Abstract
Context: Adrenal androgen excess is common in polycystic ovary syndrome (PCOS) and appears to be heritable. CYP3A7 metabolizes dehydroepiandrosterone and its sulfate (DHEAS). A promoter variant, CYP3A7*1C, which results in persistent expression in adults, was associated with reduced DHEAS levels in a previous study, which led us to consider CYP3A7*1C as a modulator of adrenal androgen excess in patients with PCOS.
Objective: The objective was to replicate the association between CYP3A7*1C and reduced DHEAS levels in PCOS patients and assess its possible role in modulating testosterone levels.
Design: Women with and without PCOS were genotyped for CYP3A7*1C, and this variant was tested for association with DHEAS and total and free testosterone.
Setting: Subjects were recruited from the reproductive endocrinology clinic at the University of Alabama at Birmingham; controls were recruited from the surrounding community. Genotyping took place at Cedars-Sinai Medical Center (Los Angeles, CA).
Participants: A total of 287 white women with PCOS and 187 controls were studied.
Main Measurements: CYP3A7*1C genotype, PCOS risk, and androgen levels were measured.
Results: PCOS subjects who carried the CYP3A7*1C variant had lower levels of serum DHEAS and total testosterone (P = 0.0006 and 0.046, respectively). The variant was not associated with PCOS risk.
Conclusion: This study replicated prior work of the association of CYP3A7*1C and decreased DHEAS in a different population of young PCOS women, providing further genetic evidence that CYP3A7 plays a potential role in modulation of DHEAS levels. Adult expression of CYP3A7 may modify the PCOS phenotype by ameliorating adrenal androgen excess.
In women with polycystic ovary syndrome (PCOS), a promoter variant in fetal life, CYP3A7*1C, results in persistent expression of CYP3A7 in adults and is associated with reduced dehydroepiandrosterone sulfate levels. Adult expression of CYP3A7 may modify the PCOS phenotype by ameliorating adrenal androgen excess.
Polycystic ovary syndrome (PCOS) is a complex common genetic disorder characterized by hyperandrogenism, menstrual dysfunction, and polycystic ovaries, affecting 5–8% of reproductive aged women (1). Although the ovaries are considered the main source of androgens in PCOS, about 20–30% of patients present with increased adrenal androgen levels, specifically elevated dehydroepiandrosterone sulfate (DHEAS) (2). Dehydroepiandrosterone (DHEA) and DHEAS are secreted from the zona reticularis of the adrenal cortex, and DHEAS is commonly used as a measure of adrenal androgen excess. DHEAS levels are modulated by heritable factors (3).
Cytochrome P450 enzymes metabolize multiple substrates including endogenous and exogenous compounds and participate in synthesis of cholesterol, steroids and other lipids (4). The CYP3A subfamily, a major subfamily of the CYP superfamily, consists of CYP3A4, CYP3A5, and CYP3A7. CYP3A4 is the most abundant P450 in the adult liver (30% of total CYP3A) (4). CYP3A5 is also present in the liver but at lower levels and is the primary extrahepatic CYP3A isoform (5). CYP3A7 is predominantly expressed in the fetus (>90% of total fetal CYP3A), and its expression is sharply down-regulated after birth (6). However, one promoter variant of the CYP3A7 gene, called CYP3A7*1C, consists of the replacement of an approximately 60-bp sequence from the analogous region of the CYP3A4 promoter, resulting in persistent CYP3A7 protein expression in the adult liver (5,7).
CYP3A7 catalyzes 16α-hydroxylation of DHEA and DHEAS, which renders them less active and easier to eliminate (8). The frequency of CYP3A7*1C in two different groups was 3–4%; heterozygous carriers had approximately 50% lower DHEAS levels (9). These data suggested that the variant CYP3A7*1C may play a role in regulating DHEAS levels, via increasing the clearance of DHEA and DHEAS, which may ameliorate adrenal androgen excess in PCOS.
The aim of this study was to replicate the association between CYP3A7*1C and reduced DHEAS levels in PCOS patients and investigate association with PCOS and hyperandrogenemia. Our results confirmed the significant association with DHEAS and suggest CYP3A7*1C has an impact on the elimination of DHEA and/or DHEAS in PCOS. CYP3A7 may function as a modulator of adrenal androgen excess, regulating DHEAS and testosterone levels.
