Abstract
The antiapoptotic molecule Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein (FLIP) inhibits Fas-mediated apoptosis by blocking activation of caspase-8. We previously showed that expression of transgenic FLIP on thyroid epithelial cells (TECs) of DBA/1 and CBA/J mice promoted earlier resolution of granulomatous experimental autoimmune thyroiditis in vivo. This study was undertaken to directly determine whether transgenic FLIP expressed on cultured TECs can protect TECs from Fas-mediated apoptosis in vitro. The results indicate that cultured TECs from DBA/1 and CBA/J mice can be sensitized in vitro by interferon-γ and TNF-α to undergo Fas-mediated apoptosis. Transgenic overexpression of FLIP protected cultured TECs of FLIP transgene (Tg)+ DBA/1 and CBA/J mice from Fas-mediated apoptosis, and FLIP small interfering RNA transfection of cultured TECs of FLIP Tg+ DBA/1 and CBA/J mice abolished the protective effect. These in vitro results are consistent with our previous in vivo studies using DBA/1 and CBA/J FLIP Tg+ mice and provide direct support for the hypothesis that transgenic expression of FLIP promotes resolution of granulomatous experimental autoimmune thyroiditis by protecting TECs from apoptosis.
APOPTOSIS IS AN active process of cell death involving the sequential activation of a series of caspases that induce cell death when stimulated by an appropriate trigger (1,2). Improper regulation of apoptosis can lead to disease (1,2). Apoptosis can be initiated through molecules such as Fas and Fas ligand (FasL), and this signal is propagated through caspase-8 and caspase-3 (1,2,3,4,5,6,7,8,9). FasL is a member of the TNF family that acts through the Fas death receptor (3,4). Fas, a widely expressed type I transmembrane protein, is also a member of the TNF receptor family (5). Both FasL and Fas are expressed by thyroid epithelial cells (TECs) in autoimmune thyroid diseases (6,7). Fas-mediated apoptosis plays an important role in many human and murine autoimmune diseases including granulomatous experimental autoimmune thyroiditis (G-EAT) (6,7,8,9,10,11,12,13,14,15,16). The antiapoptotic molecule Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein (FLIP) inhibits Fas-mediated apoptosis by blocking activation of caspase-8 (17,18). FLIP is an important inhibitor of the initial upstream steps of Fas-mediated apoptosis (17). FLIP expression was increased in lymphocytes of patients with active multiple sclerosis (18,19), Graves’ disease (7), T cells in animal models of inflammatory arthritis (18), and thyroids of mice with G-EAT (20). Fas-mediated apoptosis can function to induce autoimmune damage (10,11,21) and also to reduce autoimmune responses (8,9,14,20).
We recently demonstrated that expression of FLIP as a transgene (Tg) on TECs of DBA/1 and CBA/J mice promoted earlier resolution of G-EAT in vivo possibly by protecting TECs from Fas-mediated apoptosis (20,22). To directly determine whether transgenic FLIP expressed on TECs can protect TECs from Fas-mediated apoptosis, cultured TECs from naïve FLIP Tg+ and Tg−DBA/1 and CBA/J mice were generated. Because human TECs can be sensitized in vitro by the combination of interferon (IFN)-γ and TNF-α to undergo Fas-mediated apoptosis (23), cultured murine TECs with or without agonist anti-Fas in the presence or absence of IFN-γ and TNF-α were included in this study. The results suggested that, like human cultured TECs, normal cultured TECs from DBA/1 and CBA/J mice can be sensitized in vitro by IFN-γ and TNF-α to undergo Fas-mediated apoptosis. Transgenic overexpression of FLIP protected cultured TECs of FLIP Tg+ DBA/1 and CBA/J mice from Fas-mediated apoptosis, and FLIP small interfering RNA (siRNA) transfection of cultured TECs of FLIP Tg+ mice abolished the protective effect, directly supporting the hypothesis that FLIP expression on TECs contributes to G-EAT resolution.
Materials and Methods
Mice
DBA/1 and CBA/J FLIP transgenic mice were generated using the recombinant construct containing rat thyroglobulin promoter and FLAG (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys)-tagged mouse long form of cellular FLIP cDNA described previously (20,22). Both FLIP Tg+ mice and Tg− littermates were used for primary TEC culture. Thyroids from both male and female mice 8–10 wk of age were used. All mice were bred and maintained in accordance with University of Missouri institutional guidelines for animal care.
