Abstract
Context: High plasma adiponectin concentrations in human fetuses and neonates are unique features of early developmental stages. Yet, the origins of the high adiponectin concentrations in the perinatal period remain elusive.
Objective: This study was undertaken to identify the sources and functional properties of adiponectin in utero.
Design and Methods: Tissue specimens were obtained at autopsy from 21- to 39-wk-old stillborn human fetuses. Adipose tissue and placenta were obtained at term elective cesarean section. Adiponectin complexes and expression were measured by immunodetection and real-time PCR.
Results: Adiponectin mRNA transcripts were detected in fetal sc and omental adipose depots at lower concentrations than in maternal adipose tissue. Immunoreactive adiponectin was also observed in vascular endothelial cells of fetal organs, including skeletal muscle, kidney, and brain. The absence of adiponectin in all placental cell types and lack of correlation between maternal and umbilical adiponectin indicate that umbilical adiponectin reflects its exclusive production by fetal tissues. The most prominent forms of adiponectin in fetal plasma were high and low molecular mass (HMW and LMW) multimers of 340 and 160 kDa, respectively. The proportion of the HMW complexes was 5-fold (P < 0.001) higher in umbilical plasma than in adult. The high HMW and total adiponectin levels were associated with lower insulin concentration and lower homeostasis model of assessment of insulin resistance indices in umbilical plasma, reflecting higher insulin sensitivity of the fetus compared with adult.
Conclusions: The abundance of HMW adiponectin and its vascular expression are characteristics of human fetal adiponectin. Combined with high insulin sensitivity, fetal adiponectin may be a critical determinant of in utero growth.
Examination of adipose tissue and placenta from 21-39-week-old stillborn human fetuses reveals a high abundance of high molecular weight adiponectin in skeletal muscle, white adipose tissue, kidney, and brain, implying that like high insulin sensitivity, adiponectin may be a critical determinant of intrauterine growth.
The multimeric adiponectin protein is the most abundant hormone produced by the adipose tissue of humans and rodents (1,2). Adiponectin production is inversely related to adipose tissue mass and, once released in the peripheral blood, exerts pleiotropic insulin-sensitizing effects (3,4). Consequently, plasma adiponectin concentrations are positively correlated with overall insulin sensitivity, showing significant decreases in obesity, diabetes, or lipodystrophy (5,6,7). A lack of adiponectin has also been associated with different vascular manifestations of the metabolic syndrome (8). The low molecular weight (LMW) hexamers and the high molecular weight (HMW) 12–16-mers secreted by the adipocytes represent the predominant forms of adiponectin circulating in plasma (9). Bioactivity studies have suggested that HMW rather than LMW are the functional components of adiponectin as it relates to the regulation of insulin sensitivity (10,11). Based on its functional properties, measurements of the ratio of plasma level of HMW adiponectin to that of total adiponectin (HMW ratio) rather than total adiponectin have been proposed as a functional biomarker for the prediction of insulin resistance and the metabolic syndrome (12).
During pregnancy, plasma adiponectin concentrations are decreased compared with pre-gravid as a consequence of reduced gene expression in white adipose tissue (13,14). Adiponectin concentration is lower in gestational diabetes compared with nondiabetic pregnancy, consistent with greater insulin resistance in this condition (15). The low levels of adiponectin in pregnant women contrast with the high adiponectin concentrations in the fetus (16,17). Moreover, high adiponectin levels in neonates indicate that the hyperadiponectinemia is a common feature of early developmental stages in humans (18). In contrast to other adipocytokines that have a ubiquitous tissue distribution, adiponectin is exclusively synthesized by adipocytes in adults (1,19), but little data are available in fetal life. The aim of this study was to identify the source of fetal plasma adiponectin.