Subjects and Methods
Subjects and phenotyping
A total of 287 consecutive unrelated white patients with PCOS and 187 healthy unrelated white control women were recruited from the Birmingham, AL, area. PCOS cases were recruited from the reproductive endocrine practice of one of the investigators (R.A.) at the University of Alabama at Birmingham. Participation in research studies was offered to patients meeting inclusion criteria (premenopausal, nonpregnant, on no hormonal therapy for at least 3 months, and meeting diagnostic criteria for PCOS). PCOS was diagnosed following the 1990 National Institutes of Health consensus criteria (10).
Controls were healthy, nonhirsute women, with a history of regular menstrual cycles and no family history of hirsutism. Controls were recruited by word of mouth and advertisements in the Birmingham area through a call for healthy women without detailing further the nature of the studies.
Subjects underwent a physical examination and serum androgen measurement, per a previously described protocol (1). The same laboratory assays were used for all subjects.
All subjects gave written informed consent, and the study was performed according to the guidelines of the Institutional Review Boards of University of Alabama at Birmingham and Cedars-Sinai Medical Center.
Genotyping
In the CYP3A7*1C variant, seven base pairs are changed in a specific 60-bp region of the CYP3A7 promoter (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org). This variant is a haplotype of seven single-nucleotide polymorphism alleles (5); therefore, we used one pair of probes covering four single-nucleotide polymorphisms to give the information of all of them. The variant was genotyped using the 5′-exonuclease assay (TaqMan MGB; Applied Biosystems, Foster City, CA); duplicate genotyping of 96 samples yielded 100% concordance. The PCR primers (forward, 5′-ACCTCGGCAGTTGGCAAA-3′; reverse, 5′-GCCAATGGCTCCACTTGAGT-3′) were synthesized by Invitrogen (Carlsbad, CA). TaqMan MGB probes (probe 1, 5′-TATTCTATGtaGaATCATAc-3′; probe 2, 5′-ATATTCTATGagGtATCATAa-3′) were synthesized by Applied Biosystems. The probes were labeled at the 5′-end with 6FAM or VIC (laser-activated fluorescent dyes), and at the 3′-end with a quencher/minor groove binder. Variant nucleotides are indicated in lower case in the probe sequences. The genotyping success rate was 93.4%.
Statistical analysis
Unpaired t tests and χ2 tests were used to compare clinical characteristics and allele frequencies between women with and without PCOS; quantitative trait values were log or square root transformed as appropriate to reduce nonnormality.
Data are expressed as the median (interquartile range). Association with androgens was evaluated using analysis of covariance, adjusting for age and body mass index (BMI) by including both as independent variables in every analysis. Association with presence/absence of PCOS was evaluated using logistic regression, adjusting for age and BMI. Significance was taken at P < 0.025 to account for the effects of multiple testing, considering that we analyzed one variant against two families of traits (PCOS diagnosis and androgens), yielding a correction factor of 2 (i.e. two independent comparisons). Analyses were carried out using Statview 5.0 (SAS Institute, Cary, NC).
Results
Clinical characteristics of the subjects are presented in supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org. The overall frequency of the CYP3A7*1C variant was 2.7%. The frequency was 2.2% in PCOS women and 3.6% in controls (P = 0.21). We observed 5.2% carriers (n = 23) in the whole cohort, 4.0% in PCOS (n = 11) and 7.2% in controls (n = 12) (P = 0.14). There was only one subject homozygous for CYP3A7*1C (a PCOS subject). There was a trend to lower PCOS risk in carriers with an age- and BMI-adjusted odds ratio of 0.4 (95% confidence interval 0.13–1.18, P = 0.095).
In the entire cohort, carriers of CYP3A7*1C had significantly lower levels of DHEAS (P = 0.002), but the variant was not associated with total or free testosterone levels (Table 1).
Table 1.