Culture of primary TECs
Mouse primary TEC cultures were generated using conditions described by others (23,24,25). Individual thyroid lobes from naive DBA/1 and CBA/J mice were aseptically dissected and disrupted mechanically to 12–14 fragments and then digested for 1 h at 37 C in digestion medium consisting of 112 U/ml type I collagenase (Sigma, St. Louis, MO) and 1.2 U/ml dispase II (Roche, Indianapolis, IN) dissolved in Eagle’s MEM, shaking every 5 min (24,25). After centrifugation for 3 min at 1200 rpm, digested thyroid pellets from Tg− or Tg+ groups were pooled, resuspended, and seeded in 60-mm petri plates (Corning, Corning, NY) or eight-well chamber slides (Nalge Nunc International, Rochester, NY), and cultured at 37 C to 60–80% confluence. The culture medium was prepared as follows: Nu-Serum IV was diluted 2.5 times with F-12 medium to give a concentration of 10% fetal bovine serum, 5 μg/ml human transferrin, 10 μg/ml bovine insulin, and 3.5 ng/ml hydrocortisone. Medium was supplemented with 10 ng/ml somatostatin (Sigma) and 2 ng/ml glycyl-l-histidyl-l-lysine acetate (Sigma). The purity of the thyroid cell population was verified by staining with mouse monoclonal antibody specific for mouse pan-cytokeratin (PCK-26; Sigma) or rabbit polyclonal antibody specific for human thyroglobulin (TG) but cross-reactive with mouse TG (Santa Cruz Biotechnology, Santa Cruz, CA).
Cytokine and anti-Fas treatment
Seventy to 80% confluent cultured TECs were cultured for 4 d with 100 IU/ml IFN-γ (eBioscience, San Diego, CA) and 50 IU/ml TNF-α (eBioscience) (23). Cells were then cultured overnight with 1 μg/ml agonist anti-Fas antibody (Jo-2; BD PharMingen, San Diego, CA) (23). The amounts of cytokine and anti-Fas were based on studies using human cultured TECs (23).
Transfection with FLIP siRNA
Sixty to 70% confluent cultured TECs of FLIP Tg+ mice were transfected with 10 pmol (per well in eight-well chamber slide) murine FLIP siRNA or control siRNA (Santa Cruz Biotechnology). Each transfection was performed according to the manufacturer’s protocol. After 72 h, TECs in some chamber slides were stained with anti-FLIP (Abcam) or anti-FLAG (Abcam, Cambridge, MA) as previously described (20) to determine the transfection efficiency and others were stimulated with cytokines and anti-Fas as described above.
Confocal laser-scanning immunofluorescence microscopy
Confocal laser-scanning immunofluorescence microscopy was performed as previously described (20,22). Briefly, thyrocytes were grown to 70–80% confluence in eight-well chamber slides. Cells were washed twice in PBS, fixed in methanol at −20 C for 10 min, followed by treatment in acetone at −20 C for 1 min. After microwave antigen retrieval, cells were incubated 1 h with mouse anti-pan-cytokeratin-26 (PCK; Sigma) or rabbit anti-TG (Santa Cruz Biotechnology). After incubation with a secondary biotinylated antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), cells were washed and incubated with Alexa 488-labeled streptavidin for 30 min and visualized by Alexa 488 (Molecular Probes, Eugene, OR). Slides were observed using a Radiance 2000 confocal system (Bio-Rad Laboratories, Hercules, CA) coupled to an IX70 inverted microscope (Olympus, Tokyo, Japan). As a negative control, primary antibody was replaced with an equal amount of normal serum. These controls were always negative.
Immunohistochemistry
Immunohistochemistry (IHC) staining for FLIP, FLAG, active caspase-8, and active caspase-3 was described previously (20). Briefly, after treatment with 0.1% saponin in 1% BSA for 30 min, slides containing cultured TECs fixed in methanol and acetone were incubated with polyclonal anti-FLIP (Abcam), anti-FLAG (Abcam), antiactive caspase-8 (Imgenex, San Diego, CA), or antiactive caspase-3 (BD PharMingen) 1 h at room temperature. After incubation with a secondary biotinylated antibody (Jackson), immunoreactivity was demonstrated using the avidin-biotin complex immunoperoxidase system (Vector, Burlingame, CA) and developed using NovaRED (Vector). Slides were counterstained with hematoxylin. As a negative control, primary antibody was replaced with an equal amount of normal rabbit or goat IgG. These controls were always negative.