Patients and Methods
Tissue collection
The use of human material conformed to the principal outlined in the declaration of Helsinki. Subcutaneous and omental adipose tissue specimens (150–300 mg) from eight stillborns were obtained at postmortem examination. The gestational ages of the fetuses ranged from 21–39 wk, with birth weight of 774-3183 g. None of the stillborns showed any evidence of maceration. The postmortem examinations were performed within 5 h after demise. Tissue fragments for RNA analysis were immediately snap frozen in liquid nitrogen and stored at −80 C before processing. Additional tissue fragments of kidney, skin, adrenal with adjacent adipose tissue, and from cerebral cortex were obtained from four term fetuses (38–39 wk) as well as adipose tissue from one 21-wk-old fetus. Tissues were fixed in 4–10% formalin and embedded in paraffin for use in immunohistochemistry. Subcutaneous fat tissue (1–3 g) and blood samples were obtained in the fasting state from 56 lean and 72 obese nonlaboring women without evidence of clinical infection at elective cesarean section delivery. Placenta tissue and umbilical venous cord blood were obtained upon delivery of the placenta. The study was approved by the institution review board of MetroHealth Medical Center.
Immunohistochemistry
Adiponectin was localized in maternal and fetal paraffin-embedded tissue sections by indirect immunofluorescence. Sections were rehydrated and rinsed in a 1:20 dilution of Tris-buffered saline-Triton X-100. Normal rabbit serum solutions [20% in Tris-HCl buffer (pH 7.6)] were used to reduce the background. Mouse monoclonal antiadiponectin antibody (1:250 dilution) was added for 30 min, followed by sequential 10-min incubations with secondary fluorescein isothiocyanate, and tetramethylrhodamine isothiocyanate antibodies (United States Biological, Inc., Swampscott, MA). Anti-CD34 antibody (CHEMICON International, Inc., Tremecula, CA) was used for double staining of endothelial cells. Sections were illuminated and photographed using a fluorescent microscope (Nikon, Melville, NY). The capture of digital pictures was made with SPOT software (Diagnostic Instruments, Inc., Sterling Heights, MI).
RNA purification and cDNA synthesis
Total RNA was extracted from adipose tissue using TRIzol (Invitrogen Corp., Carlsbad, CA). Total RNA was purified from white blood cells using the RNeasy kit (QIAGEN, Inc., Valencia, CA). Integrity was verified by Agilent Technologies, Inc. (Palo Alto, CA). RNA was reversed transcribed using the Superscript II Rnase H Reverse Transcriptase system (Invitrogen). Adiponectin and actin gene expression was measured by real-time PCR using the LightCycler FastStart DNA Master SYBR Green I kit (Roche, Indianapolis, IN). Amplifications were performed in duplicate using 30 ng cDNA using the primer pairs as follows: actin (NM_001101), forward 5′-ggacttcgagcaagagatgg-3′ and reverse 5′-agcactgtgttggcgtacag-3′; and adiponectin (NM_004797), forward 5′-gcagtctgtggttctgattcc-3′ and reverse 5′-ccagtggagccatcatagtg-3′. Results normalized for actin were expressed as copy number per ng RNA calculated from an adipose tissue cDNA standard curve.
Western blots
Maternal and venous umbilical cord plasma (1 μl, ∼60 μg protein) was electrophoresed under denaturing and nondenaturing conditions, and transferred to nitrocellulose membrane. Membranes were blocked overnight then incubated for 2 h with primary antibody for rabbit polyclonal anti-adiponectin (CHEMICON International), then incubated with goat antirabbit IgG horseradish peroxidase-conjugated secondary antibody. Immunocomplexes were visualized by chemiluminescence (Amersham Biosciences Inc., Piscataway, NJ). The abundance of HMW adiponectin multimers was determined by densitometry with a gel-doc system (Bio-Rad Laboratories, Inc., Hercules, CA) and expressed as percent total adiponectin multimers. Total plasma adiponectin was determined by ELISA (LINCO Research, Inc., Billerica, MA).
Plasma assays
All plasma samples were run in duplicate in a single assay. Total plasma adiponectin was determined by ELISA. Insulin was measured by RIA kits (LINCO). Glucose was assessed by the glucose oxidase method using a glucose analyzer (Yellow Springs Instrument Inc., Yellow Springs, OH).
