Abstract
Several studies report that environmental enrichment enhances sensitivity to opioid receptor agonists in male rats. Very few studies have examined the effects of enrichment in female rats, and thus it is not clear whether females are similarly sensitive to these effects. Consequently, the purpose of the present study was to examine the effects of environmental enrichment on sensitivity to representative mu, kappa, and mixed-action opioids in female rats. Following a protocol established in males, females were obtained at weaning and randomly assigned to two groups immediately upon arrival: isolated rats were housed individually with no visual or tactile contact with other rats; enriched rats were housed in groups of four in large cages and given various novel objects on a regular basis. After 6 weeks under these conditions, the antinociceptive effects of mu (morphine, levorphanol), kappa (spiradoline, U69,593), and mixed-action (buprenorphine, butorphanol) opioids were examined in a warm-water, tail-withdrawal procedure. All the opioids examined produced dose-dependent increases in antinociception; however, no differences in opioid sensitivity were observed between the two groups. To determine whether these findings were consistent across behavioral endpoints, the antidiuretic effects of representative mu opioids, and the diuretic effects of representative kappa opioids, were examined in female rats reared under isolated or enriched conditions for 10 weeks. Similar to that seen in the antinociceptive experiment, no significant differences in opioid sensitivity were observed between groups. These data indicate that environmental enrichment does not alter sensitivity to the effects of opioid receptor agonists in female rats, and suggest that females may respond differently to environmental enrichment than males.
Keywords: antidiuresis, antinociception, diuresis, enrichment, female, isolation, opioid, rat
A large number of studies reveal that environmental manipulations during critical periods in development can influence the physical maturation of the nervous system and produce functional effects on behavior. For example, male rats reared under conditions of environmental enrichment (i.e., conditions in which rats were housed together in large groups and provided with novel objects on a regular basis) had greater cortical mass [1, 2], had greater dendritic branching on cortical neurons [3, 4], and performed better in learning and memory tasks [5, 6] than animals reared in isolation under impoverished conditions. Interestingly, these same types of manipulations can also influence an organism’s sensitivity to the neurochemical and behavioral effects of centrally acting drugs. In studies conducted with psychomotor stimulants, for instance, enriched male rats were more sensitive than isolated male rats to amphetamine-induced dopamine release and metabolism in the nucleus accumbens [7, 8], and were more sensitive to the effects of amphetamine on locomotor activity [8, 9] and conditioned reward [9].
A limited number of studies have examined the effects of environmental enrichment on sensitivity to the behavioral effects of opioids. These studies typically reported that enriched rats were more sensitive than isolated rats to a variety of opioid receptor agonists. For instance, enriched female rats were more sensitive to the aversive effects of the mu opioid morphine in the conditioned taste aversion paradigm [10], and enriched male rats were more sensitive to the rewarding effects of morphine in the conditioned place preference procedure [11]. Similarly, we reported that enriched male rats were more sensitive to the effects of the mixed-action opioids butorphanol and nalbuphine in the conditioned place preference procedure and in a test of thermal antinociception [12]. We also reported that enriched male rats were more sensitive to the effects of the kappa opioid spiradoline on measures of antinociception, diuresis, and conditioned place preference [13]. In contrast to these findings, isolated male rats were more sensitive to the effects of morphine on locomotor activity and displayed greater locomotor sensitization following repeated morphine treatment [11]. Furthermore, isolated and enriched male rats did not differ in their oral consumption of morphine in a two-bottle preference test [14], and did not differ in sensitivity to the effects of morphine and levorphanol in a thermal antinociception test [12].
Of those studies examining the effects of enrichment on opioid sensitivity, only one has used females [10]. This is a potentially significant issue, given evidence that males and females may respond differently to social and environmental manipulations. For instance, previous studies reported that females were less sensitive than males to the effects of enrichment on locomotor activity [15], social exploratory behavior [16], and behavioral recovery following brain trauma [17]. Consequently, the purpose the present study was to examine the effects of environmental enrichment on sensitivity to opioid receptor agonists in female rats. Using a protocol established in males, females were obtained at weaning and immediately divided into two groups: isolated rats were housed individually with no visual or tactile contact with other rats; enriched rats were housed in groups of four and given novel objects on a daily basis. After 6 weeks under these conditions, the antinociceptive effects of representative mu, kappa, and mixed-action opioids were examined in a warm-water, tail-withdrawal procedure. In a second experiment, the antidiuretic and diuretic effects of representative mu and kappa opioids, respectively, were examined in rats reared under isolated or enriched conditions for 10 weeks.