Determination of apoptosis
Apoptosis was determined by terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick-end labeling (TUNEL) staining using the Apoptag Plus peroxidase kit (Chemicon, Temecula, CA), following the manufacturer’s instructions. Briefly, cultured TECs were fixed and endogenous peroxidase activity was blocked in 0.3% hydrogen peroxide in PBS for 30 min. Slides were incubated 5 min at room temperature with equilibration buffer, followed by 1 h incubation at 37 C with terminal deoxynucleotidyl transferase enzyme (or reaction buffer negative controls) diluted with the reaction buffer in a humidity chamber. The terminal deoxynucleotidyl transferase reaction was stopped with stop buffer, and slides were washed with PBS before 30 min incubation with horseradish peroxidase-conjugated antidigoxigenin. After washing, color development was obtained by incubating slides with NovaRED (Vector). Slides were counterstained with hematoxylin. A positive reaction for apoptosis was characterized by red color of the nuclear or perinuclear region of the cell. To quantify the number of positive cells, all cells in five to six randomly selected high-power fields (magnification, ×400) were manually counted and expressed as a percentage of total cells.
RT-PCR
TECs cultured in 60-mm plates were washed with PBS and homogenized in TRIzol (Invitrogen, Carlsbad, CA), and RNA was extracted and reverse transcribed as previously described (16,20). To determine the relative initial amounts of target cDNA, each cDNA sample was serially diluted 1:5 and 1:25 (16,20). Hypoxanthine phosphoribosyltransferase (HPRT) was used as a housekeeping gene to verify that the same amount of RNA was amplified. PCR products were electrophoresed in 2% agarose gel, visualized by UV light after staining with ethidium bromide, and normalized between samples relative to levels of HPRT using an IS-1000 digital imaging system (Life Sciences, St. Louis, MO). Most primers used in this study have been described previously (16). Primer sequences for Fas-associated death domain (FADD) are: sense, 5′-TGGATGGGATTGAGGAGAAG-3′, antisense, 5′-AAACCACAGTCCTCACAGGG-3′. Primer sequences for Bcl-xL are: sense, 5′-TGGTCGACTTTCTCTCCTAC-3′, antisense, 5′-GAGATCCACAAAAGTGTCCC-3′.
Statistical analysis
All experiments were repeated two or three times. Statistical analysis was performed using an unpaired two-tailed Student’s t test. P < 0.05 was considered significant.
Results
Generation of PCK+TG+ murine TEC cultures
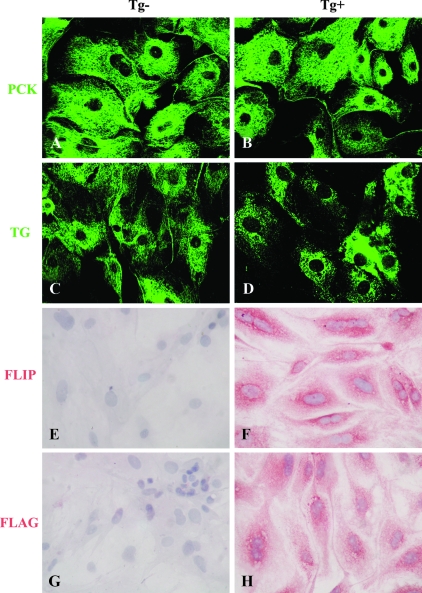
Primary TECs freshly prepared from thyroids of DBA/1 or CBA/J FLIP Tg+ mice or their Tg− littermates were pooled respectively and cultured in 8-well chamber slides in complete F-12 medium. After 7–10 d, 70–80% confluent cells were stained as described in Materials and Methods. All results obtained using thyroids from DBA/1 and CBA/J mice are identical, and shown are results from DBA/1 mice. Nearly 100% of the cultured cells were PCK+ and TG+ in both FLIP Tg− and Tg+ groups (Fig. 1, A–D), confirming their thyrocyte origin. FLIP and FLAG were not detected in cultured cells of FLIP Tg− mice (Fig. 1, E and G), whereas cultured TECs from FLIP Tg+ mice expressed FLIP and FLAG (Fig. 1, F and H). This indicates that in vitro culture conditions do not influence transgenic expression of FLIP.
Figure 1.
