Abstract
Obesity is a major risk factor for the development of type 2 diabetes, and both conditions are now recognized to possess significant inflammatory components underlying their pathophysiologies. We tested the hypothesis that the plant polyphenolic compound curcumin, which is known to exert potent antiinflammatory and antioxidant effects, would ameliorate diabetes and inflammation in murine models of insulin-resistant obesity. We found that dietary curcumin admixture ameliorated diabetes in high-fat diet-induced obese and leptin-deficient ob/ob male C57BL/6J mice as determined by glucose and insulin tolerance testing and hemoglobin A1c percentages. Curcumin treatment also significantly reduced macrophage infiltration of white adipose tissue, increased adipose tissue adiponectin production, and decreased hepatic nuclear factor-κB activity, hepatomegaly, and markers of hepatic inflammation. We therefore conclude that orally ingested curcumin reverses many of the inflammatory and metabolic derangements associated with obesity and improves glycemic control in mouse models of type 2 diabetes. This or related compounds warrant further investigation as novel adjunctive therapies for type 2 diabetes in man.
THE HIGH PREVALENCE of obesity is a major threat to the public’s health. Obesity is an important risk factor for the development of myocardial infarction, stroke, type 2 diabetes mellitus, cancer (1,2,3,4,5), female infertility (6,7,8), and pregnancy complications (9). Not surprisingly, obesity is also associated with substantially decreased health-related quality of life and increased medical expenditures (10,11,12). Given the large burden imposed by obesity on the welfare of society, it is imperative to develop new methods that can decrease its prevalence as well as reverse its detrimental physiological alterations.
There is compelling evidence that a large component of obesity-associated pathophysiology may stem from a low-grade proinflammatory state. The manifestations of this proinflammatory state include increased production of proinflammatory molecules such as TNF-α, monocyte chemoattractant protein (MCP)-1, inducible nitric oxide synthase, and plasminogen activator inhibitor-1 in adipose, liver, and muscle tissue and increased activation of inflammatory signaling pathways such as the nuclear factor-κB (NF-κB) and Jun N-terminal kinase systems in these tissues. The white adipose tissue of obese subjects becomes histologically inflamed, with evidence of hypoxia, increased adipocyte death, and infiltration of both macrophages and cytotoxic T cells into the stromal vascular space (13,14,15).
Targeted deletions of several genes important for mediating inflammatory responses protect against the development of insulin resistance and hyperglycemia in mouse models of obesity. Some of these genes encode cytokines such as TNF-α and MCP-1 (16,17). Recently several studies have shown that disruption of the gene encoding the innate immune system receptor Toll-like receptor (TLR)-4 in mice confers protection from obesity-induced inflammation and insulin resistance (18,19,20,21,22). Also, inhibition of NF-κB signaling using high-dose salicylates or conditional deletion of inhibitory-κB kinase-β in myeloid lineage cells confers protection from obesity-induced inflammation and insulin resistance in mouse models (23,24). Conversely, stimulation of hepatic NF-κB signaling with a transgene is sufficient to increase local hepatic production of proinflammatory cytokines and systemic insulin resistance (25). Therefore, compounds that attenuate the inflammatory response associated with obesity may prove useful in the medical management of patients with type 2 diabetes.
The dried ground rhizome of the perennial herb Curcuma longa, called turmeric in English, is a popular dietary spice in Asia, as used in curry. It is also an integral part of the ancient Hindu medicinal system called Ayurveda. In contrast to the maximum dietary consumption of 1.5 g per person per day in certain Southeast Asian communities, smaller quantities of turmeric tend to be used for medicinal purposes such as pain relief or wound healing (26). The polyphenol curcumin (diferuloylmethane) comprises 2–8% of most turmeric preparations and is generally regarded as its most active component, having potent antioxidant, antiinflammatory, and anticarcinogenic properties (27). Commercial grade curcumin is readily available in any health food store in the United States as a standardized 95% pure curcuminoid preparation comprised of curcumin (∼80%), desmethoxycurcumin (∼10–20%), and bisdesmethoxycurcumin (<5%). Curcumin has no known dose-limiting toxicities and has been consumed by humans in dosages up to 12 g/d without significant side effects (28).
Several in vitro and in vivo studies have demonstrated that curcumin inhibits activation of the TLR-4 and NF-κB proinflammatory signaling pathways in diverse cell types including macrophages (29,30,31,32). Genetic studies in mice have implicated both of these proinflammatory signaling pathways in the pathogenesis of type 2 diabetes. Because curcumin is an antiinflammatory compound with no known dose-limiting toxicities and it targets both the TLR-4 and NF-κB signaling pathways, we investigated whether it could attenuate the proinflammatory, endocrine and metabolic consequences of obesity. There is only a single case report that describes the beneficial effect of curcumin in a type 2 diabetic patient (33). Previous studies have established that oral curcumin treatment improves hyperglycemia in KK-Ay mice, a genetic mouse model of type 2 diabetes, as well as the streptozotocin-treated rat (34,35,36,37,38,39,40,41). One study has shown that curcumin treatment prevents macrophage activation during coculture with adipocytes in vitro (42). Here we demonstrate that oral curcumin treatment of mice made obese by a high-fat diet and genetically obese Lepob/ob mice attenuates NF-κB activation in liver and macrophage accumulation in adipose tissue; increases adiponectin production by adipose tissue; and decreases the development of insulin resistance and hyperglycemia.
Materials and Methods
Experimental animals
Wild-type and ob/ob male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were housed five to a cage and maintained on a 12-h light, 12-h dark cycle with ad libitum access to food and water. Upon receipt at age 8–10 wk, the ob/ob mice were randomized to receive a standardized diet meal of 4% fat by weight (D12450B-I; Research Diets, New Brunswick, NJ) containing either a 3% by weight admixture of curcumin or no additive. The wild-type C57BL/6J mice were received at age 3–5 wk and randomized to receive either a standardized 4% fat by weight diet or high-fat diet containing 35% fat by weight (D12492-I; Research Diets). At the age of 20 wk, these wild-type mice were further randomized with regard to the addition to their predesignated diet of a 3% by weight admixture of curcumin or no additive.