Materials and Methods
Animals
Forty-eight female Long-Evans rats were obtained at weaning (~ 21 days) from Charles River Laboratories (Raleigh, NC, USA) and randomly assigned to two groups. Isolated rats (n = 24) were housed individually in opaque cages (interior dimensions: 43 × 21 × 20 cm) that permitted no visual or tactile contact with other rats. Enriched rats (n = 24) were housed socially in groups of four in large, transparent cages (interior dimensions: 92 × 38 × 40 cm) that permitted extensive social interactions between cagemates. Rats in the latter group also were provided with supplemental enrichment by placing a variety of novel objects (e.g., ladders, PVC pipes, ping-pong balls, animal toys) in the cage each day. Activity wheels were not included in the enrichment condition, as previous studies reported that voluntary exercise produced cross tolerance to opioid receptor agonists, presumably via the release of endogenous opioid peptides during vigorous activity [18, 19, 20]. All rats were kept in a large colony room maintained on a 12-hr light/dark cycle with food and drinking water freely available in the home cages. Behavioral testing commenced 6 weeks after arrival in the antinociceptive experiment and 10 weeks after arrival in the experiment on urine output. All rats remained in their respective conditions until testing was complete. All subjects were maintained in accordance with the guidelines of the Davidson College Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals [21]. Estrous phase was allowed to cycle normally and was not monitored.
Antinociception Testing
Antinociceptive tests began in all rats 6 weeks after arrival to the laboratory. During testing, subjects (n = 8 isolated; n = 8 enriched) were restrained in acrylic restraint tubes (Fischer Scientific, Pittsburg, PA, USA) and tail-withdrawal latencies were measured with a hand-operated stopwatch (time resolution: 0.01 s). Water was maintained at 50° and 54° C via thermostat-controlled water baths (Fischer Scientific).
All antinociceptive tests were conducted according to procedures described previously [22, 23]. Briefly, rats were habituated to restraint tube confinement and the injection procedure during two 30-min habitation sessions conduced prior to the first test session. During antinociceptive tests, rats were removed from their home cages and placed into the restraint tubes with their tails hanging freely off the edge of a table. The distal 10 cm of the tail was then immersed into a cup of either 50° or 54° C water, with the order of stimulus presentation counterbalanced across subjects. The latency for the rat to withdraw its tail completely from the water was then recorded. A maximal latency of 15 s was employed in all tests to prevent tissue damage. During all tests, 3 min separated presentation of the 50° and 54° C water.
All test drugs were administered during the antinociceptive tests using a cumulative dosing procedure. In this procedure, a rat was removed from its restraint tube, administered the lowest dose of the drug, and then immediately returned to the tube. After 15 min, the latency for the rat to withdrawal its tail from the 50° and 54° C water was recorded. The rat was then administered the next dose of the drug, such that the dose increased the cumulative amount of drug received by 0.25, 0.50, or 1.00 log unit. For instance, the administration of 1.0, 2.0, 7.0, and 20 mg/kg over the course of a session yielded cumulative totals of 1.0, 3.0, 10, and 30 mg/kg. For individual rats, once a maximal response (15 s) was obtained at one temperature, no further tests were conducted at that temperature.
Representative mu, kappa, and mixed-action opioids were tested at weekly intervals in the following order: morphine (3.0, 10, 30 mg/kg), butorphanol (0.3, 3.0, 30 mg/kg), buprenorphine (0.1, 0.3, 1.0 mg/kg), spiradoline (10, 30, 56 mg/kg), levorphanol (1.0, 3.0, 10 mg/kg), and U69,593 (1.0, 3.0, 10 mg/kg). Previous studies from our laboratory showed that a 7-day interval between antinociceptive tests was sufficient to prevent the development of tolerance that could lead to systematic changes in opioid sensitivity [22, 23]. Although our previous studies were conducted only in males, other investigators reported that this testing schedule did not alter baseline measures of nociception or alter sensitivity to the antinociceptive effects of opioid receptor agonists in females [24, 25].
