Abstract
Human G protein-coupled receptor 30 (GPR30) mediates estradiol-17β (E2) activation of adenylyl cyclase in breast cancer cells and displays E2 binding typical of membrane estrogen receptors (mERs). We identified a mER in Atlantic croaker ovaries with characteristics similar to those of human GPR30. To confirm the proposed role of GPR30 as a mER in this distantly related vertebrate group, we cloned GPR30 from croaker ovaries and examined its distribution, steroid binding, and signaling characteristics. Western blot analysis showed the GPR30 protein (∼40 kDa) is expressed on the plasma membranes of croaker oocytes and HEK293 cells stably transfected with GPR30 cDNA. Plasma membranes prepared from croaker GPR30-transfected cells displayed high-affinity, limited-capacity, and displaceable binding specific for estrogens, characteristic of mERs. Consistent with previous findings with human GPR30, estrogen treatment of plasma membranes from both croaker ovaries and GPR30-transfected cells caused activation of a stimulatory G protein (Gs) resulting in increased cAMP production. Treatment with E2 as well as G-1, a specific GPR30 ligand, significantly reduced both spontaneous and progestin-induced maturation of both croaker and zebrafish oocytes in vitro, suggesting a possible involvement of GPR30 in maintaining oocyte meiotic arrest in these species. Injection of antisense oligonucleotides to GPR30 into zebrafish oocytes blocked the inhibitory effects of estrogen on oocyte maturation, confirming a role for GPR30 in the control of meiotic arrest. These findings further support our previous suggestion that GPR30 is a vertebrate mER. In addition, the results suggest GRP30 may play a critical role in regulating reentry into the meiotic cell cycle in fish oocytes.
ESTROGENS PLAY CRITICAL roles in regulating many physiological processes in reproductive and nonreproductive tissues through activation of nuclear estrogen receptors (nERs) and the resulting changes in transcription rates of a large number of estrogen-responsive genes (1). In addition to these classic genomic actions, estrogens also rapidly activate intracellular signaling pathways in a wide variety of cell types and target tissues through binding to receptors on the cell surface or attached to it (2). There is now clear evidence that some rapid estrogen actions, such as those identified in vascular endothelial cells (3,4), rat pituitary cells (5), and breast cancer cells (6,7), are mediated by nERs or nER-like forms expressed near the cell surface. In addition, there is a growing body of evidence that some rapid nongenomic estrogen actions in several other cell and tissue models, including hypothalamic neurons (8), rat pancreatic islets (9), macrophages (10), and human breast cancer cells (11), do not involve nERs and instead appear to be mediated by novel membrane estrogen receptors (mERs). The identities of most of these novel mERs, however, remain unclear.
One candidate for a novel mER, which was cloned from several mammalian species a decade ago and shown to be widely expressed in the brain and other tissues, is G protein-coupled receptor 30 (GPR30) (12,13,14,15). GPR30 has the structural characteristics of a G protein coupled receptor (GPCR), including a seven-transmembrane domain and a DRY sequence, important for receptor activation, on the second intracellular loop (12,14). The observation by Filardo and co-workers (16,17) that estrogens cause rapid activation of adenylyl cyclase and MAPK in SKBR3 cells, an ER-negative human breast cancer cell line that expresses GPR30, provided the first indication that GPR30 may function as a mER. Subsequently, it was demonstrated independently by our research group and the Prossnitz laboratory that both wild-type and recombinant human GPR30 display high-affinity, limited-capacity, specific estrogen binding characteristic of mERs (18,19). Moreover, the finding that estrogen treatment of cells transfected with GPR30 causes rapid increases in two second messengers, cAMP and calcium, further suggests that GPR30 acts as the intermediary in nonclassical rapid estrogen actions in some estrogen-responsive cells (18,19).
The publication of these papers suggesting GPR30 is a mER has stimulated considerable interest and research on this putative receptor (20,21,22,23,24,25,26). However, many basic characteristics of GPR30 remain unresolved and are surrounded by controversy. Research in our and Filardo’s laboratories indicates that human GPR30 is expressed on the plasma membrane and is coupled to a stimulatory G protein (Gs) with characteristics typical of GPCRs (18,25). In contrast, Prossnitz and co-workers (19,26) report that GPR30 is not expressed on the cell membrane but instead is highly expressed in the endoplasmic reticulum where it is activated to cause increases in intracellular free calcium. Another paper by Levin and colleagues (22) reported that GPR30 is expressed on the cell membrane of breast cancer cells but does not function as a mER in response to low estrogen concentrations, from which they concluded that GPR30 is not physiologically important for rapid estrogen signaling in breast tissues. Finally, although GPR30 has been shown to be highly expressed in breast, ovarian, and endometrial cancer tissues (20,27,28,29), its physiological functions in nonmalignant cells are unclear.
A comparative endocrine approach, in which the characteristics of the homologous proteins from distantly related vertebrate groups are compared, has proven to be valuable for determining the fundamental, evolutionarily conserved features of newly discovered novel proteins (30,31). We have identified an estrogen-binding moiety with the characteristics of a mER in the testes of a marine fish, Atlantic croaker, Micropogonias undulatus (32), and have also obtained initial evidence for the presence of a putative mER in croaker ovaries (33). Preliminary results indicate that estrogen treatment stimulates adenylyl cyclase activity in croaker ovarian tissue (34), which raises the possibility that the mER in this tissue is GPR30. Therefore, in the present study, we cloned GPR30 from Atlantic croaker ovaries and examined the localization, estrogen binding, G protein coupling, and signaling characteristics of recombinant croaker GPR30 and the ovarian mER as well as its potential role in maintenance of oocyte meiotic arrest.
Materials and Methods
Chemicals
[2,4,6,7-3H]Estradiol-17β (E2) (84 Ci/mmol) and [35S]GTPγS were purchased from Amersham Pharmacia Biotechnology (Piscataway, NJ). Nonradioactive steroids were purchased from Sigma-Aldrich (St. Louis, MO) or Steraloids Inc. (Newport, RI). G-1, the GPR30 selective ligand, was purchased from EMD Chemicals (San Diego, CA). All other chemical reagents were purchased from Sigma-Aldrich unless otherwise stated.
Fish maintenance and tissue collection
Adult young Atlantic croaker (M. undulatus) (∼50 g) were purchased from local fishermen, maintained in 4200-liter circular, recirculating seawater tanks at a temperature of 22–25 C under an 11-h light, 13-h dark photoperiod to stimulate ovarian development, and fed a mixed diet of commercial pellets and shrimp (3% of body weight/d). Mature females with a mean gonadosomatic index (gonad weight × 100/total weight) of 12–15 were used as ovarian tissue donors for the experiments and for the analysis of membrane receptor binding. Oocytes with an average diameter of 450–470 μm were considered maturationally competent [capable of undergoing oocyte maturation in response to the maturation-inducing steroid (MIS), 17α,20β,21-trihydroxy-4-pregnen-3-one (20β-S)] and suitable for in vitro oocyte maturation experiments. Fish were deeply anesthetized with quinaldine sulfate and humanely killed by severing the spinal cord following procedures approved by the University of Texas at Austin Animal Care and Use Committee. The ovaries were rapidly excised and used in experiments immediately or stored at −80 C for up to 6 months, which did not significantly affect estrogen-binding activity.
In vitro oocyte maturation bioassays
Croaker and zebrafish oocyte maturation bioassays were conducted as described previously with minor modifications (35,36,37,38). Atlantic croaker ovarian tissue fragments containing 50–70 large oocytes were incubated at 24 C in DMEM supplemented with streptomycin sulfate (100 mg/liter) and penicillin (100 mg/liter) at pH 7.6 and primed with human chorionic gonadotropin (hCG) (10 IU/ml) for 8–16 h to induce maturational competence (ability to undergo maturation when treated with progestins). The duration of priming with hCG is typically adjusted in the bioassays to achieve a low level of spontaneous maturation in the absence of exogenous progestins. However, to investigate the effects of estrogens and aromatase inhibitors on oocyte maturation due to endogenous progestin induction (i.e. under more physiological conditions), the period of gonadotropin priming was increased (overprimed) in several of the bioassays so that spontaneous maturation reached approximately 25%. The primed oocytes were subsequently treated (in triplicate) for 8–10 h with 5–100 nm 20β-S (croaker oocytes) or 17,20β-dihydroxy-4-pregnen-3-one (DHP, zebrafish oocytes), 5–500 nm of various estrogens, and aromatase inhibitors (10–500 μg/ml), alone or in combination. The disappearance of the germinal vesicle, germinal vesicle breakdown (GVBD), was determined by examination of the oocytes using a binocular microscope after treatment with clearing solution. The number of the oocytes that had undergone GVBD was counted and the percentage that had matured was calculated based on the total number of the oocytes incubated.
Preparation of plasma membranes
Ovarian plasma membranes were prepared according to the general procedure described previously (39). Briefly, 3–4.5 g of fresh ovarian tissue was homogenized in 15 ml HAED buffer (25 mm HEPES; 10 mm NaCl; 1 mm dithioerythritol; 1 mm EDTA, pH 7.6) with a Polytron Tissuemizer (Tekmar, Cincinnati, OH) and centrifuged at 1000 × g for 7 min to remove the nuclear fraction. The supernatant was centrifuged at 20,000 × g for 20 min. The pellet containing the plasma membrane fraction was further purified by resuspending it in HAED buffer, and 5 ml 1.2 m sucrose solution was added below the tissue suspension layer, followed by a centrifugation at 9600 × g for 45 min. The membrane layer was collected, washed, and then pelleted with a final centrifugation step at 20,000 × g for 20 min. The pellet then was resuspended in HAED buffer at 1 mg/ml membrane protein and kept in ice for up to 1 h until used in experiments.