Data analysis
The homeostasis model of assessment (HOMA) index of insulin sensitivity was calculated as: glucose (mmol/liter) insulin (μU/ml)/22.5. Statistical significance was determined using ANOVA, followed by the Fisher’s projected least significant difference test. Pearson’s correlation coefficient was used to determine the relationship between maternal and umbilical cord adiponectin. Values are given as mean ± sem.
Results
Detection of adiponectin in fetal tissues
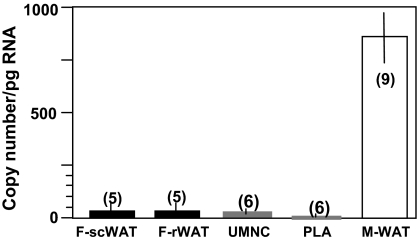
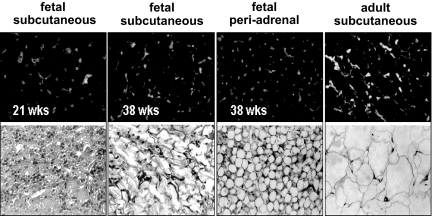
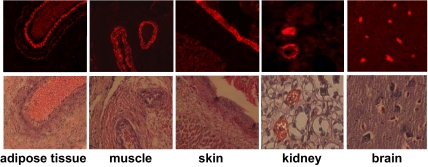
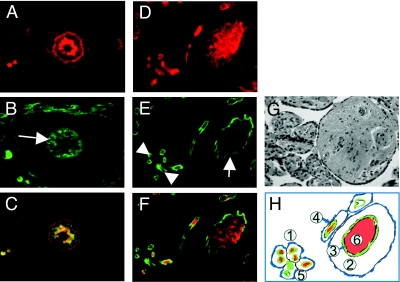
To investigate the origin of high adiponectin concentrations during fetal life, we have characterized adiponectin gene and protein expression in several fetal tissues as potential in utero sources of adiponectin. Adiponectin mRNA was detected by real-time PCR in white adipose tissue of the fetus, albeit at a much lower concentration than in maternal adipose tissue (3.8 ± 0.5 vs. 840 ± 82 copies per μg RNA). Adiponectin mRNA transcripts were also detected at a low level in umbilical white blood cells (0.03 ± 0.006 copies per ng RNA), and the copy numbers were at the limit of detection (0.005 ± 0.002 copies per ng RNA) in placental villous tissue (Fig. 1), with an average Ct value of 35. The immunoreactive adiponectin protein was identified in sc and omental adipose tissue of term and preterm fetuses (Fig. 2), and the protein identity was confirmed by Western blot of adipose homogenates (data not shown). In addition to adipose tissue, adiponectin-specific immunoreactive signals were observed in vessels and vessel walls of several fetal organs, including skeletal muscle, kidney, skin, and cortex (Fig. 3). Double labeling with CD34 identified the endothelial cells as the source of adiponectin expression in capillaries of fetal tissues but not in placental endothelial cells or any other placental cell type, including trophoblast (Fig. 4). Adiponectin immunoreactivity was abundant in the fetal blood trapped within the fetal vessels of the placental stem and terminal villi.
Figure 1.
Adiponectin mRNA expression. Results of RT-PCR determinations from individual tissue samples are normalized for actin and expressed as mean ± sem. Maternal sc white adipose tissue were from term elective cesarean section delivery (M-WAT). F-scWAT, Fetal sc white adipose tissue from 38- to 39-wk-old fetuses; F-rWAT, fetal retroperitoneal white adipose tissue from 38- to 39-wk-old fetuses; n, number of tissue samples analyzed in duplicate; PLA: placenta, UMNC, umbilical mononuclear cells.
Figure 2.