The curcumin used was Curcumin C3 Complex (Sabinsa Corp., Newark, NJ), which is a 95% standardized curcumin extract. All mice remained on their assigned diet until they were killed by CO2 asphyxiation at the age of approximately 24–28 wk. All protocols were conducted in accord with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Columbia University Animal Care and Use Committee.
Body composition analysis
The fat and lean tissue masses were calculated from living, nonanesthetized mice using a nuclear magnetic resonance (NMR) machine (Bruker, Billerica, MA). Pelleted food was weighed daily to assess total grams of food consumed per cage per day and then divided by 5 to estimate average daily food consumed per mouse.
Glucose tolerance test
After fasting in fresh cages for 16 h overnight, approximately 25 μl tail tip blood was collected to ascertain fasting glucose and insulin levels. Thereafter all mice received one unit (0.01 cc) of a 20% dextrose solution per gram of body weight by ip injection. Sample tail tip blood glucose levels were assessed using a Freestyle Flash glucometer (Abbott, Abbott Park, IL) at 15, 30, 60, and 120 min. The blood samples were centrifuged at 14,000 rpm for 10 min (4 C), and the sera were transferred to fresh tubes and frozen at −80 C for later insulin assay.
Insulin tolerance test
After fasting mice for 6 h to stabilize dietary glucose and insulin levels, approximately 25 μl tail tip blood was collected to ascertain fasting glucose and insulin levels. Mice then received 1.5 U/kg regular insulin (Lilly, Indianapolis, IN) by ip injection. Sample tail tip blood glucose levels were assessed by glucometer at 15, 30, 45, 60, and 120 min.
Immunohistochemistry
All tissues were fixed for 12–16 h at room temperature in zinc-formalin fixative (Z-Fix; Anatech Ltd., Battle Creek, MI) and embedded in paraffin. Tissue was sliced into 5-μm sections cut at 50-μm intervals and then mounted on charged glass slides, deparaffinized in xylene, and stained. Perigonadal white adipose slides were stained with a macrophage-specific F4/80 monoclonal antibody provided by E. Richard Stanley (Albert Einstein College of Medicine, New York, NY). For each mouse adipose depot, four different high-power fields from each of four different sections were analyzed. The total number of nuclei and the number of nuclei of F4/80-expressing cells were counted for each field. The fraction of F4/80-expressing cells for each sample was calculated as the sum of the number of nuclei of F4/80-expressing cells divided by the total number of nuclei in sections of a sample (SPOT version 3.3; Diagnostic Instruments Inc., Sterling Heights, MI).
Quantitative real-time PCR
Total RNA was extracted from frozen adipose tissue (100 mg) using a commercially available acid-phenol reagent (TRIzol; Invitrogen, Carlsbad, CA). For tissue samples, first-strand cDNA was synthesized using SuperScript III reverse transcriptase and random hexamer primers as described in the manufacturer’s protocol (Invitrogen). Samples of cDNA were diluted 1:25 in nuclease-free water (QIAGEN Inc., Valencia, CA). Samples from each cDNA pool were diluted 1:10, 1:30, 1:90, and 1:270 to create a standard curve for calculation of relative gene expression levels. PCR amplification mixtures (20 μl) contained 10 μl of 2× PCR SYBR Green I QuantiTect master mix (QIAGEN), 0.4 μl of a mixture of 25 μm reverse and forward primers, and 11.6 μl diluted cDNA template. Real-time quantitative PCR was carried out using the DNA Engine Opticon 2 instrument (MJ Research Inc., Waltham, MA) with the following cycling parameters: polymerase activation for 15 min at 95 C and amplification for 40 cycles of 15 sec at 94 C, 10 sec at 58 C, and 10 sec at 72 C. After amplification, melting curve analysis confirmed the presence of a single amplicon in each instance.
For expression analysis, we used prevalidated real-time PCR primers (QuantiTect; QIAGEN) for several mouse genes: ribosomal protein S3, Emr1 (F4/80 antigen), TNF-α, IL-6, and adipocyte complement-related protein (adiponectin).
For each cDNA and standard curve sample, quantitative PCRs were performed to assay the expression of each internal control gene. To calculate the normalized relative expression levels of each gene assayed in each sample, we divided the relative gene expression value for that sample by the geometric mean of the relative expression values of the control genes. Separate analyses in which relative expression values were normalized with the relative expression values of each control gene yielded similar results.
Hormone assays
After minimally fasting for 6 h to avoid any potentially acute fluctuations in glucose or leptin attributable to recent eating, mice were weighed and then had their blood glucose measured by glucometer (Glucometer Elite, Elkhart, IN) using approximately 5 μl of tail tip blood. They were then killed by CO2 asphyxiation. Their blood was then obtained by cardiac puncture, allowed to clot on ice for 3 h, and then centrifuged at 10,000 × g for 10 min. The sera were then transferred to clean vials for storage at −80 C until the day of assay. Mouse serum insulin, leptin, MCP-1, adiponectin, resistin, and tissue plasminogen activator-1 were quantified by ELISA (Linco Research Inc., St. Charles, MO). All inter- and intraassay coefficients of variation were less than 10%.
NF-κB activity assay
Nuclear protein extracts were prepared from liver tissue from control and curcumin-fed mice using the nuclear extract kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. Protein concentrations were then quantified using a Bradford assay, and equal amounts of protein were used in a colorimetric NF-κB assay specific for the activated form of p65 subunit of NF-κB (TransAM NF-κB p65 kit; Active Motif).
Statistics and definitions
Two-tailed student’s t tests were used to compare serum analytes between C57BL/6J lean and obese experimental groups. P < 0.05 was considered statistically significant. All data were examined using SigmaStat 2.0 software (Jandel Scientific, San Rafael, CA).