Extraction of plasma membranes from untransfected and GPR30-transfected HEK 293 cells was performed as described previously (18). Cells were grown in 15-cm cell culture dishes and washed two times before being harvested. Cells were collected by scraping into ice-cold HAED buffer containing 0.1% protease inhibitor cocktail (Sigma-Aldrich) and washed. The cell suspension then was sonicated with two to three short bursts on a sonicator (550 Sonic Dismembrator; Fisher Scientific, Pittsburgh, PA); the homogenate was centrifuged for 7 min at 1000 × g to remove cell debris and nuclear material, followed by a 20,000 × g centrifugation for 20 min. The resulting pellet was resuspended in HAED buffer for the binding and cAMP assays.
ER binding assays
Membrane ER binding assays were conducted following procedures published previously, and the results were calculated as the means of triplicate measurements (18). Plasma membrane preparations (∼0.2 mg protein in 250 μl) were incubated in HAED buffer with a range of concentrations (0.125–8 nm) of [3H]E2 dissolved in 250 μl HAED buffer with or without 100-fold or higher excess of nonradioactive E2 for 30 min at 4 C. Bound steroid was separated from free steroid by filtration through presoaked glass microfiber filters (Whatman GF/B, pore size 1 μm; Clifton, NJ). The filters were washed, and radioactivity was counted in a liquid scintillation counter. The dissociation constant (Kd) and binding capacity (Bmax) were determined from saturation and Scatchard analyses of specific binding. The rates of association of receptor binding were determined by incubating membranes (∼1 mg/ml membrane protein) with 2 nm [3H]E2 in triplicate for 1–60 min at 4 C in the presence or absence of 100-fold excess nonradioactive E2. For measurement of the rate of dissociation, 100-fold excess of nonradioactive E2 was added to the samples containing membrane protein and [3H]E2, and the mixture was incubated at 4 C for various times ranging from 1–60 min. Competitive binding assays were conducted with steroid competitors over the concentration range of 10−10 to 10−5 m incubated with 2 nm [3H]E2 for 30 min. Competitor binding was expressed as a percentage of maximal specific binding. Each point in all the receptor assays is the mean of three observations.
Cloning and expression of Atlantic croaker GPR30
Total RNA was extracted from Atlantic croaker ovarian tissue (0.5 g) using 1 ml TRI-reagent (Sigma-Aldrich), and the middle portion of the croaker GPR30 gene was amplified by RT-PCR with degenerate primers designed according to the sequences of human, mouse, and rat GPR30 genes. The full-length croaker GPR30 gene was obtained using 3′- and 5′-rapid amplification of cDNA ends (RACE) (Invitrogen, La Jolla, CA) and a p-GEM TA cloning system (Promega, Madison, WI). The clone was sequenced from both 3′ and 5′ ends with T7 and SP6 primers. Specific primers for the croaker GPR30 gene with EcoR1 and Notl adaptor sequences were used to amplify the croaker ovarian cDNA, and the full-length gene was cloned into pBK-CMV expression vectors (Stratagene, La Jolla, CA). After the correct sequence and orientation of the insert was confirmed by sequencing, the croaker GPR30 gene expression constructs were transfected into human HEK293 cells (American Type Culture Collection, Manassas, VA) with Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Stably transfected cell clones with high E2-binding affinities were selected using the limited dilution method and maintained under antibiotic selection. One of the clones was used in the subsequent characterization studies on recombinant GPR30. The stably transfected cell line was maintained with DMEM/Ham’s F-12 medium supplemented with 10% charcoal-stripped fetal bovine serum and 100 μg/ml gentamicin and selected with 500 μg/ml geneticin (G418) as described previously (18). Expression of croaker GPR30 in transfected cells was confirmed periodically by RT-PCR and Western blot analysis.
Sequence alignment and phylogenetic analysis
The deduced amino acid sequence obtained from the cloned Atlantic croaker cDNA was aligned with known vertebrate GPR30s and other sequences with high identities published in GenBank. Alignments and phylogenetic analyses of the GPR30s were conducted using ClustalW (1.83) (http://www.ebi.ac.uk/Tools/clustalw/index.html) and TreeTop-Phylogenetic Tree Prediction software (http:// www.genebee.msu.su/services/phtree_reduced.html). The tree type was set to PHYLIP, and a neighbor joining method was used.
RT-PCR
Total RNA was extracted either from croaker ovaries or incubated cells using the TRI-reagent (Sigma-Aldrich) followed by deoxyribonuclease treatment using a Turbo deoxyribonuclease kit (Ambion, Austin, TX) to remove any genomic DNA contamination. RT was performed using Superscript III reverse transcriptase (Invitrogen) at 50 C for 1 h. PCR was performed for 35 cycles using the Red Taq PCR MasterMix (Sigma-Aldrich) in an Eppendorf Mastercycler Gradient (Eppendorf, Hamburg, Germany). The sequences of the primers were as follows: sense, 5′-CGA TAC ATT GCC TTG GCT AAC T-3′, and antisense, 5′-GGC AAC CAG CAG ATG AAG AA-3′. The PCR cycling profiles were 94 C for 30 sec, 55 C for 30 sec, and 72 C for 60 sec, with a final extension at 72 C for 10 min. Finally, to visualize the PCR products, agarose gel electrophoresis was performed following standard procedures.
Western blot analysis and immunocytochemistry
Western blot analysis of plasma membranes from croaker ovaries or transfected cells was conducted as described previously (18) using a polyclonal antibody raised in rabbits against a synthetic 15-mer peptide (RDKLRLFIKQKASWC) derived from the C terminus of the croaker GPR30 sequence (Sigma Genosys, Woodland, TX). Plasma membranes were incubated with 5× sample loading buffer (Pierce, Rockford, IL) at room temperature for 15 min. Electrophoresis was conducted with 15–20 μg membrane protein fractions on a 10% sodium dodecyl sulfate (SDS) gel. The resulting protein bands were transferred to a nitrocellulose membrane that was washed twice with Tris-buffered saline (TBS) and blocked with 5% nonfat milk in TBS/Tween 20 (50 mm Tris; 100 mm NaCl; 0.1% Tween 20, pH 7.4) for 1 h before incubation overnight at 4 C with the croaker GPR30 antisera (1:2000). The specificity of the antibody was tested by incubating it with both specific and nonspecific antigen peptide (1–5 μg/μl antibody diluted at 1:20 in PBS) for 2 h at room temperature. The membrane was washed in TBS and then incubated with goat antirabbit IgG secondary antibody conjugated with horseradish peroxidase (Cell Signaling, Beverly, MA) for 1 h at room temperature. The blot was washed three times in TBS and then treated with enhanced chemiluminescence reagent (Pierce) and exposed to x-ray film.
Immunocytochemistry of transfected cells was conducted following a protocol described previously (31). Briefly, croaker GPR30-transfected cells were subcultured on coverslips for about 3 d until they were 70% confluent. The cells were rinsed twice with PBS and fixed with 2% paraformaldehyde and 0.25% glutaraldehyde in PBS for 15 min at 4 C. The cells were then washed briefly with PBS and blocked in 2% BSA in PBS for 1.5 h at 4 C, followed by three additional washes in PBS. The cells were incubated with the croaker GPR30 primary antibody (dilution 1:1000) or monoclonal anticadherin (plasma membrane marker) antibody (Abcam Inc., Cambridge, MA) (1:1000) in 2% BSA overnight at 4 C followed by three 5-min washes in PBS. For specificity controls, the antiserum was preabsorbed and blocked with the peptide antigen (1–5 μg peptide/μl antibody diluted in PBS) overnight at 4 C. The cells were then incubated with AlexaFluor 488 goat antirabbit and AlexaFluor 647 goat antimouse secondary antibodies (Molecular Probes, Carlsbad, CA; dilution 1:2000) followed by three 5-min washes in PBS. The coverslips were wet-mounted on slides with antifade embedding reagent (Invitrogen) and the presence of fluorescent-labeled GPR30 proteins in the cells visualized using a Nikon C1 confocal microscope.
Immunohistochemistry of croaker ovarian tissue
Fresh croaker ovarian tissue fragments were washed with PBS solution, transferred to a plastic embedding tray containing TBS tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC), and frozen at −20 C for 20 min. The embedded tissue was sectioned immediately or stored at −80 C. The frozen ovarian tissue was sectioned (thickness cryosections: 5 μm) at −20 C with a Reichert HistoSTAT, cryostat microtome. Tissue sections (four to six sections per slide) were placed on precooled (−20 C) glass slides with a camelhair brush. The sections were thawed and dried overnight at 37 C, fixed in 100% ethanol for 30 min at 4 C, dried again, rinsed with PBS, and then blocked with 2% BSA for 1 h at 4 C. GPR30 protein expression in ovarian tissue sections was detected using the same primary and secondary antibody staining procedures as those described for immunocytochemistry of the GPR30-transfected cells. The cell nuclei were localized by staining the ovarian sections for 5 min with 4′,6-diamidino-2-phenylindole (0.1 μg/ml PBS; Invitrogen). The tissue sections were mounted with antifade embedding reagent (Invitrogen), and the fluorescent signals were examined by using a Nikon fluorescent microscope.
G protein activation
G protein activation by estrogens was assayed by measuring the increase in [35S]GTPγS binding to plasma membranes as described previously (18). The estrogen response relative to the values of the no-treatment controls was enhanced in some assays by decreasing the [35S]GTPγS concentration by half to 0.25 nm and by increasing the membrane protein concentration 1.25- to 2.5-fold. The concentration of non-radiolabeled GTPγS (100 μm) was unchanged. The binding assays were repeated three times with different membrane preparations.
