Abstract
CS-17 is a murine monoclonal antibody to the human TSH receptor (TSHR) with both inverse agonist and antagonist properties. Thus, in the absence of ligand, CS-17 reduces constitutive TSHR cAMP generation and also competes for TSH binding to the receptor. The present data indicate that for both of these functions, the monovalent CS-17 Fab (50 kDa) behaves identically to the intact, divalent IgG molecule (150 kDa). The surprising observation that CS-17 competes for TSH binding to the human but not porcine TSHR enabled identification of a number of amino acids in its epitope. Replacement of only three human TSHR residues (Y195, Q235, and S243) with the homologous porcine TSHR residues totally abolishes CS-17 binding as detected by flow cytometry. TSH binding is unaffected. Of these residues, Y195 is most important, with Q235 and S243 contributing to CS-17 binding to a much lesser degree. The functional effects of CS-17 IgG and Fab on constitutive cAMP generation by porcinized human TSHR confirm the CS-17 binding data. The location of TSHR amino acid residues Y195, Q235, and S243 deduced from the crystal structure of the FSH receptor leucine-rich domain provides valuable insight into the CS-17 and TSH binding sites. Whereas hormone ligands bind primarily to the concave surface of the leucine-rich domains, a major portion of the CS-17 epitope lies on the opposite convex surface with a minor component in close proximity to known TSH binding residues.
TSH RECEPTOR (TSHR) activity and autoregulation by iodine are the two mechanisms that maintain thyroid hormone homeostasis (reviewed in Refs. 1,2,3). Nevertheless, it has long been recognized that the thyroid gland is able to maintain a low level of thyroid function in the absence of TSH. Thus, in secondary hypothyroidism, serum thyroid hormone levels are typically not as profoundly low as after total thyroid ablation or autoimmune destruction. The likely explanation for this phenomenon is that the TSHR retains modest constitutive activity in the absence of ligand, to a greater extent than the structurally related gonadotropin G protein-coupled receptors (4,5). TSHR constitutive activity would be even higher were it not for the constraint of the ectodomain, which functions as an inverse agonist (6,7). An inverse agonist suppresses the function of a receptor that exhibits ligand-independent (constitutive) activity.
Recently a murine monoclonal antibody (mAb) to the TSHR was observed to have inverse agonist activity in suppressing cAMP levels in transfected cells expressing the human TSHR (8). This mAb, CS-17, also competed for TSH binding to the TSHR. The dual properties of CS-17 in being both an inverse agonist and an antagonist is consistent with many classical competitive antagonists (reviewed in Ref. 9). Indeed, a human TSHR autoantibody with TSH-blocking activity was also found to reduce TSHR constitutive activity (10). For these reasons, it is likely that reexamination of the murine TSH-blocking mAb that have been reported in recent years (i.e. Refs. 11,12,13,14,15,16,17) will reveal that they also possess inverse agonist activity.
Inverse agonists of G protein-coupled receptor are generally small molecules developed by the pharmaceutical industry for targets such as the adrenergic, histamine, angiotensin, and dopamine receptors with small extracellular domains (ectodomains). TSHR mAb CS-17 is a rare inverse agonist in the form of an antibody, a very large molecule. The few other antibody inverse agonists that have been reported interact with the extracellular loops of the β2-adrenergic (18) and M2-muscarinic acetylcholine receptors (19). The TSHR, on the other hand, has a very large ectodomain of 397 amino acid residues after signal peptide removal (reviewed in Ref. 2). Moreover, mAb CS-17 does not bind to the extracellular loops but rather to the N-terminal portion of the TSHR ectodomain, having been generated by immunizing mice with a component approximating the TSHR A-subunit (residues 22–289 after deletion of the signal peptide) (8).
Because of these unique properties, in the present study, we explored further the interaction between CS-17 and the TSHR. We report that CS-17 inverse agonist and TSH-blocking activities are not indirect effects caused by the IgG Fc region. In addition, identification of key amino acid residues in the CS-17 epitope provides challenging insight into the TSH binding site.
Materials and Methods
TSHR mAbs
Monoclonal antibody CS-17 is one of a panel of TSHR mAb generated in our laboratory from seven fusions over a 3-yr period, as described previously (8). For IgG purification, cells were cultured in serum-free medium and the latter applied to protein G Hi-Trap columns (Pharmacia, now GE Healthcare, Piscataway, NJ). CS-17 Fab was generated from 10 mg IgG using the ImmunoPure Fab preparation kit (Pierce, Rockford, IL). Nonfunctional murine mAb 4C1 (11) was purchased from Serotec (Oxford, UK).
Culture of TSHR-expressing cells
Three different types of TSHR-expressing cells were used. One is Chinese hamster ovary (CHO) cells stably expressing the wild-type TSHR. This cell line was obtained using the human TSHR cDNA (20) with three introduced restriction sites (21) and the H601 polymorphism converted to Y601 (22).