Immunolocalization of adiponectin in fetal white adipose tissue. Upper panels, Immunoreactivity is localized in the stroma surrounding the adipocytes. Lower panels, hematoxylin-eosin counterstaining of duplicate sections. Original magnification, ×40. Sections are a representative analysis of three individual tissue samples from each depot and gestational age.
Figure 3.
Vascular expression of fetal adiponectin. Immunoreactivity is detected in vascular cells of multiple fetal tissues at 38- to 39-wk gestation. In the adipose tissue, muscle, skin, and kidney, the thickness of the labeling in the vessel walls, suggests significant staining of the media in addition to the endothelial staining (intima). In the brain, the vessels do not have a media, thus only the endothelial layer and the plasma are stained. Lower panels, Hematoxylin-eosin counterstaining of duplicate sections. Original magnification, ×100. Sections are representative of two individual tissue samples.
Figure 4.
Immunolocalization of adiponectin in fetal vascular endothelial cells. A–C, Representative tissue sections showing a small artery (arrow) in retroperitoneal adipose tissue from one 39-wk-old fetus. D–F, Tissue section of a 39-wk placenta showing fetal blood vessels in a stem villous (arrow) and terminal villi (arrowheads). A and D, Adiponectin (red) tetramethylrhodamine isothiocyanate immunoreactivity. B and E, CD34 (green) fluorescein isothiocyanate immunoreactivity. C and F, Merging adiponectin and CD34 signals (yellow) show strong adiponectin immunoreactivity in the media of a small artery of fetal adipose tissue (C) and in the blood present within the fetal circulation in the stem villous (F). Analysis of four individual sections from fetal and placenta tissue. G, Hematoxylin-eosin staining of a placental section at 39-wk gestation. H, Schematic representation of placental architecture. 1, Intervillous space where maternal circulation takes place. 2, Stem villous. 3, Endothelial cells of fetal vessels. 4, Trophoblast layer surrounding all placental villi (arrow). 5, Cluster of terminal villi showing endothelial lining around fetal vessels. 6, Fetal blood present in fetal vasculature of stem villous.
Distribution of adiponectin complexes
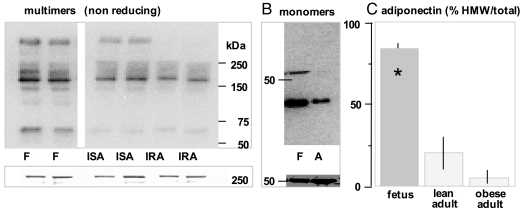
To examine the characteristics of fetal adiponectin, we measured total plasma adiponectin and quantified the absolute amount of low (LMW) and high (HMW) adiponectin multimers in umbilical and maternal venous plasma in 56 uncomplicated term pregnancies in lean women. Fetal adiponectin was detected as a protein of approximately 30 kDa under denaturing conditions, corresponding to the monomeric form of adiponectin similar to adult (Fig. 5B). However, the most prominent forms of circulating fetal adiponectin were multimeric complexes of 340 and 160 kDa corresponding to the assembly of 12 and six molecules of adiponectin with a minor representation of the 67-kDa trimers (Fig. 5A). The absolute amount of HMW was higher in umbilical than in maternal plasma (20.9 ± 8.6 vs. 4.5 ± 0.2 densitometry units; P < 0.001). The increased abundance of HMW adiponectin was reflected in the higher adiponectin indices (defined as the ratio of HMW/total adiponectin) in umbilical compared with maternal plasma: 2-fold higher than in insulin-sensitive subjects (0.35 ± 0.06 vs. 0.17 ± 0.04; P < 0.01) and 20-fold higher (0.35 ± 0.06 vs. 0.05 ± 0.02; P < 0.001) than in insulin-resistant subjects (Fig. 5C). Total adiponectin concentration was higher in umbilical compared with maternal plasma (42.4 ± 4.3 vs. 12.3 ± 1.8 μg/ml; P < 0.001), whereas HOMA indices were low in fetal plasma (1.1 ± 0.9) compared with that in insulin-sensitive and insulin-resistant pregnant women (2.2 ± 0.2 and 5.0 ± 0.4), respectively (Table 1). There was a strong negative correlation between maternal and fetal adiponectin and insulin concentrations (r = −0.54; P < 0.0001), as well as HOMA indices (r = −0.55; P < 0.0001).