Immunoprecipitation of G proteins
G proteins activated by E2 treatments were immunoprecipitated with specific G protein α-subunit antibodies as described previously (18). Ovarian cell membranes, GPR30-transfected or untransfected HEK293 cell membranes (100 μg membrane protein) were incubated for 30 min at 25 C in assay buffer (250 μl) containing 10 μm GDP, 50 mm Tris-HCl (pH 7.4), 100 mm NaCl, 5 mm MgCl2, 1 mm CaCl2, 0.6 mm EDTA, 0.1% BSA, and protease inhibitor cocktail. [35S]GTPγS was added to all assay tubes, and the non-radiolabeled GTPγS (10 μm) was added to half of them to measure nonspecific binding. The reaction mixture was incubated with 100 nm E2 or vehicle and terminated after 30 min by adding 750 μl ice-cold buffer containing 100 μm each of GDP and unlabeled GTPγS. Samples were then pelleted by centrifugation and resuspended in 200 μl of immunoprecipitation buffer containing 1% Triton X-100, 0.1% SDS, 150 mm NaCl, 5 mm EDTA, 25 mm Tris-HCl (pH 7.4), supplemented with protease inhibitor cocktail. Nonimmune serum or G protein α-subunit-specific antibodies [Santa Cruz Biotechnology, Inc., Santa Cruz, CA; rabbit IgG, inhibitory G protein (Gi), and Gs at 1:300 dilution] were incubated with the membrane mixture at 4 C for 6 h with gentle agitation. All the antibodies were precipitated by adding 50 μl protein A/G plus-agarose beads (Santa Cruz) to each sample, and the mixture was incubated overnight at 4 C with gentle shaking. The immunoprecipitates were collected after centrifugation at 12,000 × g for 2 min, and the protein A/G agarose beads were washed three times with buffer containing 50 mm HEPES (pH 7.4), 100 μm NaF, 50 mm sodium phosphate, 100 mm NaCl, 1% Triton X-100, and 0.1% SDS. The bead samples then were boiled in 0.5% SDS, and radioactivity was counted in a scintillation counter.
Coimmunoprecipitation of G protein αs-subunit with GPR30
Coimmunoprecipitation experiments were performed following procedures described previously (31). Croaker ovarian plasma membranes or GPR30-transfected HEK cells were treated with 100 nm E2 for 10 min, followed by two washes with PBS at 4 C. The cells were scraped and frozen in triethanolamine buffer containing 50 mm triethanolamine, 25 mm KCl, 5 mm MgCl2, 0.25 m sucrose, 0.1% protease inhibitor cocktail (Sigma-Aldrich) (pH 7.5) at −80 C until analyzed. Plasma membranes were prepared as described previously and resuspended in immunoprecipitation buffer (0.1 mm EDTA and 1% Triton X-100 in Ca2+- and Mg2+-free PBS, pH 7.5) to a final volume of 300 μl. The membrane suspension was incubated overnight at 4 C with goat anti-Gi and -Gs antibodies and control goat IgG (1:100; Santa Cruz Biotechnology). Plasma membranes were then incubated for an additional 2–3 h at 4 C with 20–30 μl protein A/G plus-agarose beads (Santa Cruz) in the buffer. At the end of the incubation, the beads were washed twice with 1 ml buffer; the immunoprecipitates were eluted by boiling for 10 min in SDS sample loading buffer. Samples were electrophoresed on a 10% Tris-glycine SDS-polyacrylamide gel, and proteins were blotted onto nitrocellulose membranes. Membranes were blocked with 5% nonfat milk in a TBS/Tween 20 buffer containing 50 mm Tris, 100 mm NaCl, and 0.1% Tween 20 (pH 7.4) for 1 h and then incubated at 4 C overnight with the croaker GPR30 antibody (1:2500). The membranes were subsequently washed three times with TBS and incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat antirabbit antibody (Cell Signaling, Beverly, MA). The signals then were visualized by treatment with enhanced chemiluminescence substrate (SuperSignal; Pierce) and exposed to x-ray film (Amersham Biotechnology). The presence of Gs, Gi, and GPR30 proteins in plasma membrane preparations of croaker ovaries and GPR30-transfected cells used in the coimmunoprecipitation experiments was confirmed by Western blot analysis.
Flow cytometry
The flow cytometry study was conducted as described previously (31). The croaker GPR30-transfected cells were carefully scraped from the culture plates and washed two times in cold PBS followed by low-speed centrifugation to remove any cellular debris and damaged cells. Cells were then incubated in 1% BSA in PBS for 30 min for blocking of the nonspecific binding site on the cell surface. GPR30 antibody (against the extracellular loop; sequence DPSQRTATTLRHDY; validated with Western blot, see supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org; ∼1:1000) was then added to the cell suspension in blocking solution and incubated at room temperature for 1 h followed by two washes in PBS. AlexaFluor 488 goat antirabbit IgG antibody (Alexa 488; Molecular Probes, Carlsbad, CA) in blocking solution was added to the cell suspension (1:1000) and incubated for 30 min at room temperature in the dark, followed by two washes with blocking solution. The cells were resuspended in 1 ml PBS, kept at 4 C, and analyzed within 24 h on a flow cytometer FACSCalibur (BD Biosciences, Franklin Lakes, NJ). Data were analyzed with CellQuest Pro software (BD Biosciences).
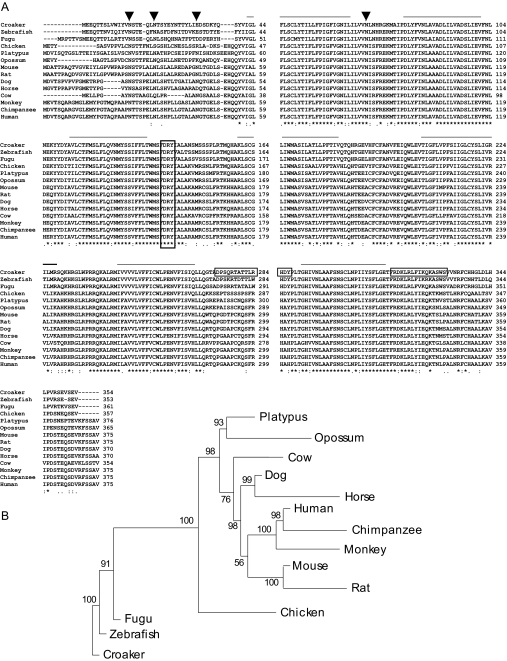
Figure 1.
Structural analysis of Atlantic croaker GPR30 protein. A, Alignment of the deduced croaker GPR30 protein amino acid sequence with GPR30 proteins of other vertebrate species. Solid lines, transmembrane domains; frame, DRY sequences; croaker framed sequences, the antigen peptides used for generating croaker GPR30 antibodies; arrows, potential N-glycosylation sites; asterisks, identical residues; colons, conserved substitutions; periods, semiconserved substitutions. B, Phylogenetic analysis of vertebrate GPR30 amino acid sequences. The lengths of the branches represent relative distance of the species. GenBank accession numbers are as follows: croaker, EU274298 (available January 1, 2008); zebrafish, XM_688551; Fugu, CAG12216.1; chicken, XP_414765.2; platypus, XP_001516051.1; opossum, XP_001378461.1; mouse, NP_084047.1; rat, NP_598257.1; dog, XP_537923.2; horse, XP_001488797.1; cow, XP_606236.3; monkey, XP_001084531.1; chimpanzee, XP_001145332.1; human, NP_001496.1. Support at each branch is indicated by the value at each node based upon 100 bootstrap replicates.
Membrane adenylyl cyclase activity measurement
Adenylyl cyclase activity in croaker ovarian tissue membrane preparations was assessed by measuring cAMP production as described previously (18). The membrane preparation (2 mg protein/ml) was incubated at room temperature in 100 μl cAMP buffer containing 20 mm KCl, 12 mm MgCl2, 3 mm EDTA, 2 mm ATP, 0.2 mm dithiothreitol, 10 mm creatine phosphate, 10 μg creatine kinase, and 20 mm HEPES (pH 7.5). The reaction was initiated by adding 100 nm E2 or an equal volume of vehicle in the cAMP buffer to membranes at room temperature and terminated by boiling the samples for 10 min followed by centrifugation at 12,000 × g. The supernatant was collected and either assayed for cAMP immediately or frozen at −80 C for future analysis. cAMP concentrations were measured using an EIA kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Microinjection of zebrafish oocytes
Because Atlantic croaker oocytes are too fragile to survive the microinjection process, we microinjected the morpholino antisense oligonucleotides to zebrafish GPR30 into zebrafish oocytes. The microinjection method has been published previously (38) and was followed in this study with some modifications. The morpholino antisense oligonucleotide to zebrafish GPR30 gene (5′-AGA GAG GTT GTC TGC TCC TCC ATA C-3′) and 5-mispair control oligonucleotide (five different nucleotides from antisense; Gene Tools, Philomath, OR) were dissolved in Danieau buffer [58 mm NaCl, 0.7 mm KCl, 0.4 mm MgSO4, 0.6 mm Ca(NO3)2, 5.0 mm HEPES (pH 7.6) in ultra-pure water] and injected into zebrafish oocytes in intact follicles (∼400–450 μm) at a final concentration of 20–25 μm. The follicles were then incubated in 1 ml 60% Leibovitz L-15 medium supplemented with 20 U/ml hCG in a 24-well plate (∼15–20 oocytes per well) at 26 C for 6 h to induce oocyte maturational competence and incubated for another 16 h in medium containing 5 nm DHP. The oocytes were scored at the end of incubation for oocyte maturation, and the maturation rates were calculated according to the number of oocytes that were undergoing GVBD.
Data analysis
Saturation curves of [3H]E2 binding were analyzed by nonlinear regression using the Prism GraphPad program (GraphPad Software, San Diego, CA). Affinity (Kd) and Bmax of [3H]E2 were calculated from nonlinear curve fitting. All the experimental data presented are means ± sem of at least three observations, and all experiments were repeated three or more times with separate batches of cells or tissues. Significant differences between paired treatment groups were analyzed by Student’s paired t test and multiple treatment groups by one-way ANOVA and Tukey’s (Sigma Stat; SPSS, Chicago, IL).