A second type is HEK293 cells stably transfected with TSHR mutants generated in the vector pcDNA5/FRT according to the protocol of the manufacturer (Invitrogen, Carlsbad, CA). For this purpose, the cDNA was excised with EcoRI (blunted with Klenow DNA polymerase) and XbaI. The vector was cut with NheI (blunted) and XbaI. The XbaI site in the vector cloning site was made available by removal of a second, downstream XbaI site by mutation without codon alteration. The mutations described in the text were introduced using the QuikChange site-directed mutagenesis kit (Stratagene, San Diego, CA). All mutations were confirmed by nucleotide sequencing. Transfections were with FuGENE HD (Roche, Indianapolis, IN) followed by hygromycin B selection. We transfected Flp-In HEK293 cells because we were unsuccessful in extended attempts with Flp-In CHO cells (both from Invitrogen) despite using a wide variety of hygromycin concentrations and numerous transfection variations. In contrast, we obtained stable cell lines for all TSHR mutants (but not the wild-type TSHR) using Flp-In HEK293 host cells selected with 100 μg/ml hygromycin. Cells were cultured in DMEM supplemented with 10% fetal calf serum, penicillin (100 U/ml), gentamicin (50 μg/ml), and Fungizone (2.5 μg/ml).
The third type is Cos-7 cells transiently transfected with the wild-type TSHR and the indicated TSHR mutants in pcDNA5/FRT. We used Cos-7 cells because transiently transfected HEK293 cells passaged the day before assay (1 d after transfection) are insufficiently adherent to permit effective washing of cell monolayers in the TSH binding protocol (see below). After transfection as described above, cells were cultured in DMEM supplemented with 10% fetal calf serum, penicillin (100 U/ml), gentamicin (50 μg/ml), and Fungizone (2.5 μg/ml) and were tested after approximately 48 h.
Cultured cell cAMP assays
CHO or HEK293 cells stably expressing the wild-type TSHR or TSHR mutants were transferred into 96-well plates. Cells from the same transfection were also plated in 6-cm culture dishes to monitor the expression level by flow cytometry (see below). For bioassay, the culture medium described above was replaced with DMEM medium supplemented with 1 mm isobutyl methylxanthine, 10 mm HEPES, and, where indicated in the text, bovine TSH (Sigma-Aldrich, St. Louis, MO). Mock-transfected COS-7 cells were included as controls. After 60 min at 37 C, the medium was aspirated and intracellular cAMP was extracted with 0.2 ml 95% ethanol. The extracts were evaporated to dryness, resuspended in 0.1 ml of Dulbecco’s PBS (pH 7.5), and samples (12 μl) assayed using the LANCE cAMP kit according to the protocol of the manufacturer (PerkinElmer, Shelton, CT).
Flow cytometry
HEK293 cells were harvested from six-well plates using 1 mm EDTA and 1 mm EGTA in PBS. After washing twice with PBS containing 10 mm HEPES (pH 7.4), 2% fetal bovine serum, and 0.05% NaN3, the cells were incubated for 30 min at room temperature in 100 μl of the same buffer containing 1 μg of either normal mouse IgG or mAb CS-17 (8). After rinsing, the cells were incubated for 45 min with 100 μl fluorescein isothiocyanate-conjugated goat antimouse IgG (1:100; Caltag, Burlingame, CA), washed, and analyzed using a FACScan flow cytofluorimeter (Beckman-Coulter, Fullerton, CA). Cells stained with propidium iodide (1 μg/ml final concentration) were excluded from analysis.
TSH binding
CHO cells stably expressing the TSHR or COS-7 cells transiently transfected with plasmids expressing the wild-type TSHR or TSHR mutants were cultured in 24-well plates. Medium was aspirated and replaced with 250 μl binding buffer (Hanks’ buffer with 250 mm sucrose substituting for NaCl to maintain isotonicity and 0.25% BSA) containing approximately 12,000 cpm 125I-TSH (BRAHMS, Berlin, Germany). The buffer was supplemented with the indicated concentrations of bovine TSH (Sigma-Aldrich), CS-17 IgG, or CS-17 Fab. After incubation for 3 h at room temperature, cells were rapidly rinsed three times with binding buffer (4 C) and solubilized with 0.2 ml 1 n NaOH, and radioactivity was then measured in a γ-counter. Nonspecific binding was determined using COS-7 cells transfected in parallel with the vector alone.
Competition by CS-17 for 125I-TSH binding to the porcine TSHR was measured using a commercial kit (Kronus, Boise, ID). CS-17 diluted in normal human serum (1 μg in 50 μl) was incubated with detergent solubilized TSHR; 125I-TSH was added (final volume of 200 μl), and the TSHR-antibody complexes were precipitated with polyethylene glycol, according to the protocol of the manufacturer. Controls included the same concentrations of normal mouse IgG and CS-1, another mAb to the TSHR with known TSH binding inhibitory activity.