Results
Male C57BL/6J mice gradually develop obesity and moderate diabetes when placed on high-fat diets. Male C57BL/6J ob/ob mice possess a spontaneous knockout mutation of the leptin gene that produces hyperphagia, decreased metabolic rate, severe obesity, and moderate diabetes, which is eventually compensated for by pancreatic β-cell hyperplasia and hyperinsulinemia (43).
Curcumin significantly improves glycemic status and insulin sensitivity in mouse models of obesity-related diabetes
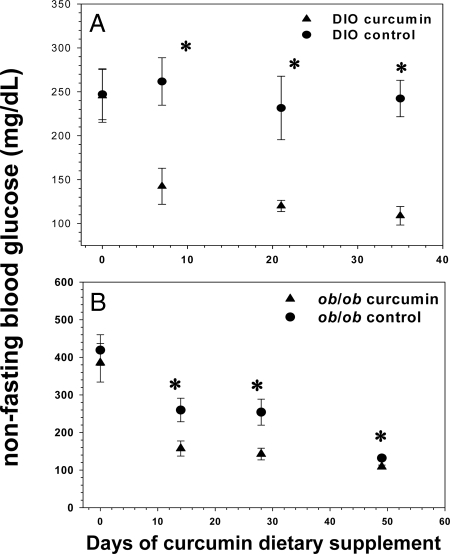
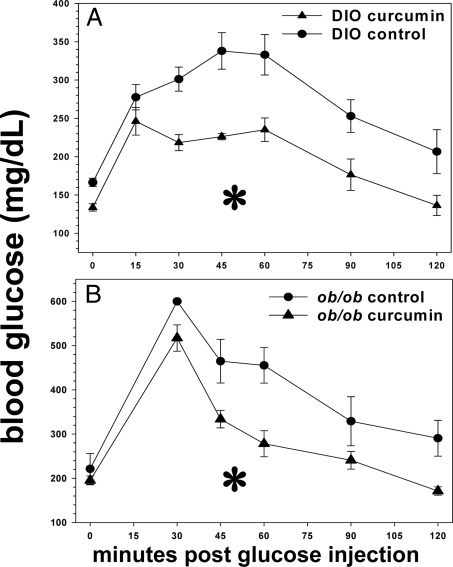
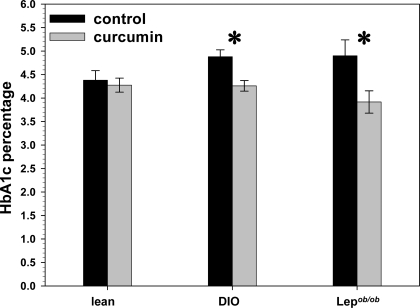
We determined that 3% dietary curcumin induces significant decreases in random-fed glucose levels in all groups. After less than 2 wk of treatment, curcumin had already decreased random-fed glucose levels in both diet-induced obesity (DIO) and ob/ob mice (Fig. 1, A and B). This pattern persisted in the DIO mice for the 5 wk duration of treatment, whereas the ob/ob mice over time eventually became euglycemic without treatment, a well-known phenomenon attributable to pancreatic islet hyperplasia (44). Both DIO and ob/ob mice treated with curcumin manifested significantly diminished areas under the curve (AUC) generated by 2 h glucose tolerance testing (Fig. 2, A and B), compared with controls. Moreover, the hemoglobin A1c (HbA1c) percentage in DIO and ob/ob mice was significantly decreased after 5 wk of curcumin treatment (Fig. 3), whereas no such decrease was noted in the lean wild-type mice, suggesting that curcumin does not induce hypoglycemia in euglycemic animals.
Figure 1.
Effect of curcumin on fed blood glucose levels in obese, diabetic mice. Dietary curcumin significantly lowered random blood glucose levels in both male C57BL/6J DIO (A) and ob/ob (B) mice over the course of a 6-wk period. Blood glucose levels in the ob/ob mice receiving the control diet normalized spontaneously over time due to their propensity to pancreatic islet hyperplasia and hyperinsulinemia (n = 5 per group). *, P < 0.05 by two-tailed t test.
Figure 2.
Effect of curcumin on glucose tolerance in obese, diabetic mice. Dietary curcumin significantly improves glucose tolerance in both male C57BL/6J DIO (A) and ob/ob (B) mice as determined by AUC of 2 h glucose tolerance test (n = 5 per group). *, P < 0.05.
Figure 3.
Effect of curcumin on HbA1c levels in obese, diabetic mice. Dietary curcumin (3%) significantly lowers HbA1c levels in male C57BL/6J DIO and ob/ob mice. The HbA1c levels of lean euglycemic mice were unaffected by curcumin (n = 5 per group). *, P < 0.05 by two-tailed t test.
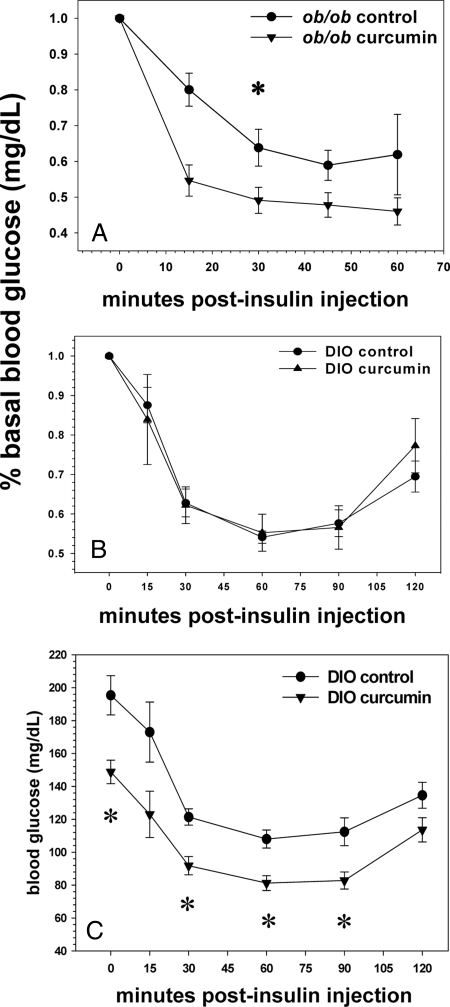
Improved insulin sensitivity was noted in the ob/ob mice as demonstrated by a decreased AUC of their percent basal glucose during a 2-h insulin tolerance test (Fig. 4A). No such difference was found for the curcumin fed DIO mice (Fig.4B), which, unlike their ob/ob counterparts, manifested blood glucose levels that were significantly lower than their control cohort to a similar degree (∼40 mg/dl) at all time points, including their fasting baseline. To portray a more accurate impression of their glycemic status, their insulin tolerance test has also been displayed using mean glucose levels for the ordinate axis rather than percent basal glucose levels (Fig. 4C).