Antidiuresis and Diuresis Testing
Beginning 10 weeks after arrival, separate groups of rats were tested for antidiuresis (n = 8 isolated; n = 8 enriched) and diuresis (n = 8 isolated; n = 8 enriched). Prior to testing, all rats were habituated to sound-attenuating operant-conditioning chambers (Med Associates, St. Albans, VT) during one 2-hr habituation session. No food or drinking water was available during any session. For the antidiuresis tests, rats were first water-loaded with 2 ml/100 g distilled water via subcutaneous injection. The rats were then administered either saline or a single dose of the test drug and placed into the operant conditioning chambers. Urine was then collected over the next 2 hr in stainless steel pans located beneath the grid floor of the chamber. For diuresis testing, normally hydrated rats were administered either saline or a single dose of the test drug and then placed into the operant conditioning chambers where urine was collected over the next 2 hr. Antidiuresis testing was conducted with saline (1 ml/kg), morphine (3.0, 10, 30 mg/kg), and levorphanol (1.0, 3.0, 10 mg/kg), whereas diuresis testing was conducted with saline, spiradoline (3.0, 10, 30 mg/kg), and U69,593 (1.0, 3.0, 10 mg/kg). All tests were separated by 48 to 72 hours.
Drugs
Butorphanol tartrate, spiradoline mesylate, levorphanol tartrate, and U69,593 were obtained from Sigma Chemical Company (St. Louis, MO, USA). Morphine sulphate and buprenorphine hydrochloride were generously supplied by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA). All drugs were dissolved in saline and injected intraperitoneally in a volume of 1.0 – 2.0 ml/kg of body weight.
Data Analysis
For the antinociceptive tests, tail-withdrawal latencies were converted to percent maximal possible effect using the following equation: % maximal possible effect = [(observed − baseline)/(15 s − baseline)] × 100. Under conditions in which a drug produced at least a 50% response, the dose estimated to produce 50% of the maximal possible effect (A50) was computed mathematically (least squares method) by log-linear interpolation [26]. For the antidiuresis and diuresis tests, urine was collected and measured in ml at the end of each 2-hr session. For all tests, group and dose effects were determined via mixed-factor, repeated-measures ANOVA, with group serving as a between-subjects factor and dose serving as the repeated measure. An alpha level of 0.05 was used for all statistical tests.
Results
Antinociception after 6 Weeks
Baseline Data
Baseline tail-withdrawal latencies were significantly greater at the low temperature than at the high-temperature [F (1, 10) = 30.306; p < 0.001), but did not differ between isolated and enriched rats (Table 1).
Table 1.
Mean (SE) baseline tail-withdrawal latenciesa of isolated and enriched rats when tested at low (50 °C water) and high (54 °C water) stimulus intensities.
| Group | 50 °C Water | 54 °C Water |
|---|---|---|
| Isolated | 4.10 (0.14) | 2.82 (0.11) |
| Enriched | 3.62 (0.18) | 2.48 (0.25) |
All data depicted in seconds
Morphine and Levorphanol
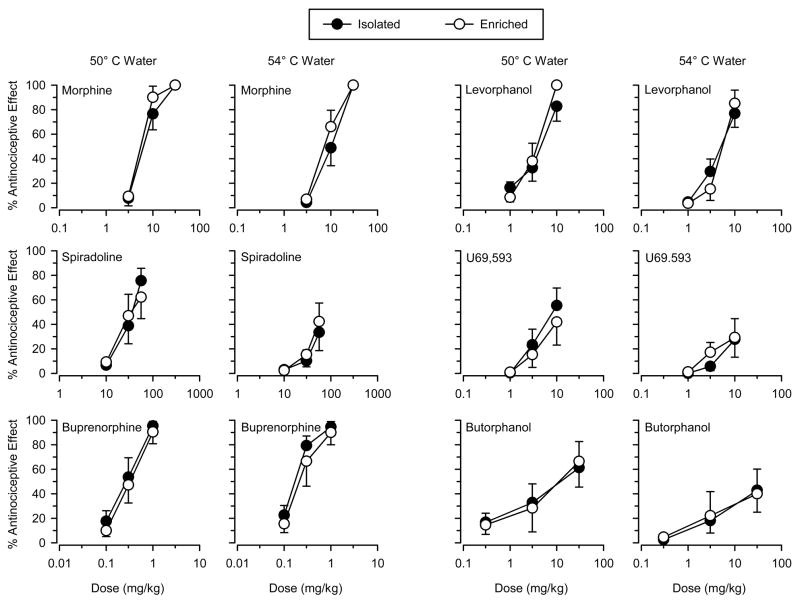
The mu opioids morphine and levorphanol produced dose-dependent increases in antinociception and produced high levels of antinociception (> 80% maximal possible effect) in both groups and at both temperatures (Figure 1). Repeated-measures ANOVA revealed significant main effects of dose for both drugs at both temperatures (p’s < 0.05), but the effects of group and the dose × group interactions were not significant. Analysis of A50 values revealed that both drugs were generally more potent at the low temperature than at the high temperature, but were similarly potent in the two groups (Table 2).