Figure 5.
Distribution of adiponectin multimers in umbilical circulation. A, upper panel, Western blot under nonreducing and nondenaturing SDS-PAGE conditions showing HMW oligomers migrating at approximately 340 kDa, LMW hexamers migrating at 160 kDa, and trimers migrating at 67 kDa. Lower panel, Ponceau staining of the 250-kDa region of the same blot. B, Western blot under heat-denaturing reducing conditions showing monomeric adiponectin protein migrating at approximately 30 kDa. C, Distribution of HMW and LMW adiponectin complexes quantified by densitometry after 1 μl plasma samples were subjected to SDS-PAGE as shown in A. Data are means ± se with n = 6 in each group from the cohort of subjects described in Table 1. *, P < 0.001 between fetal (F) and adult (A). IRA, Insulin-resistant obese adult; ISA, insulin-sensitive lean adult.
Table 1.
Anthropometric and metabolic characteristics of subjects
| Fetal (n = 56) | Insulin-sensitive adult (n = 56) | Insulin-resistant adult (n = 72) | |
|---|---|---|---|
| Gestational age at delivery | 38.8 ± 0.6 | 38.8 ± 0.6 | 39.0 ± 0.6 |
| Ponderal index (g/cm3) | 2.7 ± 0.06 | ||
| Pre-gravid BMI (kg/m2) | 21.9 ± 2.0a | 38.7 ± 4.8 | |
| Plasma insulin (μU/ml) | 7.0 ± 0.5b,c | 11.6 ± 0.8a | 25.4 ± 1.7 |
| HOMA-IR | 1.1 ± 0.09b,c | 2.2 ± 0.2a | 5.0 ± 0.4 |
| Total plasma adiponectin (μg/ml) | 42.4 ± 4.3b,c | 13.3 ± 1.8d | 10.1 ± 0.6 |
Data are means ± se. The HOMA-IR is an index of insulin resistance that is calculated as plasma glucose: (mmol/liter)/insulin(μU/ml)/22.5. BMI, Body mass index; IR, insulin-resistant obese adult; IS, insulin-sensitive lean adult.
P < 0.001 in insulin-sensitive vs. insulin-resistant adults.
P < 0.001 in fetal vs. insulin-sensitive adults.
P < 0.001 in fetal vs. insulin-resistant adults.
P < 0.05 in insulin-sensitive vs. insulin-resistant adults.
Discussion
The tissue production sites responsible for the high plasma adiponectin concentrations in early human development have remained a matter of speculation despite prior investigation (17,20). A smaller relative fetal fat mass, and pleiotropic adipokine tissue production are potential causes for the high plasma adiponectin concentrations found in the late fetus compared with those in the adult. Based on the inverse relationship between circulating adiponectin and fat mass, the smaller percent body fat in human neonates (12–15% vs. 30–40%) could simply explain the higher adiponectin concentrations. Alternatively, the high umbilical adiponectin levels may reflect additional sources of production besides adipose tissue. Several adipocytokines originally described as adipose-specific protein products actually display a ubiquitous pattern of tissue distribution. For example, leptin has a very broad tissue distribution in adults that includes stomach, brain, bone, as well as in the placenta and in several tissues of the fetus (21). In sharp contrast, the expression of the adiponectin gene is restricted to white adipose tissue in adult humans and rodents (1).