Figure 4.
Effect of curcumin on insulin tolerance in obese, diabetic mice. Curcumin improves insulin tolerance in ob/ob mice as manifested by a decreased AUC of a 2-h insulin tolerance test with blood glucose levels after insulin injection expressed as a percentage of the basal glucose level (A). The male DIO mice manifested no such differences (B) because they expressed significantly lower fasting glucose levels than their corresponding control group and after insulin injection; their glucoses at each time point thereafter remained significantly lower (C) (n = 5 per group). *, P < 0.05.
Curcumin has a beneficial effect on weight and body composition
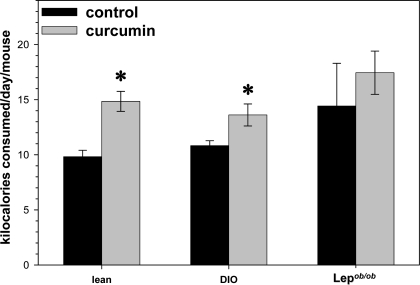
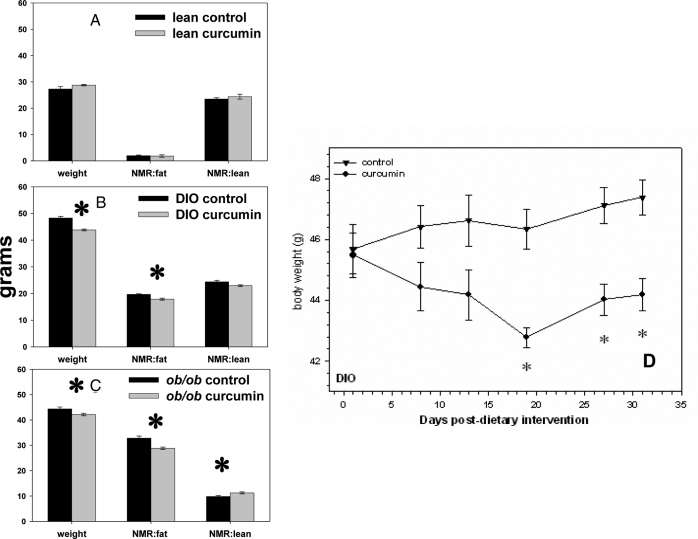
Male lean and DIO mice whose food contained 3% curcumin consumed significantly more food per day than their control cohorts, even after taking into account the percent of their food that was curcumin (Fig. 5). The curcumin-treated DIO and ob/ob mice weighed slightly but significantly less than their control cohort (Fig. 6, B and C). Curcumin treatment was also associated with significantly less body fat in DIO and ob/ob mice and more lean mass in the ob/ob mice as determined by Bruker NMR analysis (Fig. 6, B and C). Whereas the high-fat-fed DIO control mice continued to gain weight for the duration of this study, the DIO mice switched to a high-fat but curcumin-enriched diet lost a significant amount of weight by 2 wk into this study, which thereafter seemed to plateau (Fig. 6D).
Figure 5.
Effect of curcumin on food intake in mice. Dietary curcumin was associated with significantly increased food intake in lean and DIO but not ob/ob, C57BL/6J mice, even after compensating for the 3% of dietary intake that was curcumin (n = 5 per group). *, P < 0.05.
Figure 6.
Effect of curcumin on body weight and composition. Dietary curcumin (3%) is associated with significantly decreased body weight in both DIO (B) and ob/ob (C) mice. NMR scan revealed that curcumin decreased body fat in both DIO (B) and ob/ob (C) mice and was also associated with an increase in lean tissue mass in the ob/ob mice (C). D, The small decrease in weight over time that occurred in the DIO mice after being placed on a curcumin enriched diet is shown (n = 5 per group). *, P < 0.05 by two-tailed t test.
Curcumin significantly decreases adipose, hepatic, and systemic inflammation
Given curcumin’s antiinflammatory properties in other systems, we investigated the possibility that curcumin treatment would improve both local and systemic manifestations of inflammation in obese diabetic mice.
White adipose
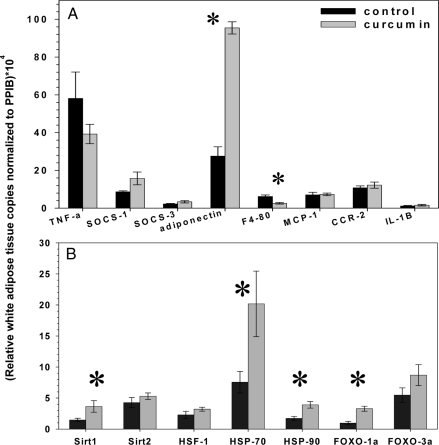
Immunohistochemistry revealed that curcumin reduced the number of macrophages present in the perigonadal white adipose tissue of DIO and ob/ob mice as determined by staining with a macrophage-specific F4/80 antibody (Fig.7). We also analyzed the effect of curcumin treatment upon the expression of several genes known to be closely correlated with the inflammatory process in perigonadal white adipose tissue using quantitative real-time PCR. Curcumin significantly decreased the expression of Emr1, the macrophage-specific gene encoding the F4/80 antigen (Fig. 8), consistent with the decreased immunohistochemical presence of macrophages seen in adipose tissue on cross-section. Curcumin treatment significantly increased perigonadal adipose expression of the genes encoding the forkhead transcription factor Foxo1 and the antiinflammatory adipokine adiponectin. These findings are consistent with recent studies that have shown decreased adipose tissue Foxo1 expression in obese mice and direct stimulation of adiponectin transcription by Foxo1 in adipocytes (45,46).