Figure 1.
Antinociceptive effects of the mu opioids morphine and levorphanol (top panels), the kappa opioids spiradoline and U69,593 (middle panels), and the mixed-action opioids buprenorphine and butorphanol (bottom panels) in isolated (filled symbols) and enriched (open symbols) rats. Tests were conducted using 50 °C and 54 °C water. Ordinates reflect tail-withdrawal latencies expressed as % maximal possible effect. Abscissas reflect dose of test drug in mg/kg of body weight. Vertical lines on data points represent the SE; where not indicated, the SE fell within the data point.
Table 2.
A50 valuesa (95% confidence limits) of test drugs in isolated and enriched rats when tested at low (50 °C water) and high (54 °C water) stimulus intensities.
| Test Drug | 50 °C Water | 54 °C Water |
|---|---|---|
| Morphine | ||
| isolated | 7.27 (5.52 – 9.59) | 9.35 (7.32 – 11.93) |
| enriched | 6.49 (5.00 – 8.41) | 8.00 (6.30 – 10.17) |
| Levorphanol | ||
| isolated | 3.84 (2.52 – 5.85) | 4.69 (3.26 – 6.76) |
| enriched | 3.20 (2.44 – 4.20) | 4.77 (3.31 – 6.89) |
| Spiradoline | ||
| isolated | 32.92 (23.46 – 44.20) | b |
| enriched | 36.07 (19.52 – 66.68) | b |
| U69,593 | ||
| isolated | 8.62 (3.81 – 19.48) | b |
| enriched | 17.28 (3.08 – 96.87) | b |
| Buprenorphine | ||
| isolated | 0.26 (0.18 – 0.38) | 0.58 (0.39 – 0.88) |
| enriched | 0.32 (0.22 – 0.46) | 0.77 (0.45 – 1.31) |
| Butorphanol | ||
| isolated | 12.39 (1.59 – 96.84) | b |
| enriched | 9.34 (1.31 – 66.76) | b |
A50 values expressed in mg/kg
could not be determined (< 50% maximal possible effect)
Spiradoline and U69,593
The kappa opioids spiradoline and U69,593 produced dose-dependent increases in antinociception, but the maximal effect differed across temperatures (Figure 1). Spiradoline produced moderate levels of antinociception (50% – 80% maximal possible effect) at the low temperature in both groups, but produced only low levels of antinociception (< 50% maximal possible effect) at the high temperature. U69,593 produced low to moderate levels of antinociception in both groups and at both temperatures. Similar to that seen with morphine and levorphanol, both drugs were similarly potent and/or effective in both groups (Table 2). Repeated-measures ANOVA revealed significant main effects of dose (p’s < 0.05) for both drugs at both temperatures, but no significant effect of group or dose × group interaction was observed.
Buprenorphine and Butorphanol
The mixed-action opioids buprenorphine and butorphanol produced dose-dependent increases in antinociception in both groups and at both temperatures (Figure 1). Buprenorphine produced high levels of antinociception at both temperatures, whereas butorphanol produced moderate levels of antinociception at the low temperature and low levels of antinociception at the high temperature. Buprenorphine was more potent at the low temperature than at the high temperature, but was similarly potent in the two groups (Table 2). Butorphanol was similarly potent in the two groups at the low temperature and similarly effective in the two groups at the high temperature. Repeated-measures ANOVA revealed significant main effects of dose (p’s < 0.05) for both drugs at both temperatures, but no significant main effect of group or dose × group interaction was observed.