Generation of PCK+TG+ murine TEC cultures Most cultured cells detected by confocal laser-scanning immunofluorescence microscopy were PCK+ and TG+ in both FLIP Tg− and Tg+ groups (A–D). FLIP and FLAG were not detected by IHC in cultured TECs of FLIP Tg− mice (E and G) but were highly expressed on cultured TECs of FLIP Tg+ DBA/1 mice (F and H). Original magnification, A–D, ×600; E and F, ×400.
Sensitization of primary TECs to Fas-mediated apoptosis is blocked by transgenic expression of FLIP
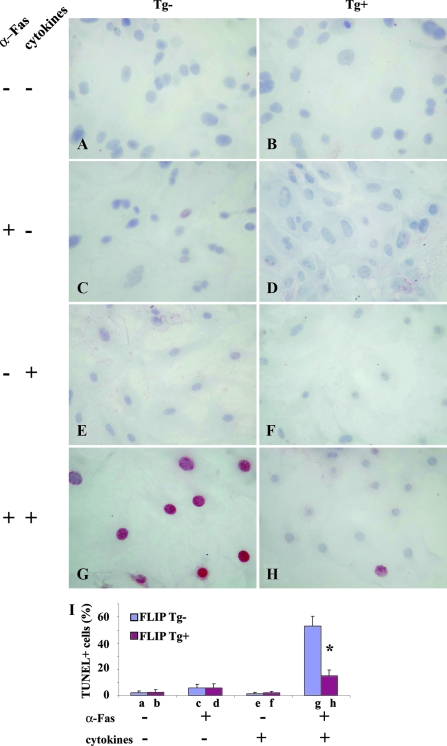
Cultured human TECs are resistant to Fas-mediated apoptosis but can be sensitized in vitro by IFN-γ and TNF-α to undergo Fas-mediated apoptosis induced by anti-Fas (23). To determine whether murine TECs can be sensitized by IFN-γ and TNF-α to undergo Fas-mediated apoptosis and whether transgenic overexpression of FLIP can protect TEC from Fas-mediated apoptosis, 70–80% confluent cultured TECs were treated with or without agonist anti-Fas (1 μg/ml) in the presence or absence of IFN-γ and TNF-α, and apoptosis was detected by TUNEL staining as described in Materials and Methods. The amounts of cytokine and anti-Fas were based on studies by others using human cultured TECs (23). No TUNEL+ cells (red) were detected in TECs of FLIP Tg− or Tg+ mice cultured in the absence of anti-Fas and cytokines (Fig. 2, A and B) or in the presence of cytokines or anti-Fas alone (Fig. 2, C–F), even using 3-fold higher concentrations of cytokines or anti-Fas (data not shown). However, when TECs were pretreated with IFN-γ (100 IU/ml) and TNF-α (50 IU/ml) and then stimulated by anti-Fas (1 μg/ml), many TUNEL+ cells (red) were detected in TEC of FLIP Tg− mice (Fig. 2G), whereas few TUNEL+ cells were detected in TEC of FLIP Tg+ mice (Fig. 2H). TUNEL+ cells (red) in five to six randomly selected high-power fields of three wells/group (magnification, ×400) were manually counted and the results are summarized in Fig. 2I (bars, a–h, correspond to Fig. 2, A–H). These results show that cultured TECs can be sensitized by IFN-γ and TNF-α to undergo Fas-mediated apoptosis. Apoptosis is inhibited by transgenic overexpression of FLIP on TECs, directly demonstrating that transgenic overexpression of FLIP protects TECs from Fas-mediated apoptosis.
Figure 2.
Sensitization of primary TECs to Fas-mediated apoptosis is blocked by transgenic expression of FLIP. Cell treatments are shown on the left. Sixty percent to 70% confluent cultured TECs from Tg− and Tg+ mice were treated with or without agonist anti-Fas (1 μg/ml) in the presence or absence of IFN-γ (100 IU/ml) and TNF-α (50 IU/ml). Apoptosis was detected by TUNEL staining (A–H). TUNEL+ cells (red) in five to six randomly selected high-power fields of three wells/group were manually counted (magnification, ×400), and the results are summarized (I). Bars (a–h) correspond to A–H. Shown are representative areas on slides. A significant difference in the percentage of TUNEL+ cells between Tg− and Tg+ TEC is indicated by the asterisk (P < 0.05). Original magnification, A–H, ×400.