Figure 7.
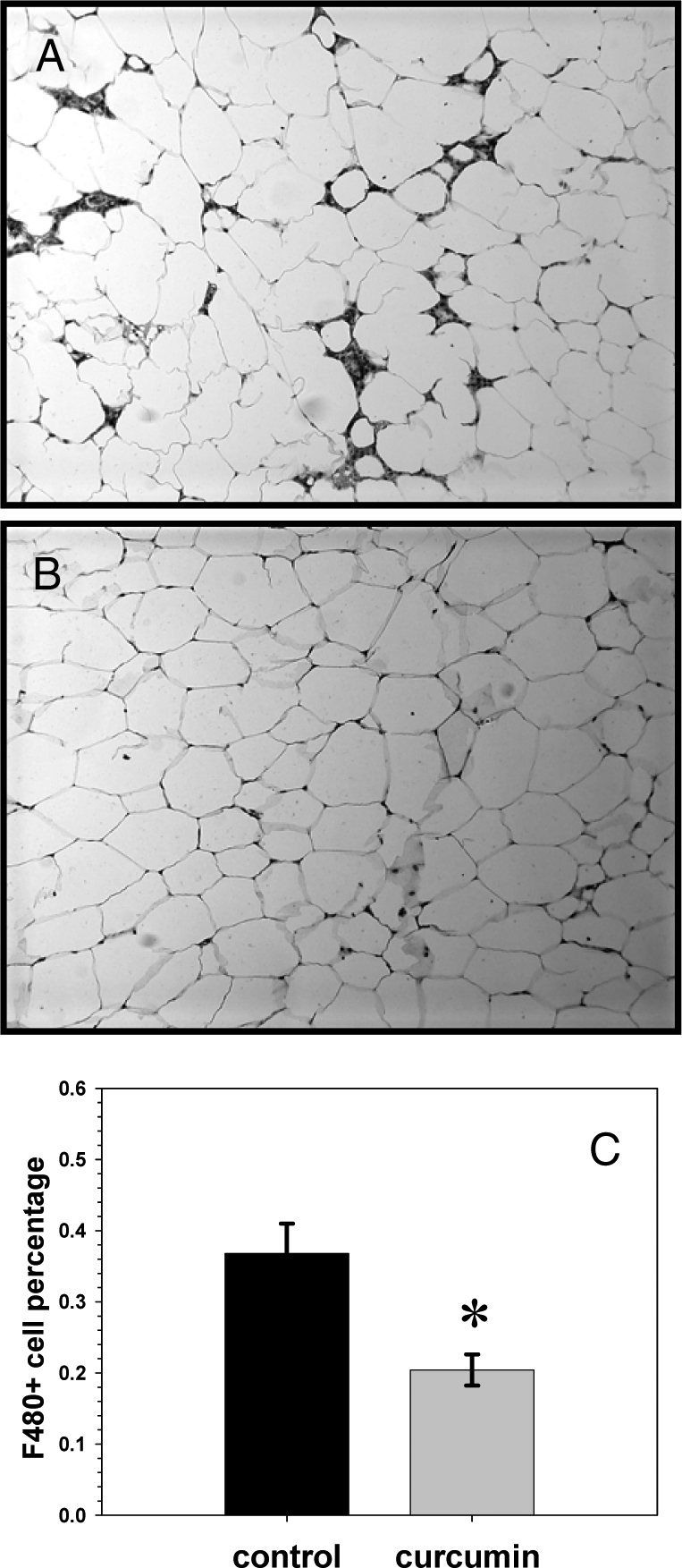
Effect of curcumin on adipose inflammation. Many adipocytes in the adipose tissue of untreated ob/ob male mice show dark staining for F4/80, a macrophage marker, whereas the adipose from those mice treated with dietary curcumin show significantly less macrophage staining (B and C).
Figure 8.
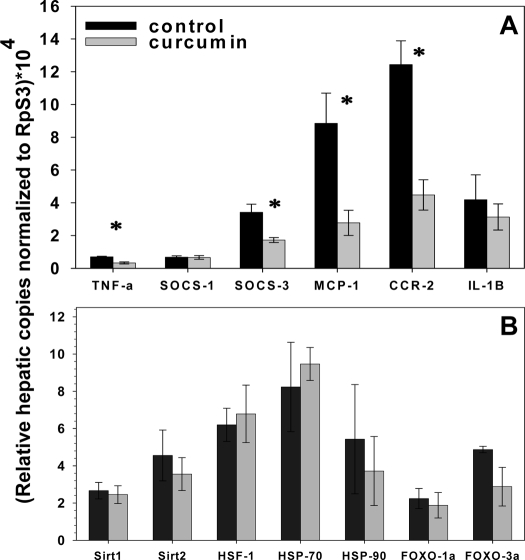
Effect of curcumin on adipose expression of cytokine and stress response genes. Dietary curcumin significantly increases expression of adiponectin (Acdc) and decreases F4/80 (Emr1) expression in perigonadal white adipose tissue of male ob/ob mice after 10 wk (A). Curcumin treatment also induced a dramatic effect on ER stress response perigonadal fat gene transcription with significant increases in the expression of Sirt1, HSP70, HSP90, and FOXO1a (B) (n = 5 per group). *, P < 0.05 by two-tailed t test.