Results
Properties of CS-17 IgG and Fab
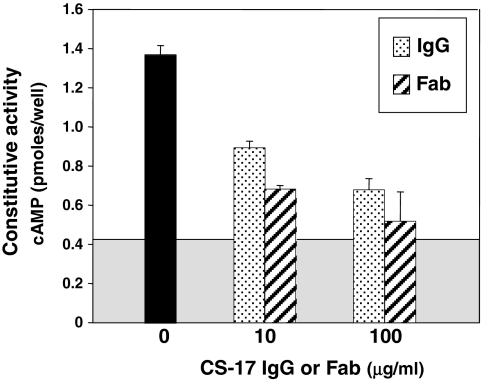
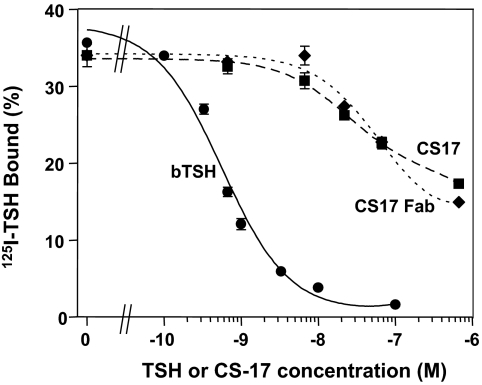
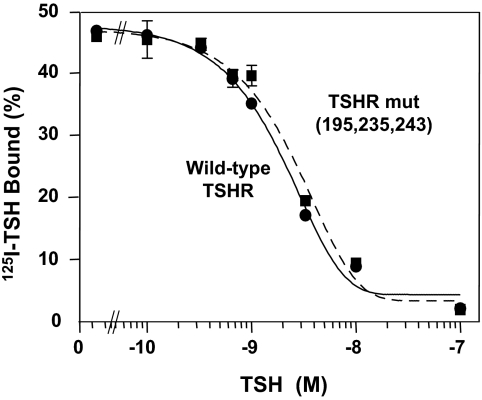
In the absence of TSH, CHO cells stably expressing the wild-type TSHR have higher cAMP levels than untransfected CHO cells (Fig. 1). As reported previously (8), this TSHR constitutive activity is reduced by mAb CS-17 IgG, in this representative experiment by 51 and 72% at 10 μg and 100 μg/ml, respectively (Fig. 1). The CS-17 Fab retained inverse agonist activity and, on a weight basis, was even more potent than the IgG. Besides suppressing TSHR constitutive activity in the absence of ligand, CS-17 IgG competes for TSH binding (8). On a molar basis, CS-17 Fab is equipotent to the intact IgG molecule in inhibiting 125I-TSH binding to CHO cells expressing the wild-type TSHR, although less effective than TSH (Fig. 2).
Figure 1.
The CS-17 Fab retains inverse agonist activity. CHO cells stably expressing the wild-type TSHR were incubated for 1 h at 37 C in the absence or presence of the indicated concentrations of CS-17 mAb or Fab. Each bar represents the mean ± sd of duplicate wells. The boxed gray area indicates the mean cAMP value in control, untransfected CHO cells. Extension of the bars beyond the boxed area represents TSHR constitutive activity. The experiment shown is representative of two experiments in the present study. Additional experiments in which CS-17 Fab inhibit TSHR constitutive activity in HEK293 cells are shown in Fig. 5.
Figure 2.
The CS-17 Fab competes for binding of TSH to the TSHR. Monolayers of CHO cells stably expressing the wild-type were incubated for 3 h at room temperature in the absence or presence of the indicated concentrations of unlabeled TSH, CS-17 mAb, or Fab. For comparison between IgG and Fab potency, concentrations are expressed in moles. Each point represents the mean ± sd of values from duplicate wells of cells. Similar data were obtained in a second experiment.
CS-17 does not bind to the porcine TSHR
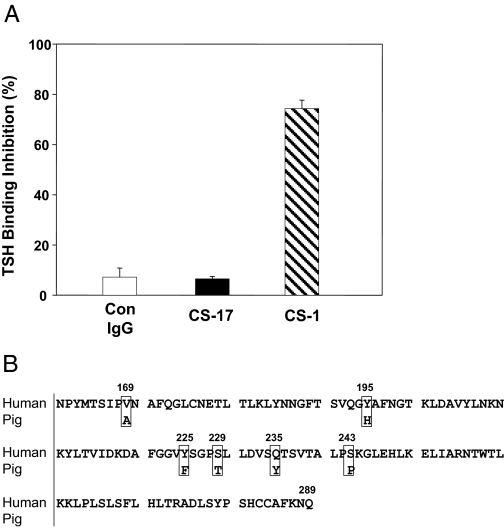
Despite competing for TSH binding to the human TSHR, CS-17 IgG (20 μg/ml) did not inhibit 125I-TSH binding to solubilized porcine TSHR relative to the same concentration of normal mouse IgG (Fig. 3A). As a positive control, another murine mAb, CS-1, generated, like CS-17, by immunization with the the human TSHR A-subunit, inhibited TSH binding by 75%.
Figure 3.
A, CS-17 does not inhibit TSH binding to the porcine TSHR. CS-17, CS-1, and normal mouse (Con) IgG (all at 20 μg/ml) were preincubated with solubilized porcine TSHR, and 125I-TSH was then added. Radiolabeled TSH complexed to the TSHR was precipitated with polyethylene glycol. CS-1 is a murine mAb, like CS-17 generated by immunization with the human TSHR A-subunit. Bars represent the mean ± se of data from triplicate experiments, each done in duplicate. B, Comparison of the primary amino acid sequences of the human (20) (accession no. M31774) and pig (37) (accession no. AY082015) TSHR between residues 161 and 289. Six amino acid differences are boxed.