Antidiuresis and Diuresis after 10 Weeks
Morphine and Levorphanol
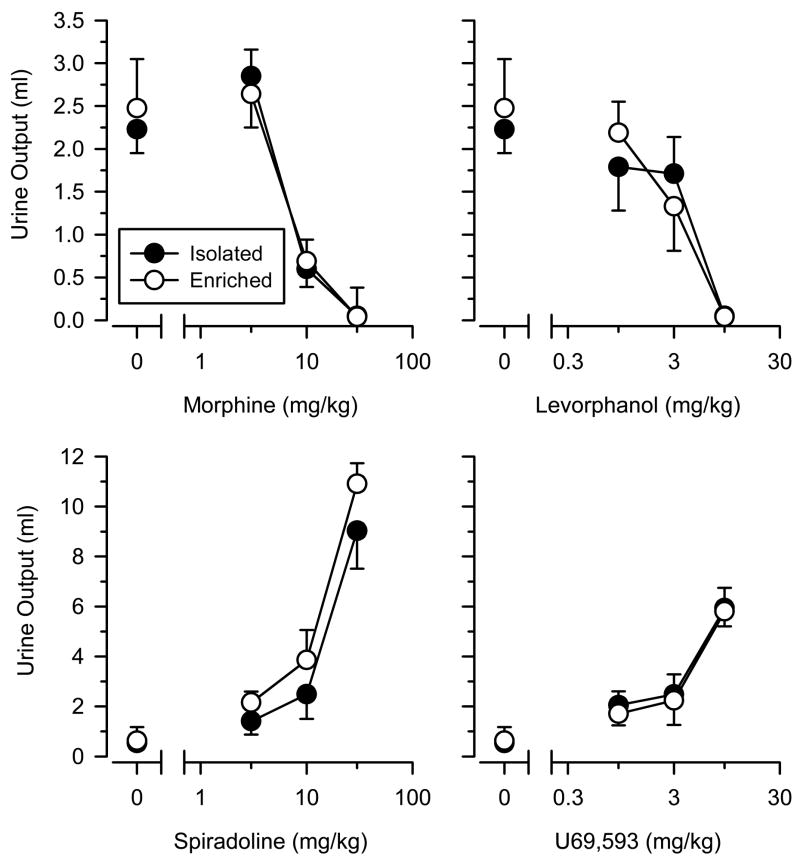
In water-loaded rats, the amount of urine collected after saline administration did not differ between isolated and enriched rats [t (14) = 0.392; p = 0.701]. Both morphine and levorphanol dose dependently decreased urine output, but no differences in sensitivity were observed between the two groups (Figure 2). Consistent with these observations, repeated measures ANOVA revealed significant main effects of dose (p’s < 0.05) for both drugs, but no main effect of group or dose × group interaction was observed.
Figure 2.
Antidiuretic effects of the mu opioids morphine and levorphanol (top panels) and the diuretic effects of the kappa opioids spiradoline and U69,593 (lower panels) in isolated (filled symbols) and enriched (open symbols) rats. For the antidiuresis tests, rats were water-loaded with 2 ml/100 g distilled water via subcutaneous injection immediately prior to the session. All rats were normally hydrated for the diuresis tests. Points above “0” represent the effects of saline (1 ml/kg). Ordinates reflect urine output (ml) as measured over a 2-hr time period. Abscissas reflect dose of test drug in mg/kg of body weight. Vertical lines on data points represent the SE; where not indicated, the SE fell within the data point.
Spiradoline and U69,593
In normally hydrated rats, urine output did not differ between isolated and enriched rats following saline administration [t (13) = 0.305; p = 0.765]. Spiradoline and U69,593 produced dose-dependent increases in urine output, but no differences in potency were observed between the two groups (Figure 2). Similar to that seen with morphine and levorphanol, repeated measures ANOVA revealed significant main effects of dose (p’s < 0.05), but no other significant effects were observed.
Discussion
Using a protocol established previously in male rats [12, 13], environmental enrichment did not influence sensitivity to the effects of a variety of opioid receptor agonists in female rats. Across a range of doses and two stimulus intensities, isolated and enriched females did not differ in sensitivity to the antinociceptive effects of the mu opioids morphine and levorphanol, the kappa opioids spiradoline and U69,593, and the mixed-action opioids buprenorphine and butorphanol. The warm-water, tail-withdrawal procedure has previously been used to demonstrate both within- and between-subject differences in opioid sensitivity based on factors such as sex [24, 27], strain [28, 29], age [22, 23], access to running wheels [18, 19], chronic exposure to opioid agonists [30, 31], and chronic exposure to opioid antagonists [32]. Thus, the failure to detect significant differences in antinociception in the present study cannot be attributed to a general insensitivity of the assay. Furthermore, all subjects remained in their respective conditions for 6 weeks prior to the commencement of behavioral testing. This period of time fully encompasses the duration of periadolescence in rats, a critical period in development during which the nervous system is particularly sensitive to social and environmental influences [33]. Finally, these effects were not limited to antinociception, as isolated and enriched females also did not differ in sensitivity to the antidiuretic effects of morphine and levorphanol, and the diuretic effects of spiradoline and U69,593.