We have addressed the possibility that multiple fetal tissues may produce adiponectin and thereby contribute to the higher concentration of circulating adiponectin during fetal life. Our results extend the original observation of Scherer’s group to fetal life showing adiponectin expression in several fat depots of the fetus (3). In addition, we show that adiponectin is highly expressed in vascular endothelial cells of fetal capillaries and in the media of larger fetal blood vessels of many tissues, including muscle, white adipose tissue, brain, and kidney (the only tissues studied to date in our laboratory). Interestingly, despite the fetal genotype of the placenta, adiponectin was not detected in placental endothelial cells lining the fetal circulation. These data provide definite support that the human placenta is not a site of endogenous adiponectin production, a finding in some but not all prior reports (22,23,24). We conclude that the adiponectin detected in human term placenta is accounted for by the adiponectin of fetal origin that circulates in the fetal vasculature of the placental villi (Fig. 3). In addition, the absence of correlation between maternal and umbilical adiponectin concentrations (y = 30.2 + 0.30x, r = 0.10; P = 0.31) supported that umbilical adiponectin primarily reflects the production by fetal tissues for lack of placental transfer. Whether or not adiponectin is produced by fetal brown adipocytes as described in vitro (25) and may also contribute to circulating adiponectin will require further investigation.
Multimer distribution
In addition to changes in body mass, insulin sensitivity is regarded as a potential confounder in adiponectin regulation (26). Data in rodents and humans indicate that adiponectin functions as an insulin sensitizer independently of the adipose tissue size (4,6,27,28). In addition, studies by Kadowaki and colleagues (9) have suggested that the multimer distribution reflects insulin sensitivity better than simply total circulating concentration. Our study shows that the HMW oligomers are the main adiponectin species circulating in fetal plasma. The higher proportion of the HMW multimers was associated with lower insulin concentrations and HOMA indices in umbilical compared with adult plasma, reflecting a high insulin sensitivity of the fetus. To the best of our knowledge, our study is the first to estimate insulin sensitivity in neonates from fasted mothers using the homeostasis model of assessment of insulin resistance (HOMA-IR). The higher insulin sensitivity at birth compared with adults is in line with that in preterm human neonates (29), suggesting that early development in humans is a period of remarkable insulin sensitivity.
Together, these data suggest that the specific fetal multimer distribution may relate to a higher bioactivity of adiponectin at early developmental stages, contributing to a higher sensitivity to insulin. The high adiponectin levels with increased bioactive HMW multimers found in the late fetus suggest that adiponectin may have different metabolic effects in utero than in the adult. Adiponectin is a potent inducer of adipogenesis, promoting preadipocyte differentiation into adipocytes, augmenting programmed gene expression for adipogenesis, and increasing lipid content and insulin responsiveness of the glucose transport system in adipocytes (30). Thus, the high adiponectin levels in the third trimester fetus may contribute to the 70% fetal fat accretion that occurs in this period (31). In addition, the angiogenic properties of adiponectin stimulating endothelial differentiation, vessel growth and remodeling (32,33) may also regulate the development of the vascular system of the fetus.
In conclusion, we show that fetal white adipose tissue and vascular endothelial cells are primary sources for adiponectin production in utero. The absence of endogenous placental adiponectin points to a distinct control of adiponectin production in the fetoplacental and maternal compartments. The abundance of circulating HMW multimers and specific vascular expression are unique characteristics of fetal adiponectin, which in combination with high insulin sensitivity may be key metabolic parameters for in utero growth.
Acknowledgments
We thank Judi Minium and Joan Lippus for skilful technical assistance with adiponectin plasma assays, and Pat Mencin for the recruitment of study subjects.
Footnotes
This work was supported by research support from the Diabetes Association of Greater Cleveland (to S.H.-d.M.) and National Institutes of Health Grant RO1-HD22965 (to P.M.C. and S.H.-d.M.).
Disclosure Information: The authors have nothing to declare.
First Published Online April 29, 2008
Abbreviations: HMW, High molecular weight; HOMA, homeostasis model of assessment; HOMA-IR, homeostasis model of assessment of insulin resistance; LMW, low molecular weight.