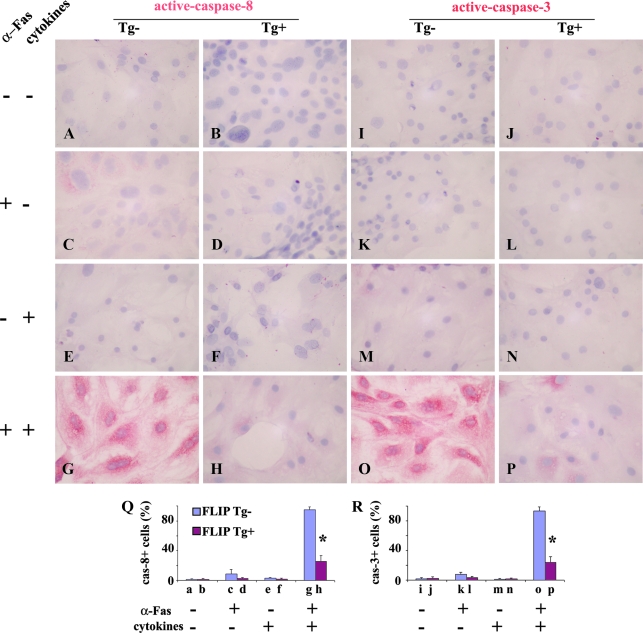
Expression of active caspase-8 and active capase-3 on cultured TECs of FLIP Tg+ and Tg− mice treated with cytokines and anti-Fas
Fas-mediated apoptosis involves activation of caspase-8 and its downstream effector, caspase-3, and FLIP inhibits Fas-mediated apoptosis by blocking the activation of caspase-8 (8,9,13). IHC was used to determine whether the level of expression of active caspase-8 and active caspase-3 differed on TECs of FLIP Tg+ and Tg− mice. Active caspase-8 was not detectable on cultured TECs of FLIP Tg− or Tg+ mice (Fig. 3, A and B) or TECs stimulated by agonist anti-Fas or cytokines (IFN-γ and TNF-α) alone (Fig. 3, C–F). When TECs pretreated with IFN-γ and TNF-α were stimulated by anti-Fas, active caspase-8 was detected on more than 90% of TECs of FLIP Tg− mice (Fig. 3G). In contrast, active caspase-8 was detected on less than 30% of TECs of FLIP Tg+ mice, and the intensity of staining was weak (Fig. 3H). The staining pattern of active caspsase-3 was essentially identical with that of active caspase-8 (Fig. 3, I–P). Active caspsase-8+ or active caspsase-3+ cells (red) in five to six randomly selected high-power fields of three wells/group were manually counted, and the results are summarized in Fig. 3Q (active caspase-8) and Fig. 3R (active caspase-3) The results showed that the increased active caspases correlated with the increased apoptosis of TECs from Tg− mice when TECs pretreated with IFN-γ and TNF-α were stimulated by anti-Fas. Transgenic overexpression of FLIP on TECs blocked activation of caspase-8 and caspase-3, thus protecting TECs from Fas-mediated apoptosis.
Figure 3.
Expression of active caspase-8 and active capase-3 on cultured TECs of FLIP Tg+ and Tg− mice treated with IFN-γ and TNF-α and anti-Fas. Cell treatments are shown on the left. The staining pattern of active caspsase-8 (A–H) was identical with that of active caspase-3 (I–P). Active caspase-8+ or caspase-3+ cells (red) in five to six randomly selected high-power fields of three wells/group (magnification, ×400) were manually counted, and the results are summarized (Q and R; bars a–p correspond to A–P). Shown are representative areas on slides. A significant difference in the percentage of caspase-8+ or caspase-3+ cells between Tg− and Tg+ TEC is indicated by the asterisk (P < 0.05). Original magnification, A–H, ×400.