Previous study of CS-17 binding to chimeric receptors containing components of the human TSHR and rat LH receptor (LHR) indicated that a significant portion of its epitope lay in the N-terminal region of the TSHR hinge (residues 261–289) (8). However, these chimeric receptor data did not exclude the possibility that additional components in a discontinuous epitope could lie further upstream, within residues 170–260 in the leucine-rich domain (LRD). Indeed, comparison of the primary amino acid sequences of the human and pig TSHR in both these regions (residues 170–289) revealed only five amino acid differences, all located in the LRD (Fig. 3B). A heterologous sixth amino acid was present closely upstream at position 169.
Identification of amino acid residues in the CS-17 epitope
We hypothesized that mutation of the human TSHR segment between amino acid residues 170 and 289 to that of the porcine TSHR should decrease or abolish CS-17 binding. Consequently, the five human TSHR amino acid residues within this region were mutated individually or in combination in the pcDNA-5/FRT expression vector. Because of its proximity, we also included V169. Having generated these constructs, we were unable to obtain stable transfectants in the preferred CHO cell line (Flp-In-CHO), but we were successful with the majority of constructs (but not the wild type TSHR) on using an HEK-293 host (Flp-In-293). CS-17 binding to these mutants was examined by flow cytometry (Fig. 4).
Figure 4.
Identification of important residues in the CS-17 epitope. The six nonhomologous residues in the human TSHR (depicted in Fig. 3B) were mutated to those of the porcine TSHR, individually and in different permutations. CS-17 binding to these mutants, stably expressed in HEK293 cells, was examined by flow cytometry using, as a benchmark, mAb 4C1 with a downstream epitope. For brevity, the mutant TSHR are identified by the positions of the mutated amino acid residues. Normal mouse IgG (Con IgG) was included as a negative control. The vertical dashed lines are drawn through the apex of the 4C1 signal to enhance visually the difference with the CS-17 signal, as shown by the horizontal arrows. These data are representative of three experiments.
Of the six individual mutations, only CS-17 binding to Y195H was clearly diminished relative to control mAb 4C1 whose epitope includes further downstream acid residues 381–384 (11). On examining combinations of mutations in various permutations (not all shown in Fig. 4), we noted a small decrease in CS-17 binding to Q235Y/S243P, unlike for Y225F/S229T. As mentioned, individually, Q235Y and S243P had minimal effects on CS-17 binding. Combination of the Q235Y/S243P double mutation with Y195H completely abolished CS-17 binding, comparable with the simultaneous mutation of all six residues. Notably, control mAb 4C1 binding remained strong in all TSHR mutants, including Q235Y (the only nonclonal stable cell line). Finally, although the absence of a stable HEK cell line expressing the wild-type TSHR did not negate identification of important residues in the CS-17 epitope, we nevertheless performed transient transfections with the wild-type TSHR and V169A in the pcDNA5/FRT vector. CS-17 and 4C1 bound to both receptors in the same proportions (data not shown), indicating that CS-17 recognized V169A in the same way as the wild-type TSHR.
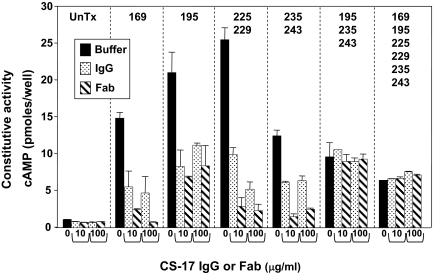
Functional consequences of conversion of the human to the pig TSHR
To complement the flow cytometry data, we examined the functional effects of converting the six nonhomologous human TSHR residues within positions 161–289 (Fig. 3B) to their porcine counterparts. The stable HEK293 cell lines expressing the TSHR mutants all displayed high ligand-independent cAMP levels (5- to 25-fold greater than untransfected cells) (Fig. 5), consistent with their high levels of expression as determined by flow cytometry (Fig. 4). CS-17 IgG and Fab at concentrations of 10 and 100 μg/ml reduced constitutive cAMP generation in proportion to the flow cytometric signals obtained with IgG. Thus, CS-17 IgG and Fab clearly inhibited constitutive cAMP generation by all TSHR mutants with the exception of the triple mutant Y195H/Q235Y/S243P and the TSHR with all six residues mutated. CS-17 and its Fab were less effective in suppressing the constitutive activity of Y195H, and an effect on the dual mutation Q235Y/S243P was not clearly evident. As expected, on a mass basis, the CS-17 Fab was more potent than IgG on susceptible TSHR.
Figure 5.
CS-17 IgG and Fab functional effects on TSHR mutations. The same HEK293 cell lines stably expressing the TSHR mutants depicted in Fig. 4 (nonhomologous human amino acid residues converted to their porcine TSHR counterparts) were incubated with the indicated concentrations of CS-17 IgG or Fab. Only the amino acid positions are indicated; substitutions are shown in Fig. 3B). UnTx, Untransfected. Bars represent the mean ± sd of values from duplicate wells. This experiment was repeated once with similar results.