Results
Cloning, sequencing, and structural analysis of Atlantic croaker GPR30 gene
Croaker ovarian GPR30 was cloned by RT-PCR using a pair of degenerate primers designed according to the human and rodent GPR30 gene sequences. A cDNA fragment of the predicted size was cloned and identified by its similarity to the mammalian GPR30 gene sequences. A full-length cDNA was cloned by 3′- and 5′-RACE with primers designed from the previously cloned cDNA fragment. A 1.5-kb cDNA clone with an open reading frame of 1062 nucleotides encoding 354 amino acids with an estimated molecular mass of 40.7 kDa was obtained. Transmembrane and hydrophobicity analyses of the deduced amino acid sequence with online programs (SOSUI, DAS, HMMTOP, etc.) clearly indicate that the croaker protein has seven transmembrane domains. Alignment of the croaker GPR30 amino acid sequence with the other GPR30s showed high homology with other species, especially in the transmembrane domains (Fig. 1A). Atlantic croaker GPR30 showed 81% similarity in the nucleotide and 87% in amino acid sequence to zebrafish GPR30 (353 amino acids), 71 and 69% in nucleotide and amino acid sequence to chicken (357 amino acids), 75 and 70% to mouse (375 amino acids), and 76 and 69% to human (375 amino acids), respectively. Similar to other GPR30 sequences, three asparagine residues located in the N terminus indicate potential N-glycosylation sites in croaker GPR30. Phylogenetic analysis of available GPR30 sequences showed several major clades of GPR30 genes in vertebrates, including distinct clades in mammals and fish (Fig. 1B).
Expression of GPR30 in croaker tissues
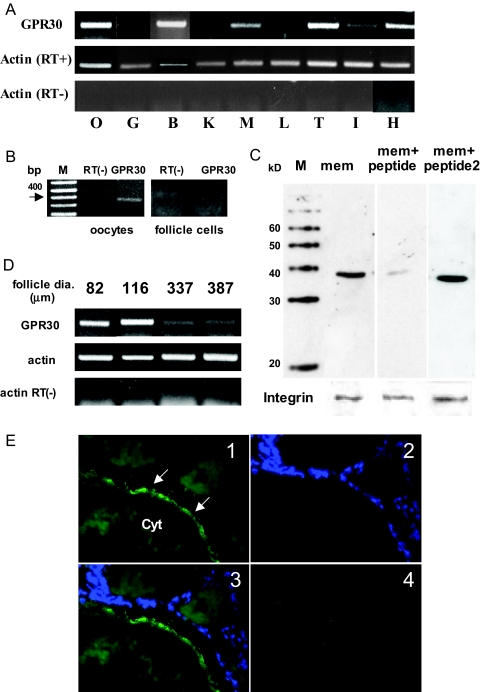
High levels of GPR30 mRNA expression were detected in brain, heart, testis, and ovary, whereas weak expression was detected in muscle and intestine (Fig. 2A). The RT-PCR showed that GPR30 expression was detected in oocytes with a PCR product of the expected size (400 bp) but not in the follicle cells surrounding the oocytes (Fig 2B). Western blot analysis of croaker ovarian plasma membranes with a croaker C-terminal GPR30 antibody showed a major band, approximately 40 kDa, that was blocked by coincubation with the antigen peptide but not with an unrelated peptide, progestin membrane receptor α (mPRα) (Fig. 2C). The abundance of croaker GPR30 mRNA varied during oocyte development. Croaker mRNA levels were highest at early stages of the oocyte development, when oocyte diameters were less than 200 μm, and were decreased in later stage oocytes with diameters greater than 300 μm (Fig. 2D). Immunohistochemistry of croaker ovarian tissue sections showed that GPR30 is expressed on the surface of oocytes (Fig. 2E1) but not in the smaller follicle cells surrounding the oocytes (Fig. 2E2 and 2E3), and no positive signals were detected using the antigen peptide-blocked antibodies (Fig. 2E4). These findings are consistent with the mRNA tissue expression results.
Figure 2.
Expression of GPR30 in Atlantic croaker tissues. A, Tissue distribution of GPR30 mRNA. B, Brain; G, gill; H, heart; I, intestine; K, kidney; L, liver; M, muscle; O, ovary; T, testis. B, Detection of GPR30 mRNA in ovarian follicle cells and oocytes. M, Molecular weight markers. C, Western blot analysis of GPR30 protein in oocyte plasma membranes. M, Molecular weight markers; mem, oocyte membrane; +peptide, GPR30 antibody preincubated with peptide antigen; +peptide2, preincubated with nonantigen peptide (mPRα). Integrin, a plasma membrane marker protein, was used as a loading control. D, GPR30 mRNA expression in croaker ovarian follicles with different oocyte diameters. RT−, Lacking reverse transcriptase. E, Immunohistochemical detection of GPR30 in ovarian tissue cryosections; 1, incubated with GPR30 antibody; 2, nuclei stained with 4′,6-diamidino-2-phenylindole, a DNA-specific dye; 3, merged; 4, incubated with GPR30 antibody that had been preincubated with antigen peptide. Arrows indicate oocyte plasma membrane. Cyt, Oocyte cytoplasm. All RT-PCR and Western blot analyses were repeated three or more times with tissues from different donors, and similar results were obtained each time.
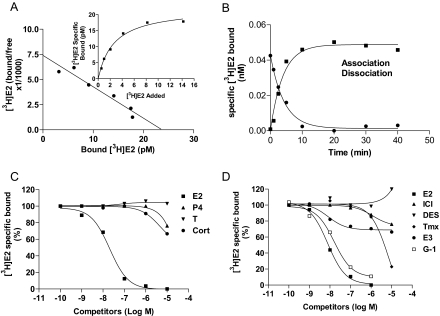
Estrogen binding to croaker ovarian plasma membranes
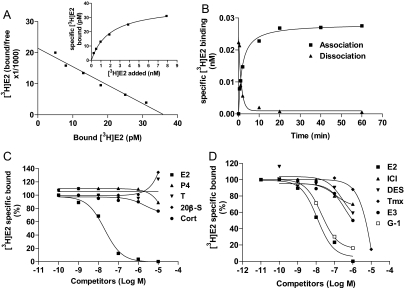
Saturation and Scatchard analyses of [3H]E2 binding to ovarian cell membrane extracts showed the presence of a single class of high-affinity, limited-capacity binding sites with a dissociation constant (Kd) of 1.97 ± 0.22 nm and binding capacity (Bmax) of 0.038 ± 0.003 nm (Fig. 3A). Association and dissociation of [3H]E2 binding to ovarian membranes were rapid with t1/2 of 1.12–1.79 min and reached equilibrium within 20 min (Fig. 3B). Competition studies showed that binding was specific for estrogens; E2 showed high binding affinity, whereas progesterone, testosterone, and 20β-S did not displace any of the bound [3H]E2. The relative binding affinities of diethylstilbestrol (DES), tamoxifen (Tmx) and ICI 182,780 were about 1/100 to 1/1000 that of E2 (Fig. 3C). The GPR30-specific ligand G-1 (40) was a very effective competitor of E2 binding with a relative binding affinity approximately one third that of E2 (Fig. 3D).
Figure 3.
Estrogen binding characteristics of Atlantic croaker ovarian plasma membranes. A, Representative saturation curve and Scatchard plot of specific [3H]E2 binding. B, Association/dissociation analysis of specific [3H]E2 binding. C, Competition curves of steroid binding expressed as a percentage of maximal specific [3H]E2 binding. D, Competition curves of binding by steroids. Cort, Cortisol; E3, estriol; ICI, ICI 182,780; P4, progesterone; T, testosterone. Each value represents the mean ± sem of three determinations. All receptor assays were repeated three to four times, and similar results were obtained in each assay.
Characteristics of recombinant croaker GPR30 stably transfected in HEK293 cells
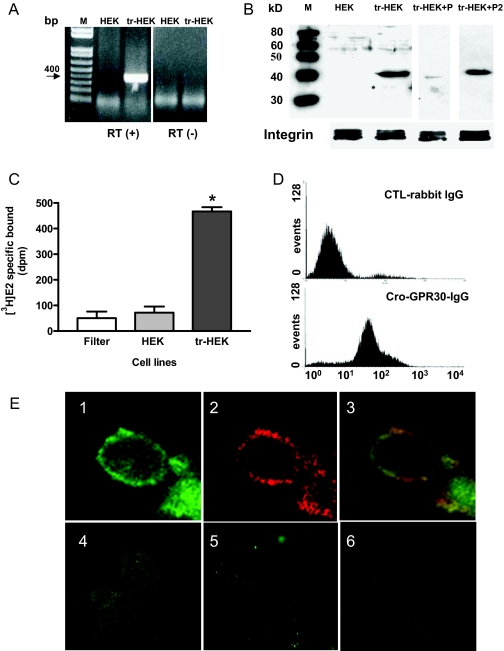
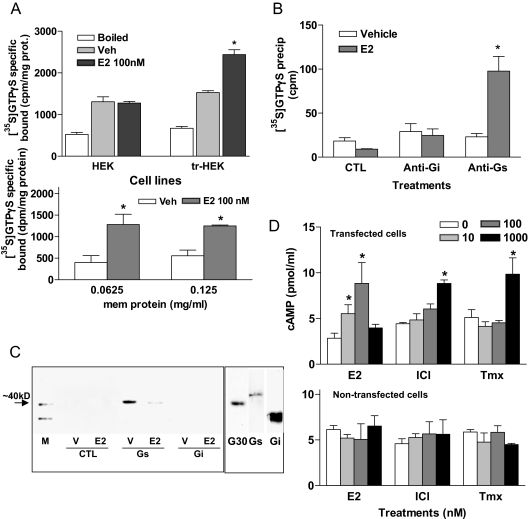
Correct expression of recombinant croaker GPR30 mRNA and protein in stably transfected HEK293 cells was confirmed by RT-PCR and Western blot analysis. A 400-bp fragment (expected size) of croaker GPR30 mRNA was amplified by RT-PCR in the transfected HEK293 cells but not in the untransfected cells (Fig. 4A). Immunoblotting with the croaker C-terminal GPR30 antibody showed the presence of a band of the correct size (∼40 kDa) on Western blots of transfected HEK293 cell plasma membranes, which was blocked by coincubation with the antigen peptide (P1) but not with an unrelated peptide (mPRα, P2) (Fig. 4B).
Figure 4.