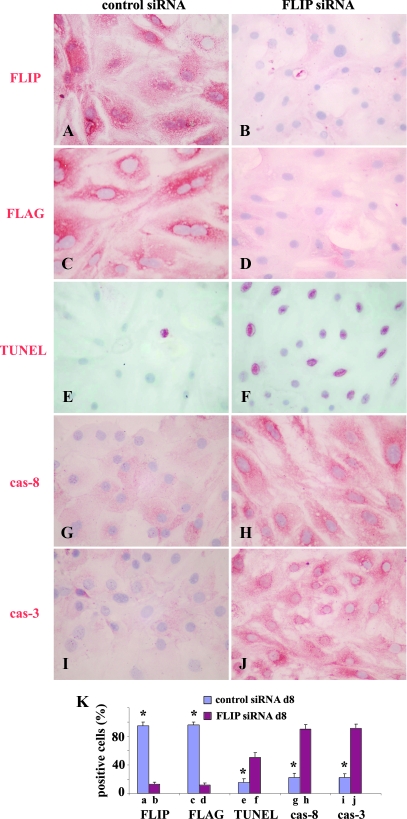
Transfection of cultured TECs of FLIP Tg+ mice with FLIP siRNA abolishes the protective effect of FLIP on Fas-mediated apoptosis
To further determine whether the protective effect of FLIP on Fas-mediated apoptosis is specifically due to overexpression of FLIP, mouse control siRNA and FLIP siRNA were used to transfect cultured TECs of FLIP Tg+ mice. Three days after transfection, FLIP and FLAG expression was examined on cultured TEC in both groups (control siRNA transfected group and FLIP siRNA transfected group). Other TECs in both groups were treated with IFN-γ and TNF-α and agonist anti-Fas. FLIP and FLAG expression, apoptosis, and active caspases were examined 8 d after transfection. FLIP and FLAG were highly expressed on more than 95% of cultured TECs of Tg+ mice 3 (data not shown) and 8 d (Fig. 4, A and C) after transfection with control siRNA, whereas FLIP and FLAG were expressed on less than 20% of TECs from Tg+ mice, and the staining intensity was weak 3 d after transfection with FLIP siRNA, corresponding to the time when IFN-γ and TNF-α treatment began (data not shown). Eight days after transfection with FLIP siRNA, less than 10% of FLIP siRNA transfected Tg+ TECs expressed FLIP and FLAG and the staining intensity was weak (Fig. 4, B and D). Consistent with the FLIP expression level, less than 15% TUNEL+ cells were detected on FLIP Tg+ TECs transfected with control siRNA (Fig. 4E), whereas more than 50% of FLIP Tg+ TECs transfected with FLIP siRNA were TUNEL+ 8 d after transfection (Fig. 4F). Active caspase-8 and active caspase-3 were detected on less than 25% of FLIP Tg+ TECs transfected with control siRNA, and the staining intensity was very weak 8 d after transfection (Fig. 4, G and I). In contrast, active caspases were detected on more than 90% FLIP Tg+ TECs transfected with FLIP siRNA, and the staining intensity was strong 8 d after transfection (Fig. 4, H and J). FLIP+, FLAG+, TUNEL+, active caspsase-8+, or active caspsase-3+ cells (red) in five to six randomly selected high-power fields of three wells/group were manually counted, and the results are summarized in Fig. 4K. The results indicate that the protective effect of FLIP on TEC apoptosis induced by anti-Fas is abolished when the protein expression of FLIP is decreased by FLIP siRNA, suggesting that protection is FLIP specific.
Figure 4.
Transfection of cultured TECs of FLIP Tg+ mice with FLIP siRNA abolishes the protective effect of FLIP on Fas-mediated apoptosis 60–70% confluent cultured TECs of FLIP Tg+ mice were transfected with 10 pmol murine FLIP siRNA (right columns) or control siRNA (left columns). Three days after transfection, TECs were cultured for 4 d with 100 IU/ml IFN-γ and 50 IU/ml TNF-α and then cultured overnight with 1 μg/ml anti-Fas (the time corresponds to 8 d after transfection). Shown are IHC and TUNEL staining 8 d after transfection with control siRNA and FLIP siRNA. FLIP and FLAG staining is shown (A–D), and TUNEL staining is shown (E and F). IHC of activecaspase-8 and active caspase-3 is shown (G–J). FLIP+, FLAG+, TUNEL+, active caspsase-8+, or active caspsase-3+ cells (red) in five to six randomly selected high-power fields of three wells/group were manually counted, and the results are summarized (K, bars a–j correspond to A–J). A significant difference in the percentage of positive cells between Tg− and Tg+ TEC is indicated by the asterisk (P < 0.05). Shown are representative areas on slides. Original magnification, A–H, ×400.