Associations of CYP3A7*1C with androgen levels
| Overalla
|
Controls
|
PCOS
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | C | P | NC | C | P | NC | C | P | |
| DHEAS (ng/ml) | 1575.5 (1596.0) | 1027.0 (794.8) | 0.002b | 966.0 (706.0) | 845.5 (622.0) | 0.27 | 2141.0 (1757.8) | 1386.0 (960.3) | 0.0006b |
| Total T (ng/dl) | 69.0 (40.0) | 60.5 (25.0) | 0.36 | 41.0 (27.3) | 49.5 (32.5) | 0.62 | 80.0 (33.5) | 70.5 (29.0) | 0.046 |
| Free T (pg/ml) | 0.70 (0.52) | 0.46 (0.46) | 0.083 | 0.36 (0.27) | 0.37 (0.18) | 0.74 | 0.85 (0.46) | 0.80 (0.39) | 0.31 |
Data are median (interquartile range). T, Testosterone; NC, noncarriers; C, carriers of CYP3A7*1C.
In the analysis of the whole cohort, PCOS status was also adjusted for, along with age and BMI.
Significant P < 0.025.
In the PCOS group, CYP3A7*1C carriers had significantly lower DHEAS (P = 0.0006) and trend to lower total testosterone (P = 0.046) (Table 1). The variant was not associated with free testosterone.
Among controls, there was a trend to reduced DHEAS level in CYP3A7*1C carriers and no effect on testosterone (Table 1).
Discussion
We confirmed that CYP3A7*1C is significantly associated with lower DHEAS levels in patients with PCOS, suggesting that this polymorphism may play a role in adrenal androgen metabolism.
Adrenal androgen excess is common in PCOS. DHEAS is increased in approximately 20% of white and approximately 30% of black PCOS patients (2) and is measured as a marker of adrenal androgen excess. DHEAS is well suited for this because it is made in large amounts by the adrenals and has a long half-life. Family studies suggested that genetic factors account for 40–50% of the overall variation in DHEAS levels in women with PCOS (the heritability index was 0.44) (3). In addition, DHEA stimulated by ACTH is correlated between PCOS patients and their sisters, further suggesting that inherited factors modulate adrenal androgen production and/or clearance in PCOS (11). For example, variants in the DHEA sulfotransferase (SULT2A1) gene were associated with DHEAS levels in women with PCOS (12). We presume that enzymes that affect DHEAS and/or DHEA clearance would also be candidate genetic modulators of adrenal androgen levels in PCOS.
CYP3A7 catalyzes 16α-hydroxylation of both DHEA and DHEAS, facilitating their elimination (8). During development, the presence of fetal CYP3A7 is important to protect the fetus against maternal DHEA effects (4). CYP3A7 expression becomes very low or undetectable after birth, whereas CYP3A4 expression is up-regulated in adult liver. However, persistent expression of CYP3A7 mRNA and protein has been identified in some human adult livers (5,6,13). About 60–70% of cases of persistent adult expression of CYP3A7 can be explained by CYP3A7 promoter variants (CYP3A7*1B and CYP3A7*1C) (5). Whereas CYP3A7*1C contains the same 60-bp homologous segment in the promoter region as that of CYP3A4, they have different substrate preferences and catalytic activities. Kinetic analysis demonstrated that CYP3A4 had a 34-fold greater 7β-hydroxylase activity than CYP3A7, whereas CYP3A7 showed 3-fold greater 16α-hydroxylase activity than CYP3A4 (14).
The functional consequences of CYP3A7*1C and the resulting CYP3A7 expression in adult liver are incompletely understood. Previously, a significant association between CYP3A7*1C and a 50% reduction in DHEAS level was observed in two groups of subjects, 208 randomly selected individuals (110 women and 98 men, mean age 66–68 yr) and 345 elderly men (mean age 78 yr) (9). This result was confirmed by our current study in a different cohort of young women. We have also shown that CYP3A7*1C is associated with a trend to decreased PCOS risk and lower testosterone, as would be expected if DHEA is being more rapidly cleared, making less DHEA available to contribute to hyperandrogenemia. That these effects were less significant is not surprising, given that testosterone and PCOS are indirectly affected by CYP3A7, whereas DHEAS is directly affected (Fig. 1). Considering the minor role of hepatic steroid sulfatase in converting DHEAS to DHEA, DHEAS cannot serve as a circulating regeneration reservoir for DHEA (15); therefore, any factor that increases DHEA clearance can have a major effect on DHEAS levels. In addition, adult expression of CYP3A7 may reduce DHEAS by acting directly on it (Fig. 1). DHEA levels were not available in our cohort, so we cannot determine whether the lowering of DHEAS is via lowered DHEA. In any case, our data provide genetic evidence that CYP3A7*1C may be a protective factor against adrenal androgen excess in PCOS.
