F, Fanelli C, Donini A 2005 Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 15:1258–1264 [DOI] [PubMed] [Google Scholar]
- Depaula AL, Macedo AL, Rassi N, Machado CA, Schraibman V, Silva LQ, Halpern A 2008 Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc 22:706–716 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W 1986 Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 63:492–498 [DOI] [PubMed] [Google Scholar]
- Morinigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J 2006 GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg 16:1594–1601 [DOI] [PubMed] [Google Scholar]
- Naslund E, Backman L, Holst JJ, Theodorsson E, Hellstrom PM 1998 Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg 8:253–260 [DOI] [PubMed] [Google Scholar]
- Valverde I, Puente J, Martin-Duce A, Molina L, Lozano O, Sancho V, Malaisse WJ, Villanueva-Penacarrillo ML 2005 Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg 15:387–397 [DOI] [PubMed] [Google Scholar]
- Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ 2007 Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund E, Gryback P, Backman L, Jacobsson H, Holst JJ, Theodorsson E, Hellstrom PM 1998 Distal small bowel hormones: correlation with fasting antroduodenal motility and gastric emptying. Dig Dis Sci 43:945–952 [DOI] [PubMed] [Google Scholar]
- Laferrère B, Tran H, Egger J, Teixeira J, McGinty J, Yap K, Bawa B, Olivan B 2007 The increase in GLP-1 levels and incretin effect after Roux-en-Y gastric bypass surgery (RYGBP) persists up to 1 year in patients with type 2 diabetes mellitus (T2DM). Obesity 15:7 (Abstract OR-21) [Google Scholar]
- Rubino F 2006 Bariatric surgery: effects on glucose homeostasis. Curr Opin Clin Nutr Metab Care 9:497–507 [DOI] [PubMed] [Google Scholar]
- Cohen RV, Schiavon CA, Pinheiro JS, Correa JL, Rubino F 2007 Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg Obes Relat Dis 3:195–197 [DOI] [PubMed] [Google Scholar]
- Horowitz M, Collins PJ, Harding PE, Shearman DJ 1986 Gastric emptying after gastric bypass. Int J Obes 10:117–121 [PubMed] [Google Scholar]
- Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B 1996 Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaikomin R, Doran S, Jones KL, Feinle-Bisset C, O’Donovan D, Rayner CK, Horowitz M 2005 Initially more rapid small intestinal glucose delivery increases plasma insulin, GIP, and GLP-1 but does not improve overall glycemia in healthy subjects. Am J Physiol Endocrinol Metab 289:E504–E507 [DOI] [PubMed] [Google Scholar]
- Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM 2006 Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290:E550–E559 [DOI] [PubMed] [Google Scholar]
- Buchan AM, Pederson RA, Koop I, Gourlay RH, Cleator IG 1993 Morphological and functional alterations to a sub-group of regulatory peptides in human pancreas and intestine after jejuno-ileal bypass. Int J Obes Relat Metab Disord 17:109–113 [PubMed] [Google Scholar]
- Cunningham KM, Daly J, Horowitz M, Read NW 1991 Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut 32:483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GC, Coulston AM, Lardinois CK, Hollenbeck CB, Moore JG, Reaven GM 1985 Moderate weight loss and sulfonylurea treatment of non-insulin-dependent diabetes mellitus. Combined effects. Arch Intern Med 145:665–669 [PubMed] [Google Scholar]
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM 1986 Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 35:155–164 [DOI] [PubMed] [Google Scholar]
- Maggio CA, Pi-Sunyer FX 1997 The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes Care 20:1744–1766 [DOI] [PubMed] [Google Scholar]
- Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D 1987 Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med 147:1749–1753 [PubMed] [Google Scholar]
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH 1991 Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 151:1334–1340 [PubMed] [Google Scholar]
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J 1994 Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 97:354–362 [DOI] [PubMed] [Google Scholar]
- Sjostrom CD, Lissner L, Wedel H, Sjostrom L 1999 Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 7:477–484 [DOI] [PubMed] [Google Scholar]
- Modigliani R, Bernier JJ 1971 Absorption of glucose, sodium, and water by the human jejunum studied by intestinal perfusion with a proximal occluding balloon and at variable flow rates. Gut 12:184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak TV, Johnson CP, Kalbfleisch JH, Roza AM, Wood CM, Weisbruch JP, Soergel KH 1995 Highly variable gastric emptying in patients with insulin dependent diabetes mellitus. Gut 37:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter LA, Ceriello A, Davidson JA, Hanefeld M, Monnier L, Owens DR, Tajima N, Tuomilehto J 2005 Postprandial glucose regulation: new data and new implications. Clin Ther 27(Suppl B):S42–S56 [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y 2003 Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88:3082–3089 [DOI] [PubMed] [Google Scholar]
- Ahren B, Pacini G, Tura A, Foley JE, Schweizer A 2007 Improved meal-related insulin processing contributes to the enhancement of B-cell function by the DPP-4 inhibitor vildagliptin in patients with type 2 diabetes. Horm Metab Res 39:826–829 [DOI] [PubMed] [Google Scholar]
- Vella A, Rizza RA 2004 Extrapancreatic effects of GIP and GLP-1. Horm Metab Res 36:830–836 [DOI] [PubMed] [Google Scholar]
- Muller WA, Faloona GR, Aguilar-Parada E, Unger RH 1970 Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283:109–115 [DOI] [PubMed] [Google Scholar]
- Drucker DJ 2002 Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology 122:531–544 [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME 2007 Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 92:4678–4685 [DOI] [PubMed] [Google Scholar]