Although CS-17 did not recognize the TSHR triple-mutant Y195H/Q235Y/S243P on flow cytometry (and consequently did not display inverse agonist activity), the question arose as to whether these three residues relevant to CS-17 binding and function affected TSH binding. HEK293 cells expressing the TSHR triple mutant bound 125I-TSH avidly, compared with untransfected cells, but competition for 125I-TSH binding required extremely high concentrations of unlabeled TSH (data not shown). These data are consistent with the phenomenon of negative cooperativity that we observed previously with CHO cells overexpressing the TSHR (23). Indeed, the flow cytometric (Fig. 4) and constitutive activity (Fig. 5) data support extremely high TSHR expression in stably transfected HEK293 cells. To study cells with fewer receptors, we transiently transfected COS-7 cells. Competition for 125I-TSH binding by unlabeled TSH was more effective, and most important, the affinities of TSH for the wild-type TSHR and the triple mutant were essentially identical (Fig. 6). These data indicate that although CS-17 IgG and Fab compete for TSH binding, the critical amino acid residues in the CS-17 epitope are distinct from residues contributing to the TSH binding site.
Figure 6.
TSHR amino acids critical for CS-17 binding are distinct from contact residues in the TSH binding site. COS-7 cells were transiently transfected with the wild-type TSHR and the TSHR triple-mutant Y195H/Q235Y/S243P. Binding of 125I-TSH was determined in the presence of the indicated concentrations of unlabeled TSH (see Materials and Methods). Each point represents the mean ± sd of duplicate wells of cells. This experiment is representative of three separate experiments with similar results.
Discussion
CS-17 is a murine mAb to the human TSHR with both inverse agonist and antagonist properties. Thus, in the absence of ligand, CS-17 reduces constitutive TSHR cAMP generation and also competes for TSH binding to the receptor (8). The present data indicate that for both of these functions, the monovalent CS-17 Fab (50 kDa) behaves identically to the intact, divalent IgG molecule (150 kDa). This information excludes the possibility that the Fc component of CS-17 is responsible for inverse agonist or TSH binding inhibitory activity by impinging on a TSHR site distinct from the antibody binding site. Monomeric and multimeric forms of TSHR exist in the plasma membrane (24). Suppression of constitutive cAMP generation by the CS-17 Fab also indicates that this function does not require the TSHR dimer. Whether CS-17 Fab inverse agonist activity occurs because of an allosteric change(s) in TSHR ectodomain conformation or whether a portion of the Fab distinct from its antigen binding site impacts elsewhere on the receptor is unknown. It is presently accepted that TSHR autoantibodies that block TSH binding do so by competition at the TSH binding site rather than through TSHR allosteric changes (reviewed in Ref. 2). For this reason, it seems more likely that the CS-17 Fab and TSH binding sites overlap, at least in part.
The observation that CS-17 competes for TSH binding to the human but not porcine TSHR enabled identification of some amino acid resideus in its epitope. Thus, replacement of three human TSHR amino acid residues (Y195, Q235, and S243) with the homologous porcine TSHR residues totally abolishes CS-17 binding as detected by flow cytometry. Of these residues, Y195 is most important, with Q235 and S243 contributing to CS-17 binding to a much lesser degree. The functional effects of CS-17 IgG and Fab on constitutive cAMP generation by porcinized human TSHR confirm the CS-17 binding data.
Although mutation of these three TSHR residues abrogated detectable CS-17 binding and inverse agonist activity, the epitope of a typical IgG antibody comprises approximately 16–20 discontinuous amino acid residues in a globular protein (25,26). Indeed, the epitope of a stimulatory human autoantibody for the TSHR A-subunit contains 22 residues (27). Therefore, CS-17 is likely to contact many more TSHR residues than Y195, Q235, and S243 in the LRD and hinge regions. The CS-17 epitope cannot extend downstream of Q289 because the latter is the C terminus of the TSHR immunogen used for CS-17 generation. In the present study, the human TSHR could not be porcinized between residues S243 and Q289 because of identity in the human and pig TSHR amino acid sequences in this region (Fig. 3B). However, previous data obtained with human TSHR-rat LHR chimeric receptors, which differ extensively in this region (21), did implicate TSHR residues between L260 and Q289 (Fig. 6 in Ref. 8). Therefore, from both the present and previous studies, we conclude that the CS-17 epitope involves TSHR residues in both the LRD and hinge regions. Incidentally, the junction between the TSHR LRD and hinge regions is imprecisely defined. The crystal structure of the FSH receptor (FSHR) LRD extends to the equivalent of TSHR residue D276 (Fig. 2C in Ref. 28), whereas the crystal structure of the TSHR LRD terminates at residue T257 (Fig. 6C in Ref. 27).