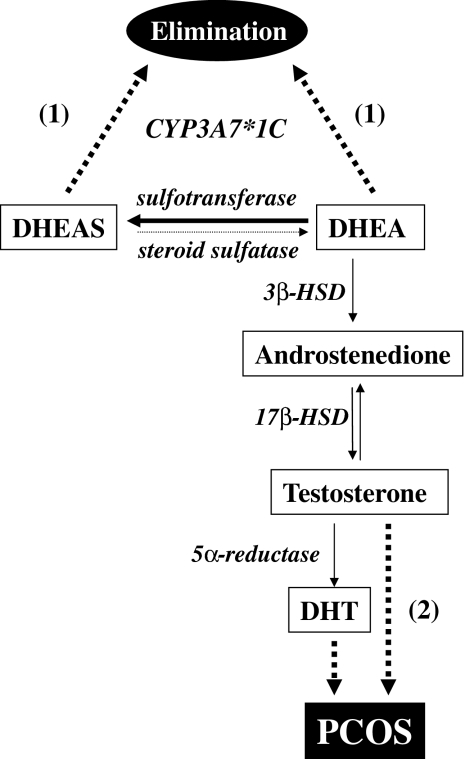
Figure 1.
Effects of CYP3A7*1C on metabolism of DHEA/DHEAS, and its indirect impact on testosterone and PCOS. DHEA is converted to DHEAS by DHEA sulfotransferase (thick arrow), but DHEAS does not serve as a reservoir for DHEA regeneration because steroid sulfatase has a minor role in conversion of DHEAS to DHEA (light arrow). Solid lines indicate normal metabolism of DHEA/DHEAS. Dashed lines indicate effects of CYP3A7*1C on androgens: (1) CYP3A7*1C can directly increase DHEA/DHEAS clearance, diverting DHEA away from conversion to active androgens, which (2) affects testosterone and PCOS indirectly. Thus, persistent adult CYP3A7 activity may influence whether adrenal androgens are eliminated or contribute to PCOS. The enzymes responsible for conversion are italicized. 3β-HSD, 3β-Hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; DHT, dihydrotestosterone.
CYP3A7*1C becomes an intriguing target for PCOS treatment based on its role in DHEA/DHEAS metabolism. CYP3A4 and CYP3A7 expression can be up-regulated by dexamethasone in human fetal liver cells (16), and CYP3A7 is inducible by rifampicin in adult human hepatocytes (17). Therefore, CYP3A7 may be pharmacologically inducible, resulting in increased DHEA and/or DHEAS clearance in PCOS. Or, in gene therapy, CYP3A7*1C could be introduced directly to patients, regulating androgen levels as a modulator, resulting in less DHEA available to contribute to hyperandrogenemia (Fig. 1). This notion deserves further investigation.
The CYP3A7*1C variant is rare and thus probably important for a minority of PCOS women. Given the considerable heritability of DHEAS levels (3), there must be other variants in different genes that also contribute to variation in adrenal androgen levels (e.g. SULT2A1, HSD11B1) (12,18).
In conclusion, this study replicated the association between CYP3A7*1C and decreased DHEAS in PCOS and found trends to reduced testosterone levels and lower PCOS risk. These results suggest adult expression of CYP3A7 may play a role in lowering DHEAS and total testosterone by increasing DHEA/DHEAS clearance and provide genetic evidence for such enzymes as modifiers of the PCOS phenotype.
Supplementary Material
Footnotes
This study was supported in part by National Institutes of Health Grants R01-HD29364 and K24-HD01346 (to R.A.) and M01-RR00425 (General Clinical Research Center grant from the National Center for Research Resources) and an endowment from the Helping Hand of Los Angeles, Inc.
Disclosure Statement: M.O.G. and N.X. have nothing to declare. R.A. has received consulting fees from Procter & Gamble, Merck & Co., and Organon.
First Published Online April 29, 2008
Abbreviations: BMI, Body mass index; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; PCOS, polycystic ovary syndrome.