Liver
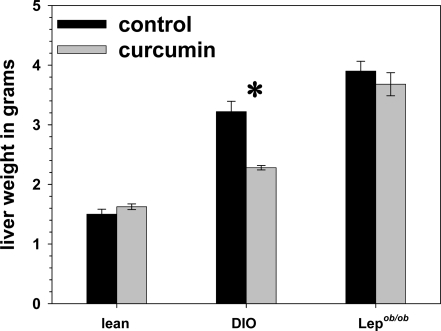
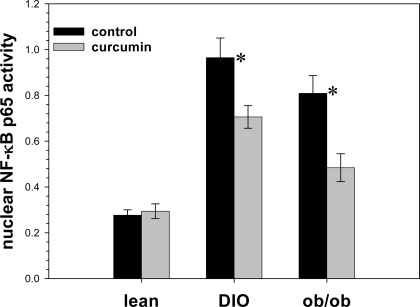
Grossly, we found that curcumin treatment was associated with significantly lighter and visibly less steatotic livers in the DIO mice (Fig. 9). The liver weights of the curcumin-fed ob/ob mice did not weigh significantly less than controls, an effect potentially attributable to the complete absence of leptin in these mice, which has been shown be associated with severe and refractory hepatosteatosis (47,48). We determined that curcumin significantly decreased the expression of hepatic TNF-α, suppressor of cytokine signaling-3, MCP-1 [chemokine (C-C motif) receptor], and chemokin (C-C motif) ligand-2 in ob/ob mice (Fig. 10). Using a specific assay for p65 activity (TransAM NF-κB p65 kit; Active Motif), we determined that there was significantly less p65 activity in liver nuclear extract samples derived from the curcumin-treated DIO and ob/ob mice, compared with those derived from untreated mice (Fig. 11). Decreased NF-κB activity may also explain the increased muscle mass seen in the curcumin-fed ob/ob mice, as NF-κB activation strongly fosters muscular atrophy, especially from disuse (49).
Figure 9.
Effect of curcumin on liver weight. Curcumin treatment was associated with significantly decreased liver weights in the DIO but not ob/ob mice (n = 5 per group). *, P < 0.05 by two-tailed t test.
Figure 10.
Effect of curcumin on expression of hepatic inflammatory genes. Dietary curcumin significantly lowers hepatic expression of the inflammatory genes TNF-α, SOCS-3, MCP-1, and CCR-2 in male ob/ob mice after 10 wk (A) (n = 5 per group). *, P < 0.05 by two-tailed t test.
Figure 11.
Effect of curcumin on expression of hepatic NF-κB activity. Dietary curcumin significantly decreases hepatic NF-κB activity after 10 wk (n = 5 per group). *, P < 0.05 by two-tailed t test.
Systemic
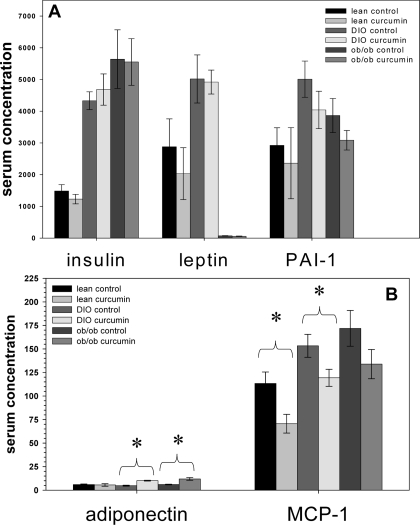
Serum adiponectin levels were significantly higher in both the curcumin-treated DIO and ob/ob mice (Fig. 12). This is consistent with the adipose expression data and their improved findings on the insulin tolerance test (Fig. 4). The serum levels of MCP-1, a monocyte chemoattractant factor secreted by adipocytes, were decreased by curcumin in all experimental groups, reaching significance in the lean and DIO groups. This is consistent with the diminished infiltration of macrophages noted immunohistochemically in the perigonadal tissue of the obese mice in this study.
Figure 12.
Effect of curcumin on serum markers of inflammation. Dietary curcumin significantly increased serum adiponectin levels in C57BL/6J DIO and ob/ob mice and decreased serum MCP-1 concentrations in wild-type lean and DIO C57BL/6J mice (n = 5 per group). *, P < 0.05 by two-tailed t test. PAI, Plasminogen activator inhibitor.
Discussion
These studies demonstrate that oral curcumin therapy ameliorates many of the inflammatory consequences of obesity in murine obesity models. Compared with control obese animals, obese animals treated with curcumin had decreased NF-κB activity in liver tissue, an effect associated with decreased hepatic expression of inflammatory molecules. Curcumin-treated obese mice also had decreased macrophage infiltration into adipose tissue, increased Foxo1, and adiponectin expression in adipose tissue and higher circulating adiponectin levels. These antiinflammatory effects of curcumin were associated with improved glycemic status in the treated animals as determined by blood glucose levels, HbA1c, and glucose and insulin tolerance tests. Curcumin was also associated with a small but significant decrease in body weight and fat content despite either a maintenance or increase in total daily kilocalories. This suggests that curcumin has beneficial effects on body composition. In fact, curcumin was noted to increase the lean tissue mass of ob/ob mice, an effect that may be due to curcumin’s ability to inhibit the activity of NF-κB, a molecule known to play a key role in the pathophysiology of muscle atrophy, especially from disuse (50).
Interestingly, dietary curcumin was found to dramatically increase the expression of adiponectin as manifested by increases in mRNA and serum protein levels. This may underlie to a large degree the antidiabetogenic effect that curcumin was shown to exert in this study because treatment with adiponectin has been found to improve insulin sensitivity in animal models of insulin resistance (51). Recent studies suggest that adiponectin is protective against both atherosclerosis and generalized inflammation, two processes in which macrophages are known to play a major role (52,53). This raises the possibility that the decrease in adipose tissue macrophage infiltration we noticed in the curcumin-fed obese mice, as evidenced by histology as well as decreased Emr1 expression, may have stemmed from curcumin’s enhancement of adipose tissue adiponectin production.