References
- Hu E, Liang P, Spiegelman BM 1996 AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703 [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y 1999 Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83 [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE 2001 The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953 [DOI] [PubMed] [Google Scholar]
- Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G 2003 Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 52:1779–1785 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T 2003 The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946 [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA 2001 Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- Haque WA, Shimomura I, Matsuzawa Y, Garg A 2002 Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab 87:2395–2398 [DOI] [PubMed] [Google Scholar]
- Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T 2004 Adiponectin as a biomarker of metabolic syndrome. Circ J 68:975–981 [DOI] [PubMed] [Google Scholar]
- Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T 2003 Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem 278:40352–40363 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE 2003 Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 278:9073–9085 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE 2004 Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162 [DOI] [PubMed] [Google Scholar]
- Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T 2006 Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 29:1357–1362 [DOI] [PubMed] [Google Scholar]
- Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-De Mouzon S 2006 Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 49:1677–1685 [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE 2003 Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52:268–276 [DOI] [PubMed] [Google Scholar]
- Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA 2004 Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand 83:341–347 [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Walker JD, Havel PJ, Hamilton BA, Calder AA, Johnstone FD, Scottish Multicenter Study of Diabetes Pregnancy 2003 Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care 26:2244–2249 [DOI] [PubMed] [Google Scholar]
- Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, Kanety H 2003 Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab 88:5656–5660 [DOI] [PubMed] [Google Scholar]
- Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, Shoham A, Sivan E 2007 Determining the source of fetal adiponectin. J Reprod Med 52:774–778 [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF 1995 A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749 [DOI] [PubMed] [Google Scholar]
- Pardo IM, Geloneze B, Tambascia, MA, Barros-Filho AA 2004 Hyperadiponectinemia in newborns: relationship with leptin levels and birth weight. Obes Res 12:521–524 [DOI] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, Lepercq J, Catalano P 2006 The known and unknown of leptin in pregnancy. Am J Obstet Gynecol 194:1537–1545 [DOI] [PubMed] [Google Scholar]
- Corbetta S, Bulfamante G, Cortelazzi D, Barresi V, Cetin I, Mantovani G, Bondioni S, Beck-Peccoz P, Spada A 2005 Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab 90:2397–2402 [DOI] [PubMed] [Google Scholar]
- Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, Vatish M, Randeva HS 2006 Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia 49:1292–1302 [DOI] [PubMed] [Google Scholar]
- Caminos JE, Nogueiras, R, Gallego R, Bravo S, Tovar S, García-Caballero T, Casanueva FF, Diéguez C 2005 Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab 90:4276–4286 [DOI] [PubMed] [Google Scholar]
- Viengchareun S, Zennaro MC, Pascual-Le Tallec L, Lombes M 2002 Brown adipocytes are novel sites of expression and regulation of adiponectin and resistin. FEBS Lett 32:345–50 [DOI] [PubMed] [Google Scholar]
- Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS 2003 Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 88:4823–4831 [DOI] [PubMed] [Google Scholar]
- Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, Youngren JF, Havel PJ, Pratley RE, Bogardus C, Tataranni PA 2002 Plasma adiponectin levels are not associated with fat oxidation in humans. Obes Res 10:1016–1020 [DOI] [PubMed] [Google Scholar]
- Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, Reaven PD 2004 Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes 53:585–590 [DOI] [PubMed] [Google Scholar]
- Farrag HM, Nawrath LM, Healey JE, Dorcus EJ, Dorcus EJ, Rapoza RE, Oh W, Cowett RM 1997 Persistent glucose production and greater peripheral sensitivity to insulin in the neonate vs. the adult. Am J Physiol 272(1 Pt 1):E86–E93 [DOI] [PubMed] [Google Scholar]
- Fu Y, Luo N, Klein RL, Garvey WT 2005 Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46:1369–1379 [DOI] [PubMed] [Google Scholar]
- Enzi G, Zanardo V, Caretta F, Inelmen EM, Rubaltelli F 1981 Intrauterine growth and adipose tissue development. Am J Clin Nutr 34:1785–1790 [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K 2004 Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279:1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang R, Mu H, Li M, Yao Q, Chen C 2006 Adiponectin promotes endothelial cell differentiation from human peripheral CD14+ monocytes in vitro. J Cell Mol Med 10:459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]