Expression of recombinant croaker GPR30 in transfected HEK293 cells. A, Analysis of GPR30 mRNA in transfected (tr-HEK) and nontransfected (HEK) HEK293 cells by RT-PCR. B, Western blot analysis of recombinant croaker GPR30 protein in cell plasma membranes of transfected and nontransfected HEK293 cells. M, Molecular weight markers; P, antigen peptide, P2, unrelated peptide (mPRα). Integrin, a plasma membrane marker protein, was used as a loading control. C, Comparison of [3H]E2 specific binding to the plasma membranes of nontransfected and transfected HEK293 cells. Filter indicates glass fiber filter only. *, P < 0.05; n = 4. D, Flow cytometry detection of cell surface fluorescence staining in HEK293 cells transfected with croaker GPR30 cDNA using the antibody against the second extracellular loop of croaker GPR30. E, Immunocytochemical detection of recombinant croaker GPR30 protein localized on the plasma membrane of HEK cells by confocal microscopy: 1, croaker GPR30; 2, cadherin, a plasma membrane marker protein; 3, merge; 4, nontransfected HEK cells; 5, transfected cells with antibody preincubated with antigen peptide; 6, control IgG. All RT-PCR and Western blot analyses were repeated three or more times with different batches of cells, and similar results were obtained each time.
Significant amounts of specific [3H]E2 binding to plasma membranes of croaker GPR30-transfected HEK293 cells were detected in a single-point receptor assay, whereas [3H]E2 binding to nontransfected control HEK293 cells was negligible and similar to the background binding to the glass fiber filter alone (Fig. 4C). Flow cytometry of transfected cells that had not been treated with fixatives showed cell surface expression of recombinant GPR30 using an antibody generated against an extracellular domain of croaker GPR30, whereas negligible immunofluorescence was detected using the control IgG (Fig. 4D). Immunocytochemistry of the transfected HEK293 cells with the croaker GPR30 antibody and a plasma membrane marker, cadherin, showed they are colocalized, suggesting that recombinant GPR30 is primarily located in the plasma membrane of the host HEK293 cells (Fig. 4E1–3). No immunocytochemical staining with the GPR30 antibody was observed in untransfected HEK293 cells, or in the transfected cells coincubated with the GPR30 antibody and the antigen peptide (Fig. 4E4–6).
Estrogen binding to plasma membranes of HEK293 cells transfected with croaker GPR30 cDNA
Saturation analysis of recombinant GPR30 showed high-affinity (Kd 2.66 ± 0.18 nm), limited-capacity (Bmax 0.022 ± 0.002 nm) specific [3H]E2 binding, and Scatchard plots showed a single binding site (Fig. 5A). Association and dissociation of [3H]E2 binding to plasma membranes of transfected cells were rapid and completed within 10 min (Fig. 5B). Competition studies showed E2 had high binding affinity for the receptor, whereas progesterone and cortisol had low binding affinities (less than 0.01% that of E2), and testosterone did not cause any displacement of [3H]E2 (Fig. 5C). The relative binding affinities of the GPR30-specific ligand G-1 and Tmx were approximately one third and 1/1000 that of E2, respectively (Fig. 5D). Other estrogenic and antiestrogenic compounds, ICI 182,780, and estriol caused less than 50% displacement of [3H]E2 at concentrations of 10−5 m, whereas DES caused no displacement of [3H]E2 at this concentration.
Figure 5.
Estrogen binding characteristics of recombinant croaker GPR30 to plasma membranes of transfected HEK293 cells. A, Representative saturation curve and Scatchard plot of specific [3H]E2 binding; B, association/dissociation analysis of specific [3H]E2 binding; C, competition curves of steroid binding expressed as a percentage of maximal specific [3H]E2 binding; D, competition curves of binding by estrogens (see Fig. 3 for key for steroid abbreviations). Each value represents the mean ± sem of three determinations. All receptor assays were repeated three times, and similar results were obtained in each assay.
G protein activation and signal transduction by the croaker ovarian mER and recombinant croaker GPR30
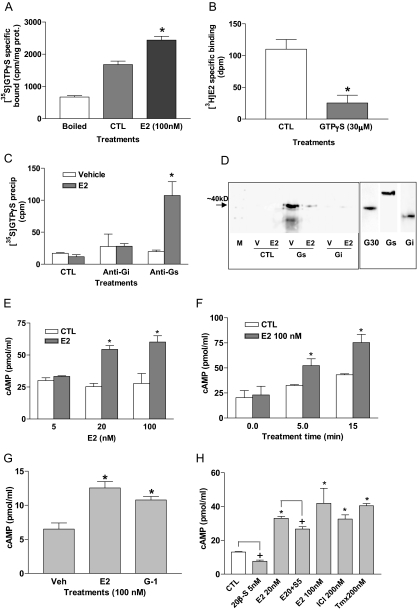
E2 (100 nm) treatment of ovarian membranes significantly increased specific [35S]GTPγS binding compared with that observed after vehicle treatment or boiling the membranes (P < 0.05), suggesting that estrogen activates G proteins through the ovarian mER (Fig. 6A). Preincubation of a croaker ovarian membrane suspension with 30 μm GTPγS significantly decreased specific E2 binding (Fig. 6B), suggesting that the croaker mER directly couples to a G protein. The G protein activated by estrogen was immunoprecipitated with a Gs α-subunit antibody, but not with an Gi antibody, indicating that the ovarian mER activates a Gs (Fig. 6C). Coimmunoprecipitation experiments using the Gs antibody followed by Western blot analysis with the croaker GPR30 antibody suggest that the GPR30 in ovarian membranes is coupled to a Gs, consistent with the identity of the ovarian mER as GPR30. In contrast, no coupling of the GPR30 protein to the Giα subunit was detected in the coimmunoprecipitation assay (Fig. 6D). Ligand-induced activation of a GPCR results in exchange of GTP for GDP on the α-subunit of the heterotrimeric G protein, causing it to dissociate into the α- and βγ-subunits, resulting in a loss of their coupling to the receptor. Consequently, G protein antibodies can no longer immunoprecipitate GPCRs once they have been activated. Therefore, as predicted, treatment with 100 nm E2 resulted in decreased amounts of GPR30 protein detected on the Western blot (Fig. 6D), consistent with decreased coupling of the receptor to the Gs after ligand activation. The finding that production of cAMP by ovarian membranes, a measure of adenylyl cyclase activity, was significantly increased in a concentration- and time-dependent manner by E2 treatment is consistent with the results showing croaker ovarian GPR30 activates Gs. (Fig. 6, E and F). Treatment of croaker oocyte membranes with 100 nm E2 or the GPR30-specific ligand G-1 caused a significant increase in cAMP production (Fig. 6G). The GPR30 agonists Tmx and ICI 182,780 also increased cAMP production by oocyte membranes, whereas 20β-S decreased both untreated and E2-mediated cAMP production (Fig. 6H).
Figure 6.
Activation of G proteins and adenylyl cyclase and coimmunoprecipitation of GPR30 with G proteins in croaker ovarian plasma membranes. A, Effects of 100 nm E2 treatment (20 min) on specific [35S]GTPγS binding to G proteins in ovarian plasma membranes. Boiled indicates that membrane samples were boiled for 15 min before assay. *, P < 0.05; n = 4. B, Effects of 30 μm GTPγS treatment for 20 min at 4 C on specific [3H]E2 binding to ovarian plasma membranes. *, P < 0.05. C, Immunoprecipitation of [35S]GTPγS bound to G proteins activated by 100 nm E2 with specific anti-Gs and anti-Gi antibodies or control IgG. CTL, Control nonimmunized IgG; Vehicle, membranes treated with media; E2, membranes incubated with 100 nm E2. *, P < 0.05. D, Coimmunoprecipitation of 100 nm E2-activated G proteins with specific G protein α-subunit antibodies (Gs, Gi) and croaker GPR30 antibody (left panel) and detection of GPR30 (G30), Gs, and Gi proteins in croaker ovarian membranes (right panel). M, Size marker; V, vehicle; CTL, control IgG. n = 4. E and F, Dose response and time course of cAMP production in response to E2 treatments in croaker ovarian plasma membranes. *, P < 0.05 compared with respective control (CTL). G and H, Effects of treatment with E2, G-1, 20β-S, ICI 182,780 (ICI) and Tmx on cAMP production by croaker oocyte membranes. G: *, P < 0.05 compared with vehicle (Veh) control. H: *, P < 0.05 compared with control (CTL); +, P < 0.05 compared with respective paired group not treated with 20β-S or E20 + 5S (20 nm E2 + 5 nm 20β-S). Each value represents the mean ± sem of three determinations. All assays were repeated three times with ovarian tissue from different donors, and similar results were obtained in each assay.
A similar pattern of G protein activation and cAMP production by estrogens to that seen in the ovarian membranes was observed in cells transfected with croaker GPR30. Specific [35S]GTPγS binding to transfected cell membranes was significantly increased by E2 (100 nm) treatment compared with that of the control nontreatment group in an experiment that used the original binding assay protocol (18), whereas no G protein activation after estrogen treatment was observed in the untransfected cells (Fig. 7A, top). Increased [35S]GTPγS binding to transfected cell membranes after estrogen treatment was also detected when the modified assay procedure was followed. Estrogen-induced [35S]GTPγS binding was more than 2-fold that of the untreated controls under these assay conditions (Fig. 7A, bottom). The G protein activated by estrogen was immunoprecipitated with a Gs α-subunit antibody, but not with a Gi antibody, indicating that croaker GPR30 activates a Gs (Fig. 7B). A coimmunoprecipitation experiment showed strong GPR30 protein bands in samples immunoprecipitated with a Gs antibody, but not with a Gi antibody or control IgG. Similar to the coimmunoprecipitation results with the croaker ovarian membrane, E2 pretreatment (100 nm) significantly decreased the amount of the GPR30 protein immunoprecipitated due to the uncoupling of Gs subunit from GPR30 protein in response to E2 activation of the receptor (Fig. 7C). Treatment of transfected cell membranes with E2 caused a concentration-dependent increase in cAMP production at concentrations of 10 and 100 nm. Higher concentrations (1000 nm) of ICI 182,780 and Tmx were also effective in significantly increasing cAMP production (Fig. 7D, upper panel), whereas none of these treatments increased cAMP production in the nontransfected HEK cells (Fig. 7D, lower panel).