Obesity is associated with increased levels of endoplasmic reticulum (ER) stress in adipose and liver tissue, and compounds that act as chemical chaperones and relieve ER stress also ameliorate insulin resistance and hyperglycemia in mouse models of obesity. The fact that curcumin therapy prevents ER accumulation of mutant proteins and Schwann cell apoptosis in mouse models of peripheral neuropathies indicates that it may also act as a chemical chaperone in obese mice and thereby decrease insulin resistance. In this study, curcumin treatment induced a dramatic effect on ER stress response perigonadal fat gene transcription with significant increases in the expression of Sirt1, heat shock protein (HSP)-70, HSP90, and Forkhead box O (FOXO)-1a. The histone deacetylase SIRT1 increases adiponectin transcription in adipocytes by activating Foxo1 and enhancing Foxo1 and CCAAT/enhancer-binding protein-α interaction (46). Both Foxo1 and SIRT1 protein levels were found to be significantly lower in perigonadal adipose from DIO mice, compared with normal-weight mice. A reversal of this process may help to explain the increased adiponectin noted in association with curcumin feeding (54).
Curcumin has been explored as a potential therapeutic modality for a large number of diseases. It suppresses proliferation and induces apoptosis in a wide array of cancer cell lines. Preclinical studies in animal models have shown that curcumin prevents chemical-induced colitis, muscle degeneration after traumatic injury and lipopolysaccharide-induced cytokine release, disseminated intravascular coagulation, and hepatocellular injury. In mouse models of inherited peripheral neuropathies, curcumin prevents accumulation of mutant proteins in the ER and thereby prevents Schwann cell apoptosis. Results from early human trials have been encouraging. A recent randomized, multicenter, double-blind, placebo-controlled trial in humans with ulcerative colitis showed that addition of 1 g of curcumin twice a day to standard maintenance therapy significantly decreased relapse rates and disease activity assessed clinically and endoscopically (55).
Given the fact that curcumin is poorly absorbed from the gastrointestinal tract and has an excellent safety profile, we felt comfortable starting with a high dosage because we wanted to determine early on if oral curcumin at any dose could improve diabetes. Based on an average weight of 40 g and a daily food consumption of approximately 2 g, the mice would have consumed approximately 1.5 g curcumin per kilogram per day. Although such an oral dose would be impractical in humans, the mice were noted to tolerate this dosage quite well without any noticeable detrimental effects on behavior or appetite. Organs appeared grossly normal. The kidneys, liver, and heart manifested either an improved or unchanged histopathology.
Early studies in humans examining systemic markers of curcumin bioactivity have shown that dosages as low as 3.6 g/d are sufficient to decrease responsiveness of circulating leukocytes to lipopolysaccharide (56). Therefore, curcumin may have significant antiinflammatory effects on human subjects on a reasonable dosing schedule. Newer and more innovative ways to achieve therapeutic blood levels of curcumin should also be investigated. For example, there are data demonstrating that coingestion of curcumin with other natural phytochemicals such as piperine has the potential to increase the oral absorption of curcumin dramatically (57). In addition, given the fact that curcumin is a low-molecular-weight and highly lipophilic molecule, it is possible that it may be absorbed easier via the transdermal rather than oral route.
In conclusion, our studies revealed that high dosages of oral curcumin safely treat diabetes in several mouse models of obesity-associated diabetes. Curcumin also greatly ameliorated inflammation at the cellular and biochemical level in white adipose tissue of obese mice. Lastly, given the fact that DIO and ob/ob curcumin-treated mice ate more, weighed the same or less, and possessed higher lean body mass content, curcumin also has a favorable effect on body composition, an effect that in itself may also contribute to improved glycemic status. Given these promising preclinical findings, we believe curcumin holds potential as an adjuvant treatment for diabesity complications and deserves further investigation.
Footnotes
This work was supported by Women’s Reproductive Health Research Scholarship National Institutes of Health 5 K12 HD001275-04 (to D.V.T.).
Disclosure Statement: S.P.W., R.L., and D.V.T. have nothing to declare.
First Published Online April 10, 2008
Abbreviations: DIO, Diet-induced obesity; ER, endoplasmic reticulum; FOXO, Forkhead box O; HbA1c, hemoglobin A1c; HSP, heat shock protein; MCP, monocyte chemoattractant protein; NF-κB, nuclear factor-κB; NMR, nuclear magnetic resonance; TLR, Toll-like receptor.