In order to increase the ecological validly of the study, estrous phase was allowed to cycle normally and was not manipulated. Although some studies reported that opioid sensitivity differed across phases of the estrous cycle [34, 35, 36], these effects were typically small and not apparent in all rat strains [37]. When effects were observed, they typically accounted for only a small percentage (< 5% in the tail-withdrawal procedure) of the total variance in responsiveness across females [38]. Given that we previously reported that environmental enrichment increased the potency and/or effectiveness of some opioids by a factor of 3 or more [12, 13], it is unlikely that variations in opioid sensitivity due to the estrous cycle was responsible for the lack of significant effects reported in the present study.
Only a few studies have examined the effects of environmental enrichment on sensitivity to morphine and other mu opioids, and those studies often reported conflicting findings. For instance, environmental enrichment was reported to increase [10, 11], decrease [11], and not affect [12, 14] sensitivity to morphine. At present, it is unclear whether those conflicting findings can be traced to sex differences in the effects of environmental enrichment, as only one of the previous studies employed the use of females [10]. However, cross-study comparisons suggest that sex differences in the effects of environmental enrichment may be minimal in regard to mu opioids. For example, we previously reported that environmental enrichment did not alter sensitivity to the antinociceptive effects of morphine and levorphanol in male rats [12], which is consistent with the data reported in the present study with females.
To our knowledge, only one study has examined the effects of environmental enrichment on sensitivity to kappa opioids. In that study, we reported that enriched male rats were more sensitive than isolated male rats to the antinociceptive, diuretic, and aversive effects of spiradoline [13]. A number of factors other than sex differed between that study and the present investigation, so it is not known whether sex was the sole factor contributing to the differences reported between the two studies. Regardless, given the magnitude and generality of the effects reported in our previous study, sex must be considered as a potential variable that serves to modulate the effects of environmental enrichment on sensitivity to opioids with a kappa component of action.
Only two studies have examined the effects of environmental enrichment on mixed-action opioids, and both of those studies reported large differences in sensitivity between isolated and enriched rats. In an initial study, we reported that enriched male rats were more sensitive than isolated male rats to the mixed-action opioid nalorphine in the warm-water, tail-withdrawal procedure [13]. The differences in sensitivity were quite dramatic, as isolated rats required a dose 1000-fold higher to produce the same antinociceptive response seen in enriched rats. In a second study, we reported that enriched male rats were more sensitive to the antinociceptive and conditioned rewarding effects of the mixed-action opioids buprenorphine, butorphanol, and nalbuphine [12]. The differences in sensitivity seen with butorphanol and nalbuphine were particularly robust, as both of those drugs produced agonist-like effects in enriched rats while functioning as antagonists in isolated rats. When such findings are considered in light of the present data obtained with females, they suggest that sex may be an important factor mediating the effects of environmental enrichment on sensitivity to opioids possessing multiple (i.e., mu and kappa) components of action.
Despite the large literature on the effects of environment enrichment on adult behavior, relatively few studies have examined these effects in females, and even fewer have specifically examined sex as a variable. When both males and females were included in the experimental design, they typically reported that females were less sensitive to enrichment than males [15, 16, 17]. The reasons that females were less responsive to the behavioral effects of enrichment are not known, but it is worth noting that histological data have revealed sex differences in the anatomical effects of environmental enrichment, and these data suggested that enrichment was stressful for males but not females [39]. Consistent with this possibility, environmental enrichment increased plasma concentrations of the stress hormone corticosterone in socially housed male mice provided with supplemental environmental enrichment [40]. Although corticosterone negatively modulates mu-opioid antinociception [41, 42, 43], evidence suggests that it positively modulates kappa-opioid antinociception, as dexamethasone, a glucocorticoid receptor agonist, increases the antinociceptive effects of the kappa opioid U50,488 [44]. Hence, if environmental enrichment leads to an elevation of corticosterone in males but not females, then enrichment would be expected to increase sensitivity to kappa receptor agonists in male rats but not female rats, via activation of central glucocorticoid receptors. Although speculative, this explanation is consistent with a large body of literature reporting sex differences in the HPA axis and stress reactivity [for reviews, see 45, 46], and would account for the sex differences we reported for kappa and mixed-action opioids across studies.