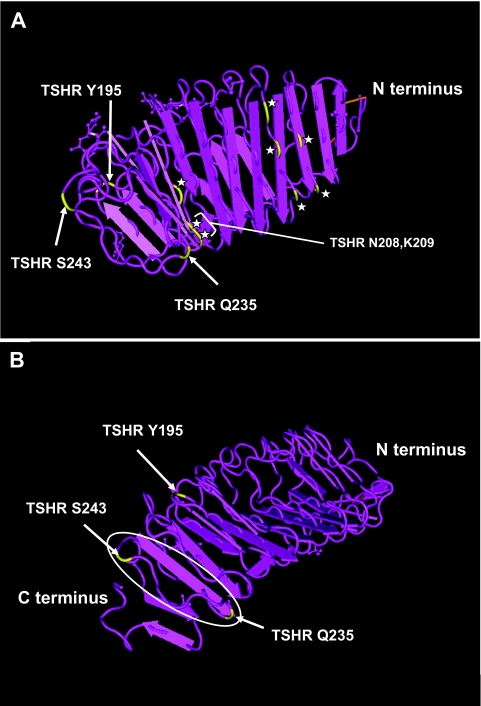
Even though only a few specific TSHR residues, Y195 and to a lesser degree Q235 and S243, have been identified as contributing to the CS-17 epitope, the location of these residues on the crystal structure of the TSHR LRD (27) provides valuable insight into the CS-17 and TSH binding sites. First, consistent with their contribution to a surface epitope, all three residues are solvent accessible, that is, they are on the surface of the molecule. Unlike mutations of buried core residues, mutations of surface residues are less likely to cause severe conformational changes with potential allosteric effects. Indeed, even though mutation of TSHR residues Y195, Q235, and S243 abrogates detectable CS-17, TSH binding affinity is unaltered. Because data on the TSHR LRD structure are yet to be deposited in the data banks, the position of the relevant TSHR residues are depicted on the FSHR LRD (Fig. 7, A and B). Two of the three TSHR amino acid residues implicated in CS-17 binding (Y195 and S243) are on the opposite, convex surface of the tubular, crescent-shaped TSHR LRD (Fig. 6C, Ref. 27). Taken together with the chimeric TSH-LHR data, we suggest that CS-17 exerts its inverse agonist activity by anchoring to the dorsum of the conformationally rigid LRD (28) and stabilizing the adjacent hinge region that makes contact with the TSHR extracellular loops (29,30,31) more proximally involved in signal transduction.
Figure 7.
Ribbon diagram of the FSHR LRD depicting amino acid residues contributing to the CS-17 epitope on the closely homologous TSHR. Data are from the National Center for Biotechnology Information, Molecular Modeling DataBase (no. 31464; Protein Data Base (1XWD), derived from a published report (28). A, The FSHR LRD viewed from its β-strand-rich concave surface. TSHR residues Y195, Q235, and S243 (arrows) are localized according to the homologous residues in the FSHR (C188, R227, and S235, respectively) (28). Residues in the TSH binding site determined by mutagenesis (34) are indicated by stars. The two residues closest to Q235 (N208 and K209) are identified. The other stars represent TSHR residues K58, E61, R80, Y82, I85, and Y206. B, The FSHR LRD viewed from the C terminus of its opposite, convex surface. TSHR residues Y195, Q235, and S243 mapped on this structure are indicated by arrows. Q235 and S243 are on the same loop connecting β-strands 9 and 10 (ellipse). The hinge region begins after β-strand 10 and is absent in the FSHR crystal structure.
The location of TSHR residue Q235 is intriguing for two reasons. First, it is at the extreme periphery of the epitope for monoclonal thyroid-stimulating antibody (TSAb) M22 (Fig. 3B, Ref. 27). Second, the question arises as to how mutation of Q235 influences the CS-17 epitope. Q235 is on the rim of the LRD and is potentially accessible to CS-17 and also contacting Y195 and S243 (Fig. 7 B). Another possible explanation is that both Q235 and S243 are on the loop connecting β-strands 9 and 10 (Fig. 6C, Ref. 27), indicated by an ellipse in Fig. 7B. As noted above, individual mutations of Q235 and S243 do not influence CS-17 binding (Fig. 4). Only their combined substitution slightly reduces CS-17 binding. Therefore, CS-17 may contact residues between Q235 and S243, with an allosteric alteration in conformation of the joining loop consequent to mutation of both peripheral residues. Regardless of whether Q235 is directly or indirectly involved in the CS-17 epitope, the latter clearly involves the convex portion of the TSHR LRD.
Because CS-17, including its Fab, competes for TSH binding, the relationship between the CS-17 and TSH binding sites is of interest. Present information on the latter site is incomplete. Although data on the crystal structure of FSH complexed with the FSHR LRD are available (28), the TSHR LRD has been crystallized with a stimulating antibody but not with TSH (27). Consequently, only indirect information is available on the TSH binding site. Initial chimeric receptor studies revealed discontinuous ligand binding sites extending over much of the TSHR ectodomain (32) with a major component in the mid region between amino acid residues 201–211 (33). Extensive mutagenesis data refined this domain to residues to Y206, N208, and K209 and also identified more upstream residues K58, E61, R80, Y82, and I85 (34). These TSHR residues on the concave β-strand surface are depicted on the structurally homologous FSHR LRD (stars in Fig. 7A). Based on the size of the footprint of FSH on its receptor (25 residues) (28), many additional TSHR residues in the TSH binding site remain to be identified.
How then does CS-17 compete for TSH binding to the opposite, concave surface of the TSHR LRD? First, Q235 is positioned very close to N208 and K209 (Fig. 7A). Second, the FSH α- and β-chains wrap partially around the FSHR LRD rim that corresponds to the location of Q235 (the rim at the C termini of the β-strands on the concave surface) (Fig. 1 in Ref. 28 and Fig. 7A). Therefore, there could be partial overlap between CS-17 and TSH binding in this region. Third, as mentioned above, many TSH binding residues on the TSHR remain unidentified. Finally, only the isolated LRD of the FSHR has been crystallized. It has been suggested that this component contains a cryptic FSH binding site that is obscured when other portions of the FSHR ectodomain are present (35). With the holoreceptor, the glycoprotein hormones may bind to different sites including the LRD rims (36).