Figure 7.
Activation of G proteins and adenylyl cyclase and coimmunoprecipitation of GPR30 with G proteins in croaker GPR30-transfected HEK293 cell plasma membranes. A, Effects of 100 nm E2 treatment (20 min) on specific [35S]GTPγS binding to G proteins in membranes of transfected HEK293 cell plasma membranes; top, results obtained using original assay procedure (18); bottom, results obtained using modified protocol described in Materials and Methods. Boiled indicates that membrane samples were boiled for 15 min before assay. *, P < 0.05; n = 4. B, Immunoprecipitation of [35S]GTPγS bound to G proteins activated by 100 nm E2 with specific anti-Gs and anti-Gi antibodies or control IgG. CTL, Control nonimmunized IgG. *, P < 0.05 compared with respective vehicle. C, Coimmunoprecipitation of 100 nm E2-activated G protein with specific G protein antibodies (Gs, Gi) and croaker GPR30 antibody (left) and detection of GPR30 (G30), Gs, and Gi protein in GPR30-transfected HEK 293 cell plasma membranes (right). M, Size marker; V, vehicle; CTL, control IgG. D, Effects of treatment with E2, Tmx, and ICI 182,780 (ICI) on cAMP level changes in the plasma membranes of HEK 293 cells transfected with croaker GPR30 cDNA (upper panel) and the nontransfected control HEK cell membranes (lower panel). *, P < 0.05 compared with respective 0 nm control (Tukey’s). Each value represents the mean ± sem of three to four determinations. All assays were repeated three times with ovarian tissue from different donors, and similar results were obtained in each assay.
Effects of estrogens and aromatase inhibitor treatments on maturation of Atlantic croaker oocytes in an in vitro assay
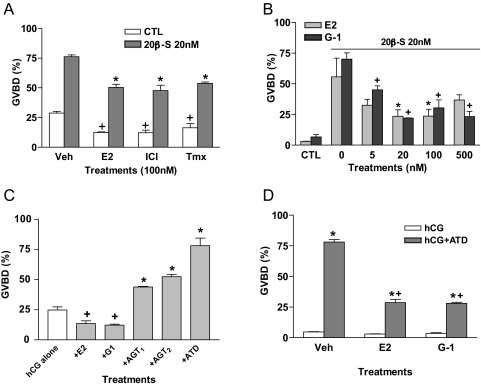
Treatment with E2, Tmx, and ICI 182,780 significantly decreased both spontaneous (gonadotropin overprimed) and 20β-S-induced maturation of croaker oocytes (Fig. 8A). The specific GPR30 agonist G-1 was as effective as E2 in partially blocking the oocyte maturation response to 20β-S over a range of concentrations (Fig. 8B).
Figure 8.
Effects of estrogens and aromatase inhibitors on hormone-induced maturation of croaker oocytes in an in vitro bioassay. The oocytes were primed with 10 U/ml hCG for 8–16 h and then treated with steroids for 8 h. A, Effects of E2, ICI 182,780 (ICI), and Tmx treatments (100 nm) on spontaneous (hCG-overprimed, white bars) and 20 nm 20β-S-induced (gray bars) GVBD of croaker oocytes. +, P < 0.05 compared with vehicle control (CTL, Veh) group; *, P < 0.05 compared with 20β-S alone (20β-S, Veh) group. B, Effects of different concentrations of E2 and G-1 on 20 nm 20β-S-induced GVBD of croaker oocytes. * and +, P < 0.05 compared with respective 20β-S alone (0) groups (Tukey’s). Control (CTL) was hCG-primed alone. C, Effects of hCG alone and hCG in the presence of E2, G-1, and aromatase inhibitors on spontaneous (hCG-overprimed, non-20β-S-induced) GVBD of croaker oocytes. AGT1, 50 μg/ml; AGT2, 500 μg/ml; ATD, 10 μg/ml. + and *, P < 0.05 compared with hCG alone. D, Effects of E2 and G-1 (100 nm) on the GVBD of croaker oocytes primed with hCG alone and hCG in the presence of ATD (10 μg/ml). *, P < 0.05 compared with respective hCG-alone treatment; +, P < 0.05 compared with hCG+ATD treated with vehicle (Veh). Each value represents the mean ± sem of three observations with 80–150 incubated oocytes in each observation. All assays were repeated three or more times with ovarian tissue from different donors, and similar results were obtained in each assay.
Based on the results of the estrogen treatment experiments, we hypothesized that endogenous estrogens inhibit maturation of croaker oocytes. Estrogen production by teleost oocytes can be effectively blocked by aromatase inhibitors such as 1,4,6-antrostatriene-3,17-dione (ATD) and aminoglutethimide (AGT) (41). Therefore, if our hypothesis is correct, treatment with aromatase inhibitors should increase spontaneous oocyte maturation. The results show that treatment with the aromatase inhibitors AGT (50 μg/ml) and ATD (10 μg/ml) was very effective in potentiating oocyte maturation in response to hormonal stimulation by hCG (overprimed), causing a 2- to 3-fold increase in the percentage of oocytes that completed oocyte maturation. In contrast, the oocyte maturation response to gonadotropin was significantly impaired after treatment with E2 and G-1 (Fig. 8C). Treatment with the aromatase inhibitor ATD was equally effective in promoting maturation of oocytes that had not been overprimed with gonadotropin and showed low levels of maturation (Fig. 8D). Over 75% of these gonadotropin-treated oocytes completed oocyte maturation when estrogen production was inhibited by ATD, which suggests that endogenous estrogens have an important physiological role in regulating oocyte maturation. The finding that oocyte maturation in response to ATD/hCG was attenuated by treatment with G-1 as well as with E2 suggests these estrogen effects are mediated through GPR30 (Fig. 8D).
Effects of microinjection with GPR30 antisense oligonucleotides on maturation of zebrafish oocytes
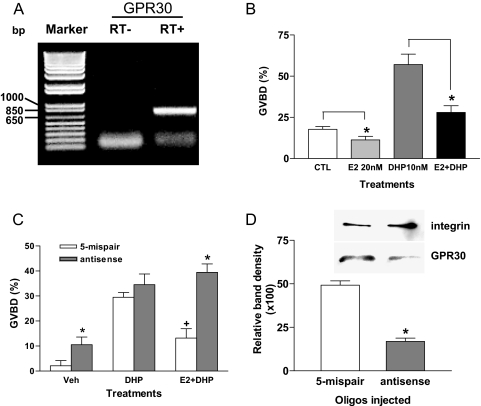
Microinjection of morpholino antisense oligonucleotides to mPRα into zebrafish oocytes has proven to be an effective approach to study the involvement and function of steroid receptors in oocyte maturation (38). GPR30 mRNA expression was detected by RT-PCR in zebrafish ovarian tissues with PCR products of the expected size (Fig. 9A). Treatment with E2 (20 nm) significantly decreased both spontaneous and DHP-induced maturation of zebrafish oocytes (Fig. 9B). The GPR30-specific ligand G-1 was also effective in inhibiting GVBD (results not shown). Therefore, we microinjected GPR30 antisense oligonucleotides into zebrafish oocytes to explore the role of GPR30 in oocyte maturation. As predicted, microinjection of morpholino antisense GPR30 RNA oligos into zebrafish oocytes resulted in significant increases in GVBD in the vehicle group (Fig. 9C), which was associated with a decrease in GPR30 protein expression (Fig. 9D). Similarly, GVBD was significantly increased after treatment with DHP and E2 in zebrafish oocytes that had been microinjected with GPR30 antisense oligonucleotides compared with that in oocytes injected with the 5-mispair oligonucleotides (Fig. 9B). It is concluded from these experiments that GPR30 is directly involved in maintenance of meiotic arrest in zebrafish oocytes.
Figure 9.
The effect of E2 on GVBD of normal and antisense oligonucleotide-injected zebrafish oocytes. A, Expression of GPR30 mRNA in zebrafish ovarian tissue. B, Effects of 20 nm E2 on spontaneous (CTL) and DHP-induced (10 nm) GVBD. *, P < 0.05 compared with respective control not treated with E2. C, Comparison of the effects of microinjection of zebrafish oocytes with either antisense morpholino oligonucleotide to zebrafish GPR30 or its 5-mispair control oligonucleotide during the hCG priming stage on spontaneous GVBD (hCG-primed only, Veh), DHP-induced (10 nm) GVBD, or DHP-induced GVBD in the presence of 100 nm E2. *, P < 0.05 compared with respective 5-mispair CTL group; +, P < 0.05 compared with DHP-treated groups. A total of 20–30 oocytes were counted in each treatment group for each experiment. D, Relative intensity of GPR30-immunoreactive bands on a Western blot of cell membranes prepared from zebrafish oocytes microinjected with antisense and 5-mispair oligonucleotides. Integrin loading control was used for the membrane samples and detected with integrin antibody. *, P < 0.05 compared with 5-mispair oligonucleotide control. Microinjection experiments were repeated three times with oocytes from different donors and similar results were obtained in each assay.
Discussion
The results show that the croaker GPR30 gene and protein display high sequence homologies with mammalian GPR30s. A major finding of this study is that recombinant croaker GPR30, like the human counterpart, is expressed on the plasma membranes of transfected cells and has the estrogen-binding characteristics of a mER. Moreover, the same signaling pathway as that observed with human GPR30, involving activation of a Gs and adenylyl cyclase, is transduced through recombinant and wild-type croaker GPR30. These results suggest that the estrogen binding and estrogen signaling functions of GPR30 arose early in vertebrate evolution, before the divergence of the teleosts from the tetrapods, at least 200 million years ago. The finding that estrogen membrane signaling through GPR30 has been conserved for such a long period in two distantly related vertebrate groups, mammals and fish, suggests that this is a fundamental function of GPR30 in vertebrates and likely its major physiological role. Previously, we have shown that the progestin signaling functions of another novel seven-transmembrane steroid receptor, mPRα, are also highly conserved in fish and mammals (31). The high degree of conservation of nontraditional estrogen and progesterone signaling pathways mediated through these novel membrane receptors suggests they have important endocrine roles in vertebrate physiology. Finally, evidence was obtained in the present study that one critical physiological function mediated through GPR30 is regulation of meiotic cell cycle progression, maintaining croaker and zebrafish oocytes in meiotic arrest.