References
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ 2003 Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638 [DOI] [PubMed] [Google Scholar]
- Kannel WB, Cupples LA, Ramaswami R, Stokes 3rd J, Kreger BE, Higgins M 1991 Regional obesity and risk of cardiovascular disease: the Framingham Study. J Clin Epidemiol 44:183–190 [DOI] [PubMed] [Google Scholar]
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS 2002 Obesity and the risk of heart failure. N Engl J Med 347:305–313 [DOI] [PubMed] [Google Scholar]
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA 2001 Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 161:1581–1586 [DOI] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH 1999 The disease burden associated with overweight and obesity. JAMA 282:1523–1529 [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn J, Chua SC 2004 Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology 145:1238–1247 [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn JE, Chua SC 2007 Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes 31:395–402 [DOI] [PubMed] [Google Scholar]
- Metwally M, Li TC, Ledger WL 2007 The impact of obesity on female reproductive function. Obes Rev 8:515–523 [DOI] [PubMed] [Google Scholar]
- Guelinckx I, Devlieger R, Beckers K, Vansant G 2008 Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 9:140–150 [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Liu K, Yan LL, Pirzada A, Garside DB, Schiffer L, Dyer AR, Greenland P, Stamler J 2003 Body mass index in middle age and health-related quality of life in older age: the Chicago heart association detection project in industry study. Arch Intern Med 163:2448–2455 [DOI] [PubMed] [Google Scholar]
- Hassan MK, Joshi AV, Madhavan SS, Amonkar MM 2003 Obesity and health-related quality of life: a cross-sectional analysis of the US population. Int J Obes Relat Metab Disord 27:1227–1232 [DOI] [PubMed] [Google Scholar]
- Arterburn DE, Maciejewski ML, Tsevat J 2005 Impact of morbid obesity on medical expenditures in adults. Int J Obes Relat Metab Disord 29:334–339 [DOI] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS 2005 Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46:2347–2355 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV 2007 Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes 32:451–463 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM 1993 Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS 1997 Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610–614 [DOI] [PubMed] [Google Scholar]
- Misonou Y, Asahi M, Yokoe S, Miyoshi E, Taniguchi N 2006 Acrolein produces nitric oxide through the elevation of intracellular calcium levels to induce apoptosis in human umbilical vein endothelial cells: implications for smoke angiopathy. Nitric Oxide 14:180–187 [DOI] [PubMed] [Google Scholar]
- Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y 2007 Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun 354:45–49 [DOI] [PubMed] [Google Scholar]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y 2007 Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 27:84–91 [DOI] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ 2007 Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56:1986–1998 [DOI] [PubMed] [Google Scholar]
- Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC 2007 C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50:1267–1276 [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M 2005 IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198 [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE 2001 Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293:1673–1677 [DOI] [PubMed] [Google Scholar]
- Arafa HM 2005 Curcumin attenuates diet-induced hypercholesterolemia in rats. Med Sci Monit 11:BR228–BR234 [PubMed] [Google Scholar]
- Sharma RA, Gescher AJ, Steward WP 2005 Curcumin: the story so far. Eur J Cancer 41:1955–1968 [DOI] [PubMed] [Google Scholar]
- Shishodia S, Sethi G, Aggarwal BB 2005 Curcumin: getting back to the roots. Ann NY Acad Sci 1056:206–217 [DOI] [PubMed] [Google Scholar]
- Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE 2006 Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB 2007 Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB-regulated gene products. Cancer Res 67:3853–3861 [DOI] [PubMed] [Google Scholar]
- Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A 2007 Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol 73:1434–1445 [DOI] [PubMed] [Google Scholar]
- Gradisar H, Keber MM, Pristovsek P, Jerala R 2007 MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol 82:968–974 [DOI] [PubMed] [Google Scholar]
- Bharti AC, Takada Y, Aggarwal BB 2004 Curcumin (diferuloylmethane) inhibits receptor activator of NF-κB ligand-induced NF-κB activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol 172:5940–5947 [DOI] [PubMed] [Google Scholar]
- Srinivasan M 1972 Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci 26:269–270 [PubMed] [Google Scholar]
- Suresh Babu P, Srinivasan K 1998 Amelioration of renal lesions associated with diabetes by dietary curcumin in streptozotocin diabetic rats. Mol Cell Biochem 181:87–96 [DOI] [PubMed] [Google Scholar]
- Sajithlal GB, Chithra P, Chandrakasan G 1998 Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol 56:1607–1614 [DOI] [PubMed] [Google Scholar]
- Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK 1999 Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen 7:362–374 [DOI] [PubMed] [Google Scholar]
- Srivivasan A, Menon VP, Periaswamy V, Rajasekaran KN 2003 Protection of pancreatic β-cell by the potential antioxidant bis-o-hydroxycinnamoyl methane, analogue of natural curcuminoid in experimental diabetes. J Pharm Pharm Sci 6:327–333 [PubMed] [Google Scholar]
- Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB 2005 Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci 46:2092–2099 [DOI] [PubMed] [Google Scholar]
- Kumar PA, Suryanarayana P, Reddy PY, Reddy GB 2005 Modulation of α-crystallin chaperone activity in diabetic rat lens by curcumin. Mol Vis 11:561–568 [PubMed] [Google Scholar]
- Mahesh T, Balasubashini MS, Menon VP 2005 Effect of photo-irradiated curcumin treatment against oxidative stress in streptozotocin-induced diabetic rats. J Med Food 8:251–255 [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Agrewala JN, Chopra K 2006 Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol 536:256–261 [DOI] [PubMed] [Google Scholar]
- Woo HM, Kang JH, Kawada T, Yoo H, Sung MK, Yu R 2007 Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci 80:926–931 [DOI] [PubMed] [Google Scholar]
- Leibel RL, Chung WK, Chua Jr SC 1997 The molecular genetics of rodent single gene obesities. J Biol Chem 272:31937–31940 [DOI] [PubMed] [Google Scholar]
- Bock T, Pakkenberg B, Buschard K 2003 Increased islet volume but unchanged islet number in ob/ob mice. Diabetes 52:1716–1722 [DOI] [PubMed] [Google Scholar]
- Subauste AR, Burant CF 2007 Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am J Physiol Endocrinol Metab 293:E159–E164 [DOI] [PubMed] [Google Scholar]
- Qiao L, Shao J 2006 SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein α transcriptional complex. J Biol Chem 281:39915–39924 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI 2002 Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109:1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P 2005 Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41:753–760 [DOI] [PubMed] [Google Scholar]
- Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK 1999 Systemic administration of the NF-κB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol 277:C320–C329 [DOI] [PubMed] [Google Scholar]
- Baghdiguian S, Richard I, Martin M, Coopman P, Beckmann JS, Mangeat P, Lefranc G 2001 Pathophysiology of limb girdle muscular dystrophy type 2A: hypothesis and new insights into the IκBα/NF-κB survival pathway in skeletal muscle. J Mol Med 79:254–261 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T 2001 The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Osei SY 2008 Adipokines in obesity. Front Horm Res 36:182–197 [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mangalat D, Korbut R 2006 Adipocytokines—novel link between inflammation and vascular function? J Physiol Pharmacol 57:505–528 [PubMed] [Google Scholar]
- Qiang L, Wang H, Farmer SR 2007 Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-Lα. Mol Cell Biol 27:4698–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, Yamada M, Iwaoka Y, Kanke K, Hiraishi H, Hirayama K, Arai H, Yoshii S, Uchijima M, Nagata T, Koide Y 2006 Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 4:1502–1506 [DOI] [PubMed] [Google Scholar]
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP 2004 Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10:6847–6854 [DOI] [PubMed] [Google Scholar]
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS 1998 Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64:353–356 [DOI] [PubMed] [Google Scholar]