The question has arisen as to the potential clinical value of CS-17 in the treatment of Graves’ disease. Hyperthyroidism in this disorder is caused by potent agonists, TSAb, and suppression of inverse agonist activity is unlikely to be of therapeutic value. An antagonist that competes for TSAb binding to the TSHR (a TSHR blocker) may be more useful in treating hyperthyroidism. However, in most circumstances, conventional antithyroid drugs are likely to be as effective, certainly cheaper, more easily administered, and more amenable to titration. Furthermore, a therapeutic TSHR blocking antibody is also likely to block TSH action, requiring the addition of T4. Although CS-17 is also a TSH antagonist, this activity is relatively weak. In our opinion, as discussed previously (8), an inverse agonist such as CS-17 may have greater clinical value as an alternative to TSH suppression by T4 in metastatic thyroid carcinoma.
In summary, the fortuitous observation that CS-17 binds to the human but not the porcine TSHR has enabled identification of a number of key residues in its epitope. The location of this epitope on the convex surface of the LRD as well as the hinge region provides insight into the mechanism of CS-17 inverse agonist activity as well as the TSH binding site.
Acknowledgments
We thank BRAHMS (Hennigsdorf, Germany) for generously providing radiolabeled TSH. We are also grateful for contributions by Dr. Boris Catz (Los Angeles, CA).
Footnotes
This work was supported by National Institutes of Health Grants DK 19289 (to B.R.) and DK 54684 (to S.M.M.).
Disclosure statement: The authors have nothing to disclose.
First Published Online April 3, 2008
Abbreviations: CHO, Chinese hamster ovary; FSHR, FSH receptor; LHR, LH receptor; LRD, leucine-rich domain; mAb, monoclonal antibody; TSAb, thyroid-stimulating antibody; TSHR, TSH receptor.
References
- Vassart G, Dumont JE 1992 The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM 1998 The thyrotropin receptor: Interaction with thyrotropin and autoantibodies. Endocr Rev 19:673–716 [DOI] [PubMed] [Google Scholar]
- Ingbar SH 1972 Autoregulation of the thyroid. Response to iodide excess and depletion. Mayo Clin Proc 47:814–823 [PubMed] [Google Scholar]
- Cetani F, Tonacchera M, Vassart G 1996 Differential effects of NaCl concentration on the constitutive activity of the thyrotropin and the luteinizing hormone/chorionic gonadotropin receptors. FEBS Lett 378:27–31 [DOI] [PubMed] [Google Scholar]
- Feng X, Muller T, Mizrachi D, Fanelli F, Segaloff DL 2008 An IL2 residue confers different basal constitutive activities to the human lutropin receptor and human thyrotropin receptor through structural communication between IL2 and Helix 6, via Helix 3. Endocrinology 149:1705–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Phuong K, Tong T, Fremont V, Chen J, Narayan P, Puett D, Weintraub BD, Szkudlinski MW 2000 The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology 141:3514–3517 [DOI] [PubMed] [Google Scholar]
- Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S 2002 Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol 16:736–746 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2007 Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148:2375–2382 [DOI] [PubMed] [Google Scholar]
- Bond RA, Ijzerman AP 2006 Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 27:92–96 [DOI] [PubMed] [Google Scholar]
- Sanders J, Betterle C, Evans M, Sanders P, Roberts E, Bhardwaja A, Bolton J, Richards T, Kiddie A, Young S, Coco G, Zanchetta R, Chen S, Furmaniak J, Rees Smith B 2007 A high affinity TSH receptor blocking type human monoclonal antibody. Hormone Research 68(Suppl 3):16 (Abstract) [Google Scholar]
- Johnstone AP, Cridland JC, Da Costa CR, Nussey SS, Shepherd PS 2003 A functional site on the human TSH receptor: a potential therapeutic target in Graves’ disease. Clin Endocrinol (Oxf) 59:437–441 [DOI] [PubMed] [Google Scholar]
- Seetharamaiah GS, Wagle NM, Morris JC, Prabhakar BS 1995 Generation and characterization of monoclonal antibodies to the human thyrotropin (TSH) receptor: antibodies can bind to discrete conformational or linear epitopes and block TSH binding. Endocrinology 136:2817–2824 [DOI] [PubMed] [Google Scholar]
- Lenzner C, Morgenthaler NG 2003 The effect of thyrotropin-receptor blocking antibodies on stimulating autoantibodies from patients with Graves’ disease. Thyroid 13:1153–1161 [DOI] [PubMed] [Google Scholar]
- Costagliola S, Bonomi M, Morgenthaler NG, Van Durme J, Panneels V, Refetoff S, Vassart G 2004 Delineation of the discontinuous-conformational epitope of a monoclonal antibody displaying full in vitro and in vivo thyrotropin activity. Mol Endocrinol 18:3020–3024 [DOI] [PubMed] [Google Scholar]
- Ando T, Latif R, Daniel S, Eguchi K, Davies TF 2004 Dissecting linear and conformational epitopes on the native thyrotropin receptor. Endocrinology 145:5185–5193 [DOI] [PubMed] [Google Scholar]