The high degree of nucleotide and amino acid sequence identities between croaker GPR30 and its mammalian homologs in the transmembrane and some intra- and extracellular domains suggests that these regions have been conserved because they are critical for receptor function. In contrast, there is little homology in the N-terminal and distal C-terminal domains, which are shorter in fishes, suggesting these regions may not be involved in conserved functions such as estrogen binding and G protein coupling. All GPCRs are thought to be glycoproteins and with the exception of the melatonin receptors contain at least one N-glycosylation site in the extracellular N terminus (42). Both mammalian and nonmammalian GPR30s with the possible exception of bovine GPR30 have three potential N-glycosylation sites in their N-terminal domains, two of which are at conserved positions.
The cysteine residues in the extracellular loops of GPR30, like those in other GPCRs, are thought to form disulfide bridges that maintain receptor structure. Human GPR30 potentially can form two disulfide bridges because it has one cysteine residue bordering the first extracellular loop in the third transmembrane domain, two in the second extracellular loop, and a fourth in the third extracellular loop. However, the GPR30s of croaker and other nonmammalian species can form only one disulfide bridge because they have only two cysteines, one bordering the first extracellular loop and another in the second extracellular loop. The finding that croaker GPR30 has similar estrogen binding and signal transduction functions as human GPR30 suggests that a second disulfide bridge between the second and third extracellular loops is not critical for these functions. The DRY (Asp-Arg-Tyr) sequence in the second intracellular loop of many GPCRs belonging to the class A or rhodopsin-like subfamilies (42) is conserved in all the vertebrate GPR30s. The highly conserved DRY sequence has been shown to have an important role in these GPCRs for signal transduction, including retention of G protein coupling and response to receptor agonists (43).
There are conflicting reports on the subcellular location of human GPR30 and the site of its activation to initiate signal transduction pathways. Estrogens can freely diffuse into cells and, therefore, potentially can activate receptors both at the cell surface and intracellularly. Prossnitz and co-workers (19) report that human GPR30 is primarily located in the endoplasmic reticulum and that the estrogen binding activity is localized in the microsomal fraction of transfected cells. However, we have obtained extensive evidence that wild-type and recombinant human GPR30s, like other GPCRs, are expressed on the plasma membranes of SKBR3 and HEK293 cells, respectively, where they bind estrogens resulting in activation of G proteins (18,25). The present results of the Western blot and immunocytochemical analyses demonstrating that wild-type and recombinant croaker GPR30 are predominantly expressed and function on the cell membrane are in agreement with our earlier findings with human GPR30 (18). Other research groups have also shown that mammalian GPR30s are expressed in the plasma membrane (21). It is concluded that both fish and mammalian GPR30s function through a classical GPCR mechanism by binding ligands on the cell surface resulting in activation of G proteins in the plasma membrane and their associated second messengers, in this case membrane-bound adenylyl cyclase. However, an additional action of GPR30 inside the cell to activate other pathways as outlined by Prossnitz remains a possibility.
Another controversy surrounds the importance of GPR30 as a mER to transduce estrogen signals intracellularly. The ability of human GPR30 to bind estrogens and activate second messengers was reported around the same time by two research groups working independently (18,19). However, another research group failed to detect significant estrogen binding or activation of second messenger pathways in several breast cancer cell lines expressing human GPR30, from which they concluded that GPR30 is not a physiologically important mER in these tissues (22). The current results, showing that GPR30 from a distantly related vertebrate species, Atlantic croaker, has all the steroid binding and signal transduction characteristics of a mER, support our earlier conclusions on the functions of GPR30. Moreover, we demonstrate for the first time the involvement of GPR30 as a mER in a novel estrogen-dependent physiological process in normal cells. It is concluded from these studies that GPR30 is a functional mER in vertebrates.
The characteristics of ER binding and G protein activation of croaker GPR30 are very similar to those of human GPR30 we described earlier. Wild-type ovarian mER and recombinant croaker GPR30 displayed the highest binding affinities for E2 with Kd values of 1.97 and 2.66 nm, respectively, similar to those of the human wild-type (Kd 2.70 nm) and recombinant GPR30 (Kd 3.3 nm) (18). Binding to croaker GPR30 is highly specific for estrogens and antiestrogens, other steroids showing negligible binding affinity for the receptor. Overall, the binding affinities of estrogens and antiestrogens for the recombinant croaker GPR30 and ovarian membrane are similar to their affinities for the human receptor but differ from their affinities for the three croaker nuclear ERs, ERα, ERβb1, and ERβb2 (44). A specific agonist for human GPR30, G-1 (40), was shown to bind to both recombinant croaker GPR30 and the ovarian membrane preparation with high affinity, approximately one third that of E2, whereas DES was a weak competitor of E2 binding to both receptors. These competitive binding studies strongly suggest that the mER identified in ovarian membrane preparations is GPR30. This conclusion is supported by the signal transduction studies showing that estrogen treatment of croaker ovarian membranes results in a similar activation of a stimulatory G protein and increased adenylyl cyclase activity as that observed with recombinant croaker and human GPR30.
One of the most interesting findings of the present study is that the GPR30 protein is highly expressed on croaker oocyte plasma membranes and is associated with specific estrogen binding. To our knowledge, there are no previous reports describing high expression of GPR30 in vertebrate oocytes. A recent study showed that GPR30 is predominantly expressed in the granulosa and theca cells of the hamster ovary (45). In contrast, there is extensive evidence for the presence of specific progestin receptors in fish and amphibian oocytes and their involvement in progestin induction of oocyte maturation (38,46,47,48). During the initial period of hormone-dependent oogenesis in fish, the ovarian follicle produces large amounts of E2, which induces the hepatic synthesis of vitellogenin, the yolk protein precursor that is sequestered by the growing oocytes (49). The oocytes are arrested at the first meiotic prophase during this period of oocyte growth in fish and other species by high intracellular levels of cAMP that are maintained by membrane-bound adenylyl cyclases (50,51). A surge in gonadotropin secretion in the periovulatory period in fishes causes a switch in the steroidogenic pathway in the ovarian follicles to the production of progestins, termed MISs, which induce the resumption of meiosis and the other events associated with oocyte maturation such as GVBD by causing intra-oocyte cAMP concentrations to decline (50,52,53). It has been demonstrated that the MIS in croaker and a closely related species, spotted seatrout, 20β-S, induces this decline in cAMP levels through binding to a specific progestin receptor on the oocyte plasma membrane, resulting in activation of a pertussis-sensitive Gi (54). Moreover, clear evidence has been obtained that the MIS receptor in croaker, seatrout, goldfish, and zebrafish is a novel seven-transmembrane receptor, mPRα, which can couple to Gi. The identification of an ER on croaker oocyte membranes that activates a stimulatory G protein suggested to us that GPR30 might have an opposite role, preventing oocyte maturation and meiotic resumption. In support of this idea, there are a few reports of inhibitory effects of E2 on hormonal induction of oocyte maturation in fish (55,56). The present results demonstrate that E2, the GPR30 agonists, Tmx, ICI 182,780, and G-1, were all effective in significantly decreasing maturation of croaker and zebrafish oocytes in vitro in response to their MISs. On the other hand, treatment with aromatase inhibitors caused marked increases in spontaneous and hormonally induced oocyte maturation. These aromatase inhibitor experiments provide the first clear evidence that endogenous estrogen production by full-grown fish ovaries is physiologically important in the regulation of oocyte maturation. Taken together, the results of the present study are consistent with an inhibitory role of estrogens in the control of meiotic cell cycle progression in croaker and zebrafish oocytes, attenuating oocyte maturation in response to the MIS. Finally, the estrogen specificity experiments and the microinjection experiments with antisense oligonucleotides to GPR30 directly implicate the receptor in mediating this estrogen action.
The mechanisms by which estrogens cause a decrease in the percentage of full-grown oocytes that mature in response to the MIS were not investigated in the present study. However, the results suggest this is partially attributable to an increase in adenylyl cyclase activity mediated by the α-subunit of the Gs-coupled to GPR30. Treatment with GPR30 agonists increased production of cAMP by croaker ovarian membranes and reversed the down-regulation of adenylyl cyclase activity by 20β-S. These estrogen treatments also decreased both spontaneous and 20β-S-induced oocyte maturation. Although cotreatment with estrogens prevented the down-regulation of cAMP production in response to 20β-S, it only partially blocked the action of 20β-S to induce oocyte maturation. We have previously shown that 20β-S induction of oocyte maturation in croaker is also dependent upon the activation of a phosphoinositide 3-kinase/AKT pathway, presumably through the βγ-subunits of the Gi (57). Therefore, the present results are consistent with estrogen interference through GPR30 with only the inhibitory adenylyl cyclase component of the multiple signaling pathways induced by 20β-S through mPRα during oocyte maturation. The involvement of another GPCR, the orphan receptor GPR3, in the maintenance of meiotic arrest through activation of a stimulatory G protein has been demonstrated in mammalian and zebrafish oocytes (58,59). An additional orphan GPCR, GPR12, also appears to be involved in the maintenance of meiotic arrest through activation of Gs (60). Injection of GPR3 and GPR12 mRNA into mouse oocytes blocked spontaneous oocyte maturation, whereas expression of these two receptors in Xenopus oocytes prevented progesterone-induced meiotic maturation (60). Therefore, multiple GPCRs coupled to Gs could potentially participate in the maintenance of oocyte meiotic arrest in fish and other vertebrates.