In conclusion, isolated and enriched female rats did not differ in sensitivity to the antinociceptive effects of representative mu, kappa, and mixed-action opioids. Isolated and enriched females also did not differ in sensitivity to the antidiuretic effects of mu opioids and the diuretic effects of kappa opioids. These data indicate that environmental enrichment does not alter sensitivity to the effects of opioid receptor agonists in female rats, and suggest that females may respond differently to environmental enrichment than males.
Acknowledgments
This study was supported by US Public Service Grant DA14255 (to M.A.S). Additional support was provided by the Howard Hughes Medical Institute, the Duke Endowment, and Davidson College. The authors wish to thank Amy Becton for expert animal care and maintenance. Portions of these data were presented at the 37th annual Society for Neuroscience meeting in San Diego, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of Brain. Science. 1964;146:610–9. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 2.Bennett EL, Rosenzweig MR, Diamond MC. Rat brain: effects of environmental enrichment on wet and dry weights. Science. 1969;163:825–6. doi: 10.1126/science.163.3869.825. [DOI] [PubMed] [Google Scholar]
- 3.Davies CA, Katz HB. The comparative effects of early-life undernutrition and subsequent differential environments on the dendritic branching of pyramidal cells in rat visual cortex. J Comp Neurol. 1983;218:345–50. doi: 10.1002/cne.902180310. [DOI] [PubMed] [Google Scholar]
- 4.Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi S, Ohashi Y, Ando S. Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. J Neurosci Res. 2002;70:340–6. doi: 10.1002/jnr.10442. [DOI] [PubMed] [Google Scholar]
- 6.Segovia G, Yagüe AG, García-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res Bull. 2006;70:8–14. doi: 10.1016/j.brainresbull.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Bardo MT, Valone JM, Robinet PM, Shaw WB, Dwoskin LP. Environmental enrichment enhances the stimulant effect of intravenous amphetamine: Search for a cellular mechanism in the nucleus accumbens. Psychobiology. 1999;27:292–9. [Google Scholar]
- 8.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 9.Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–64. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 10.Smith JK, Neill JC, Costall B. The influence of postweaning housing conditions on drug-induced conditioned taste aversion. Pharmacol Biochem Behav. 1998;59:379–86. doi: 10.1016/s0091-3057(97)00370-5. [DOI] [PubMed] [Google Scholar]
- 11.Bardo MT, Robinet PM, Hammer RF., Jr Effect of differential rearing environments on morphine-induced behaviors, opioid receptors and dopamine synthesis. Neuropharmacology. 1997;36:251–59. doi: 10.1016/s0028-3908(96)00139-6. [DOI] [PubMed] [Google Scholar]
- 12.Smith MA, Chisholm KA, Bryant PA, Greene JL, McClean JM, Stoops WW, Yancey DL. Social and environmental influences on opioid sensitivity in rats: importance of an opioid’s relative efficacy at the mu-receptor. Psychopharmacology. 2005;181:27–37. doi: 10.1007/s00213-005-2218-2. [DOI] [PubMed] [Google Scholar]
- 13.Smith MA, Bryant PA, McClean JM. Social and environmental enrichment enhances sensitivity to the effects of kappa opioids: studies on antinociception, diuresis and conditioned place preference. Pharmacol Biochem Behav. 2003;76:93–101. doi: 10.1016/s0091-3057(03)00189-8. [DOI] [PubMed] [Google Scholar]
- 14.Hill SY, Powell BJ. Cocaine and morphine self-administration: effects of differential rearing. Pharmacol Biochem Behav. 1976;5:701–4. doi: 10.1016/0091-3057(76)90315-4. [DOI] [PubMed] [Google Scholar]
- 15.Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res. 2005;165:187–96. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Pena Y, Prunell M, Dimitsantos V, Nadal R, Escorihuela RM. Environmental enrichment effects in social investigation in rats are gender dependent. Behav Brain Res. 2006;174:181–7. doi: 10.1016/j.bbr.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci Lett. 2002;334:165–8. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology. 2003;168:426–34. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- 19.Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacol Biochem Behav. 2006;85:12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiolo Behav. 2001;74:245–51. doi: 10.1016/s0031-9384(01)00577-7. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 22.Smith MA, Gray JD. Age-related differences in sensitivity to the antinociceptive effects of opioids in male rats. Influence of nociceptive intensity and intrinsic efficacy at the mu receptor. Psychopharmacology. 2001;156:445–53. doi: 10.1007/s002130100750. [DOI] [PubMed] [Google Scholar]