- Sanders J, Allen F, Jeffreys J, Bolton J, Richards T, Depraetere H, Nakatake N, Evans M, Kiddie A, Premawardhana LD, Chirgadze DY, Miguel RN, Blundell TL, Furmaniak J, Smith BR 2005 Characteristics of a monoclonal antibody to the thyrotropin receptor that acts as a powerful thyroid-stimulating autoantibody antagonist. Thyroid 15:672–682 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Gianoukakis AG, Salehi S, Moorhead J, Rao PV, Khan MZ, McGregor AM, Smith T, Banga JP 2006 Monoclonal pathogenic antibodies to the TSH receptor in Graves’ disease with potent thyroid stimulating activity but differential blocking activity activate multiple signaling pathways. J Immunol 176:5084–5092 [DOI] [PubMed] [Google Scholar]
- Peter JC, Eftekhari P, Billiald P, Wallukat G, Hoebeke J 2003 scFv single chain antibody variable fragment as inverse agonist of the β2-adrenergic receptor. J Biol Chem 278:36740–36747 [DOI] [PubMed] [Google Scholar]
- Peter JC, Wallukat G, Tugler J, Maurice D, Roegel JC, Briand JP, Hoebeke J 2004 Modulation of the M2 muscarinic acetylcholine receptor activity with monoclonal anti-M2 receptor antibody fragments. J Biol Chem 279:55697–55706 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Kaufman KD, Seto P, Rapoport B 1989 Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun 165:1184–1190 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Wadsworth HL, Chazenbalk GD, Russo D, Seto P, Rapoport B 1991 Thyrotropin-luteinizing hormone/chorionic gonadotropin receptor extracellular domain chimeras as probes for TSH receptor function. Proc Natl Acad Sci USA 88:902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichurin P, Pichurina O, Chazenbalk GD, Paras C, Chen CR, Rapoport B, McLachlan SM 2002 Immune deviation away from Th1 in interferon-γ knockout mice does not enhance TSH receptor antibody production after naked DNA vaccination. Endocrinology 143:1182–1189 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B 1996 Evidence for negative cooperativity among human thyrotropin receptors overexpressed in mammalian cells. Endocrinology 137:4586–4591 [DOI] [PubMed] [Google Scholar]
- Latif R, Graves P, Davies TF 2001 Oligomerization of the human thyrotropin receptor. Fluorescent protein-tagged hRSHR reveals post-translational complexes. J Biol Chem 276:45217–45224 [DOI] [PubMed] [Google Scholar]
- Davies DR, Padlan EA, Sheriff S 1990 Antibody-antigen complexes. Annu Rev Biochem 59:439–473 [DOI] [PubMed] [Google Scholar]
- Padlan EA 1994 Anatomy of the antibody molecule. Mol Immunol 31:169–217 [DOI] [PubMed] [Google Scholar]
- Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Miguel RN, Blundell TL, Furmaniak J, Smith BR 2007 Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 17:395–410 [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA 2005 Structure of human follicle-stimulating hormone in complex with its receptor. Nature 433:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinau G, Jaschke H, Neumann S, Lattig J, Paschke R, Krause G 2004 Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem 279:51590–51600 [DOI] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jaeschke H, Neumann S, Krause G, Paschke R 2006 Significance of ectodomain cysteine boxes 2 and 3 for the activation mechanism of the thyroid-stimulating hormone receptor. J Biol Chem 281:31638–31646 [DOI] [PubMed] [Google Scholar]
- Mizutori Y, Chen C-R, McLachlan SM, Rapoport B 2008 The thyrotropin receptor hinge region is not simply a scaffold for the leucine-rich domain but contributes to ligand binding and signal transduction. Mol Endocrinol 22:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama Y, Russo D, Chazenbalk GD, Wadsworth HL, Rapoport B 1990 Extracellular domain chimeras of the TSH and LH/CG receptors reveal the mid-region (amino acids 171–260) to play a vital role in high affinity TSH binding. Biochem Biophys Res Commun 173:1150–1156 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Russo D, Wadsworth HL, Chazenbalk GD, Rapoport B 1991 Eleven amino acids (Lys-201 to Lys-211) and 9 amino acids (Gly-222 to Leu-230) in the human thyrotropin receptor are involved in ligand binding. J Biol Chem 266:14926–14930 [PubMed] [Google Scholar]
- Smits G, Campillo M, Govaerts C, Janssens V, Richter C, Vassart G, Pardo L, Costagliola S 2003 Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J 22:2692–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Bernard MP, Cao D, Myers RV, Kerrigan JE, Moyle WR 2007 Follitropin receptors contain cryptic ligand binding sites. Mol Cell Endocrinol 260–262:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle WR, Xing Y, Lin W, Cao D, Myers RV, Kerrigan JE, Bernard MP 2004 Model of glycoprotein hormone receptor ligand binding and signaling. J Biol Chem 279:44442–44459 [DOI] [PubMed] [Google Scholar]
- Igarashi M, Nagata A 2003 Molecular cloning, sequencing and functional expression of porcine thyrotropin (TSH) receptor cDNA1). Clin Chem Lab Med 41:796–803 [DOI] [PubMed] [Google Scholar]