The observation that GPR30 expression is greatest in mid-vitellogenic croaker oocytes suggests that it likely has a major physiological role before oocyte maturation. In support of this, circulating levels of its ligand E2 are also maximal in fishes during this stage of oogenesis (49). Meiosis is arrested at prophase during this entire period of estrogen-dependent oocyte growth. Therefore, in addition to their critical functions in promoting vitellogenesis and follicular development, we propose that estrogens also prevent precocious maturation of the oocytes during their period of vitellogenic growth through activation of GPR30. The results of the present study show that a partial inhibitory effect of estrogens on the resumption of meiosis persists in fully grown oocytes, even though oocyte GPR30 expression has declined by this time. The physiological significance of this inhibitory estrogenic action is unclear but may be important for synchronizing the maturation of the many thousands of fish oocytes that are released in a single spawn, delaying it until production of the MIS is sufficient to overcome this inhibitory effect. Additional experiments will be required to test these hypotheses.
Supplementary Material
Footnotes
This research was funded by National Institutes of Health Grant ESO12961 (to P.T.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 17, 2008
Abbreviations: AGT, Aminoglutethimide; ADT, 1,4,6-antrostatriene-3,17-dione; DES, diethylstilbestrol; DHP, 17,20β-dihydroxy-4-pregnen-3-one; E2, estradiol-17β; Gi, inhibitory G protein; GPCR, G protein-coupled receptor; GPR-30, G protein-coupled receptor 30; Gs, stimulatory G protein; GVBD, germinal vesicle breakdown; hCG, human chorionic gonadotropin; mER, membrane estrogen receptor; MIS, maturation-inducing steroid; mPRα, progestin membrane receptor α; nER, nuclear estrogen receptor; 20β-S, 17α,20β,21-trihydroxy-4-pregnen-3-one; SDS, sodium dodecyl sulfate; TBS, Tris-buffered saline; Tmx, tamoxifen.
References
- Hewitt SC, Korach KS 2002 Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord 3:193–200 [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP 2004 Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 3:27–41 [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW 1999 Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR 1997 17β-Estradiol regulation of human endothelial cell basal nitric oxide release, independent of Ca2+ mobilization. Circ Res 81:855–892 [DOI] [PubMed] [Google Scholar]
- Norfleet AM, Thomas ML, Gametchu B, Watson CS 1999 Estrogen receptor-α detected on plasma membrane of aldehyde-fixed GH3/B6/F10 rat piuitary tumor cells by enzyme-linked immunocytocehistry. Endocrinology 140:3805–3814 [DOI] [PubMed] [Google Scholar]
- Song RX, McPherson RA, Adam L, Bao M, Hupnik R, Kumar R, Santen RJ 2002 Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol Endocrinol 16:116–127 [DOI] [PubMed] [Google Scholar]
- Razindi M, Pedram A, Levin ER 2000 Plasma membrane receptors signal to antiapoptosis in breast cancer. Mol Endocrinol 14:1434–1447 [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan, TS, Ronnekleiv OK, Kelly MJ 2003 Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neuroscience 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B 2000 Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci USA 97:11603–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benten WP, Stephan C, Lieberherr M, Wunderlich F 2001 Estradiol signaling via sequestrable surface receptors. Endocrinology 142:1669–1677 [DOI] [PubMed] [Google Scholar]
- Chen X, Danes C, Lowe M, Herliczek TW, Keyomarsi K 2000 Activation of the estrogen-signaling pathway by p21(WAF1/CIP1) in estrogen receptor-negative breast cancer cells. J Natl Cancer Inst 92:1403–1413 [DOI] [PubMed] [Google Scholar]
- Owman C, Blay P, Nilsson C, Lolait SJ 1996 Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun 228:285–292 [DOI] [PubMed] [Google Scholar]
- Bonini JA, Anderson SM, Steiner DF 1997 Molecular cloning and tissue expression of a novel orphan G protein-coupled receptor from rat lung. Biochem Biophys Res Commun 234:190–193 [DOI] [PubMed] [Google Scholar]
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ 1997 Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45:607–617 [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch, KR, Heng HH, Kolakowski Jr LF, George SR 1998 Discovery of three novel G-protein-coupled receptor genes. Genomics 47:310–313 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR 2000 Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Graeber CT, Quinn JA 2006 Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res 12:6359–6366 [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y 2006 G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346:904–910 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J 2006 Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol 102:175–179 [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Yu HP, Frink M, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH 2007 G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am J Pathol 170:1210–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P 2007 Activation of the novel estrogen receptor, GPR30, at the plasma membrane. Endocrinology 148:3236–3245 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, Prossnitz ER 2007 Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol 2:536–544 [DOI] [PubMed] [Google Scholar]
- Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER 2007 GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol 196:386 e1–e9; discussion e9–e11 [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M 2006 The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646 [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M 2007 G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- Takei Y, Ogoshi M, Inoue K 2007 A reverse pharmacology approach for identification of novel osmoregulatory and cardiovascular hormones in vertebrates. Front Neuroendocrinol 28:143–160 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C 2007 Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinology 148:705–718 [DOI] [PubMed] [Google Scholar]
- Loomis AK, Thomas P 2000 Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor. Biol Reprod 62:995–1004 [DOI] [PubMed] [Google Scholar]
- Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K 2006 Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids 71:310–316 [DOI] [PubMed] [Google Scholar]
- Thomas P, Zhu Y, Pang Y 2003 Current knowledge of the nature and identity of progestin and estrogen membrane receptors in fish gonads. In: Watson CS, ed. The identities of membrane steroid receptors. Boston: Kluwer Academic; 131–137 [Google Scholar]
- Patino R, Thomas P 1990 Induction of maturation of Atlantic croaker oocytes by 17α,20β,21-trihydroxy-4-pregnen-3-one in vitro: consideration of some biological and experimental variables. J Exp Zool 255:97–109 [DOI] [PubMed] [Google Scholar]
- Patino R, Thomas P 1990 Effects of gonadotropin on ovarian intrafollicular processes during the development of oocyte maturational competence in a teleost, the Atlantic croaker. Evidence for two distinct stages of gonadotropic control of final oocyte maturation. Biol Reprod 43:818–827 [DOI] [PubMed] [Google Scholar]
- Trant JM, Thomas P 1988 Structure-activity relationships of steroids in inducing germinal vesicle breakdown of Atlantic croaker oocytes in vitro. Gen Comp Endocrinol 71:307–317 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AM, Thomas P 2004 Biochemical characterization of a membrane androgen receptor in the ovary of the Atlantic croaker (Micropogonias undulatus). Biol Reprod 71:146–155 [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER 2006 Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- Lee PS, Pankhurst NW, King HR 2006 Effects of aromatase inhibitors on in vitro steroidogenesis by Atlantic salmon (Salmo salar) gonadal and brain tissue. Comp Biochem Physiol A Mol Integr Physiol 145:195–203 [DOI] [PubMed] [Google Scholar]
- Strader CD, Fong TM, Tota MR, Underwood D, Dixon RA 1994 Structure and function of G protein-coupled receptors. Annu Rev Biochem 63:101–132 [DOI] [PubMed] [Google Scholar]
- Rovati GE, Capra V, Neubig RR 2007 The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol 71:959–964 [DOI] [PubMed] [Google Scholar]
- Hawkins MB, Thomas P 2004 The unusual binding properties of the third distinct teleost estrogen receptor subtype ERβ are accompanied by highly conserved amino acid changes in the ligand binding domain. Endocrinology 145:2968–2977 [DOI] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK 2007 Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology 148:4853–4864 [DOI] [PubMed] [Google Scholar]
- Liu Z, Patino R 1993 High-affinity binding of progesterone to the plasma membrane of Xenopus oocytes: characteristics of binding and hormonal and developmental control. Biol Reprod 49:980–988 [DOI] [PubMed] [Google Scholar]
- Tokumoto M, Nagahama Y, Thomas P, Tokumoto T 2006 Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen Comp Endocrinol 145:101–108 [DOI] [PubMed] [Google Scholar]
- Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller JL 2007 The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol Endocrinol 21:664–673 [DOI] [PubMed] [Google Scholar]
- Khan I, Thomas P 1999 Fish, female reproductive cycle. In: Knobil E, Neill J, eds. The encyclopedia of reproduction. Vol III. New York: Academic Press; 552–564 [Google Scholar]
- Jalabert B, Fostier A, Breton B, Weil C 1991 Oocyte maturation in vertebrates. In Pang PKT, Schriebman MP, eds. Vertebrate endocrinology: fundamentals and biomedical implications. New York: Academic Press; 1–396 [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A 2002 Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol 187:153–159 [DOI] [PubMed] [Google Scholar]
- Haider S, Baqri SS 2000 Cyclic AMP-mediated control of oocyte maturation in the catfish, Clarias batrachus (Bloch): effects of 17α,20β-dihydroxy-4-pregnen-3-one and phosphodiesterase inhibitors. Indian J Exp Biol 38:967–973 [PubMed] [Google Scholar]
- Jalabert B, Finet B 1986 Regulation of oocyte maturation in rainbow trout, Salmo gairdneri: role of cAMP in the mechanism of action of the maturation inducing steroid (MIS), 17α-hydroxy,20β-dihydroprogesterone. Fish Physiol Biochem 2:65–74 [DOI] [PubMed] [Google Scholar]
- Pace MC, Thomas P 2005 Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev Biol 285:70–79 [DOI] [PubMed] [Google Scholar]
- Jalabert B 1975 [Modulation of 17-α-hydroxy-20β-dihydroprogesterone or gonadotropic extract activity on the in vitro intrafollicular maturation of rainbow trout (Salmo gairdnerii) oocytes by various steroids lacking maturing activity]. C R Acad Sci Hebd Seances Acad Sci D 281:811–814 (French) [PubMed] [Google Scholar]
- Goetz FW 1983 Hormonal control of oocyte final maturation and ovulation in fishes. In: Hoar WS, Randal DJ, Donaldson EM, eds. Fish physiology. Vol IXB. New York: Academic Press; 117–170 [Google Scholar]
- Pace MC, Thomas P 2005 Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod 73:988–996 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA 2004 The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306:1947–1950 [DOI] [PubMed] [Google Scholar]
- Kalinowski RR, Berlot CH, Jones TL, Ross LF, Jaffe LA, Mehlmann LM 2004 Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein mediated pathway. Dev Biol 267:1–13 [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M 2005 The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 287:249–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.