- 23.Smith MA, French AM. Age-related differences in sensitivity to the antinociceptive effects of kappa opioids in adult male rats. Psychopharmacology. 2000;166:255–64. doi: 10.1007/s00213-002-1102-6. [DOI] [PubMed] [Google Scholar]
- 24.Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology. 2000;150:430–42. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- 25.Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6:372–83. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- 26.Tallarida RJ, Murray RB. Manual of pharmacologic calculations for computer programs. Berlin Heidelberg New York: Springer; 1987. [Google Scholar]
- 27.Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–78. [PubMed] [Google Scholar]
- 28.Morgan D, Cook CD, Picker MJ. Sensitivity to the discriminative stimulus and antinociceptive effects of mu opioids: role of strain of rat, stimulus intensity, and intrinsic efficacy at the mu opioid receptor. J Pharmacol Exp Ther. 1999;289:965–75. [PubMed] [Google Scholar]
- 29.Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ. Sex and rat strain determine sensitivity to kappa opioid-induced antinociception. Psychopharmacology. 2002;160:170–81. doi: 10.1007/s00213-001-0949-2. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Barrett AC, Picker MJ. Antinociceptive effects of opioids following acute and chronic administration of butorphanol: influence of stimulus intensity and relative efficacy at the mu receptor. Psychopharmacology. 1999;143:261–9. doi: 10.1007/s002130050945. [DOI] [PubMed] [Google Scholar]
- 31.Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology. 2001;158:154–64. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- 32.Smith MA, McClean JM, Greene JL. Enhanced sensitivity to the antinociceptive effects of kappa opioids in naltrexone-treated rats: dose- and time-dependent effects. Behav Pharmacol. 2003;14:641–7. doi: 10.1097/00008877-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 34.Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–27. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- 35.Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behav Brain Res. 2007;177:126–33. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shane R, Bernal SY, Rozengurtel S, Bodnar RJ. Estrus phase differences in female rats in morphine antinociception elicited from the ventrolateral periaqueductal gray. Int J Neurosci. 2007;117:811–22. doi: 10.1080/00207450600910259. [DOI] [PubMed] [Google Scholar]
- 37.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–89. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 38.Craft RM, Bernal SA. Sex differences in opioid antinociception: kappa and ‘mixed action’ agonists. Drug Alcohol Depend. 2001;63:215–28. doi: 10.1016/s0376-8716(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 39.McConnell P, Uylings HB, Swanson HH, Verwer RW. Sex differences in effects of environmental stimulation on brain weight of previously undernourished rats. Behav Brain Res. 1981;3:411–5. doi: 10.1016/0166-4328(81)90010-3. [DOI] [PubMed] [Google Scholar]
- 40.Haemisch A, Voss T, Gartner K. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol Behav. 1994;56:1041–8. doi: 10.1016/0031-9384(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 41.Ratka A, Sutanto W, De Kloet ER. Long-lasting glucocorticoid suppression of opioid-induced antinociception. Neuroendocrinology. 1988;48:439–44. doi: 10.1159/000125046. [DOI] [PubMed] [Google Scholar]
- 42.Capasso A, Di Giannuario A, Loizzo A, Pieretti S, Sorrentino L. Central interaction of dexamethasone and RU-38486 on morphine antinociception in mice. Life Sci. 1992;51:PL139–43. doi: 10.1016/0024-3205(92)90521-p. [DOI] [PubMed] [Google Scholar]
- 43.Lim G, Wang S, Zeng Q, Sung B, Mao J. Spinal glucocorticoid receptors contribute to the development of morphine tolerance in rats. Anesthesiology. 2005;102:832–7. doi: 10.1097/00000542-200504000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Pieretti S, Di Giannuario A, Domenici MR, Sagratella S, Capasso A, Sorrentino L, Loizzo A. Dexamethasone-induced selective inhibition of the central mu opioid receptor: functional in vivo and in vitro evidence in rodents. Br J Pharmacol. 1994;113:1416–22. doi: 10.1111/j.1476-5381.1994.tb17155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 46.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–13. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]