References
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Kumar A, Woods KS, Bartolucci AA, Azziz R 2005 Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 62:644–649 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Goodarzi MO, Guo X, Rotter JI, Azziz R 2006 Heritability of dehydroepiandrosterone sulfate in women with polycystic ovary syndrome and their sisters. Fertil Steril 86:1688–1693 [DOI] [PubMed] [Google Scholar]
- Stevens JC 2006 New perspectives on the impact of cytochrome P450 3A expression for pediatric pharmacology. Drug Discov Today 11:440–445 [DOI] [PubMed] [Google Scholar]
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E 2001 Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27:383–391 [DOI] [PubMed] [Google Scholar]
- Komori M, Nishio K, Kitada M, Shiramatsu K, Muroya K, Soma M, Nagashima K, Kamataki T 1990 Fetus-specific expression of a form of cytochrome P-450 in human livers. Biochemistry 29:4430–4433 [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Beach DL, Guzelian PS 1994 Selective expression of cytochrome P450 CYP3A mRNAs in embryonic and adult human liver. Pharmacogenetics 4:11–20 [DOI] [PubMed] [Google Scholar]
- Miller KK, Cai J, Ripp SL, Pierce WM, Rushmore TH, Prough RA 2004 Stereo- and regioselectivity account for the diversity of dehydroepiandrosterone (DHEA) metabolites produced by liver microsomal cytochromes P450. Drug Metab Dispos 32:305–313 [DOI] [PubMed] [Google Scholar]
- Smit P, van Schaik RH, van der Werf M, van den Beld AW, Koper JW, Lindemans J, Pols HA, Brinkmann AO, de Jong FH, Lamberts SW 2005 A common polymorphism in the CYP3A7 gene is associated with a nearly 50% reduction in serum dehydroepiandrosterone sulfate levels. J Clin Endocrinol Metab 90:5313–5316 [DOI] [PubMed] [Google Scholar]
- Zawadzki JK, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam GR, eds. Polycystic ovary syndrome. Cambridge, UK: Blackwell Scientific Publications; 377–384 [Google Scholar]
- Goodarzi MO, Guo X, Yildiz BO, Stanczyk FZ, Azziz R 2007 Correlation of adrenocorticotropin steroid levels between women with polycystic ovary syndrome and their sisters. Am J Obstet Gynecol 196:398 e391–e395; discussion 398 e395–e396 [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Antoine HJ, Azziz R 2007 Genes for enzymes regulating dehydroepiandrosterone sulfonation are associated with levels of dehydroepiandrosterone sulfate in polycystic ovary syndrome. J Clin Endocrinol Metab 92:2659–2664 [DOI] [PubMed] [Google Scholar]
- Sim SC, Edwards RJ, Boobis AR, Ingelman-Sundberg M 2005 CYP3A7 protein expression is high in a fraction of adult human livers and partially associated with the CYP3A7*1C allele. Pharmacogenet Genomics 15:625–631 [DOI] [PubMed] [Google Scholar]
- Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ 2003 Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther 307:573–582 [DOI] [PubMed] [Google Scholar]
- Hammer F, Subtil S, Lux P, Maser-Gluth C, Stewart PM, Allolio B, Arlt W 2005 No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: in vivo and in vitro studies. J Clin Endocrinol Metab 90:3600–3605 [DOI] [PubMed] [Google Scholar]
- Maruyama M, Matsunaga T, Harada E, Ohmori S 2007 Comparison of basal gene expression and induction of CYP3As in HepG2 and human fetal liver cells. Biol Pharm Bull 30:2091–2097 [DOI] [PubMed] [Google Scholar]
- Greuet J, Pichard L, Bonfils C, Domergue J, Maurel P 1996 The fetal-specific gene CYP3A7 is inducible by rifampicin in adult human hepatocytes in primary culture. Biochem Biophys Res Commun 225:689–694 [DOI] [PubMed] [Google Scholar]
- Gambineri A, Vicennati V, Genghini S, Tomassoni F, Pagotto U, Pasquali R, Walker BR 2006 Genetic variation in 11β-hydroxysteroid dehydrogenase type 1 predicts adrenal hyperandrogenism among lean women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:2295–2302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.