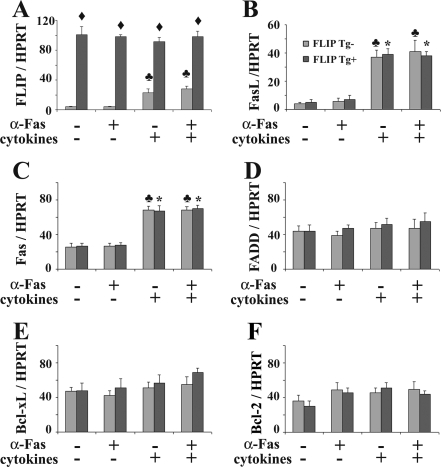
mRNA expression of pro- and antiapoptotic molecules on cultured TECs of FLIP Tg+ and Tg− mice treated with IFN-γ and TNF-α and anti-Fas
The balance between pro- and antiapoptotic molecules plays an important role in apoptosis. To determine whether pro- and antiapoptotic molecules are involved in cytokine-sensitized Fas-mediated apoptosis of TECs, mRNA expression of major pro- and antiapoptotic molecules on cultured TECs of FLIP Tg+ and Tg− mice was determined by RT-PCR. FLIP mRNA was not detected on cultured TEC of FLIP Tg− mice but was highly expressed on cultured TECs of FLIP Tg+ mice. Agonist anti-Fas had no effect on the FLIP mRNA expression level. FLIP mRNA expression by cultured TECs of FLIP Tg− mice was greater in TECs pretreated with IFN-γ and TNF-α, but the level was much lower than on cultured TECs of FLIP Tg+ mice (Fig. 5A). FasL mRNA was undetectable on cultured TECs of FLIP Tg+ or Tg− mice with or without agonist anti-Fas but was comparably increased in both groups when TECs were pretreated with IFN-γ and TNF-α (Fig. 5B). TECs of FLIP Tg+ and Tg− mice did not differ in their expression of FasL or Fas (Fig. 5, B and C). FADD mRNA, B cell leukemia/lymphoma extra long (Bcl-xL) mRNA, and B cellleukemia/lymphoma-2 (Bcl-2) mRNA were also similar on cultured TECs of FLIP Tg+ and Tg− mice with or without treatment with agonist anti-Fas or IFN-γ and TNF-α (Fig. 5, D–F). These results indicate that up-regulation of the proapoptotic molecules FasL and Fas correlated with cytokine-induced sensitization of TECs to Fas-mediated apoptosis. Overexpression of antiapoptotic FLIP protects TECs of FLIP Tg+ from Fas-mediated apoptosis, even though the IFN-γ and TNF-α pretreated TECs have increased FasL and Fas.
Figure 5.
mRNA expression of pro- and antiapoptotic molecules on cultured TECs of FLIP Tg+ and Tg− mice treated with IFN-γ and TNF-α and anti-Fas. mRNA was extracted from cultured TEC of FLIP Tg+ or Tg− mice as described in Materials and Methods. FLIP, FasL, Fas, FADD, Bcl-xL, and Bcl-2 mRNAs are shown (D–F). Results are expressed as the mean ratio of molecule densitometric U to HPRT ± sem (×100) of four to five plates/group and are representative of three independent experiments. A significant difference between cultured TECs of Tg+ and Tg− mice is indicated by the diamond (P < 0.05). A significant difference between cultured TECs of Tg− mice pretreated with and without cytokines is indicated by the club (P < 0.05). A significant difference between cultured TECs of Tg+ mice pretreated with and without cytokines is indicated by the asterisk (P < 0.05).
Discussion
Increasing evidence suggests that FLIP, an inhibitor of caspase-8, plays an important role in many human and murine autoimmune diseases including experimental autoimmune thyroiditis (7,14,15,18,19,26). Previous studies by ourselves and others indicate that transgenic expression of FLIP on TECs has no effect on thyroid histology or function (20,22,27), but expression of transgenic FLIP on TECs promotes earlier resolution of G-EAT in vivo by protecting TECs from Fas-mediated apoptosis (20,22). The results of this study directly demonstrate that cultured TECs from CBA/J or DBA/1 mice are resistant to Fas-mediated apoptosis but can be sensitized in vitro by proinflammatory cytokines to undergo Fas-mediated apoptosis. Overexpression of transgenic FLIP protects cultured TECs from Fas-mediated apoptosis, and FLIP siRNA transfection of cultured TEC of FLIP Tg+ mice abolishes this protective effect. To our knowledge, this is the first report of cytokine-sensitized Fas-mediated apoptosis of mouse TECs and also the first attempt to study whether overexpression of transgenic FLIP protects cultured TECs of FLIP Tg+ mice from Fas-mediated apoptosis.
The mechanism by which the combination of IFN-γ and TNF-α sensitizes TECs to undergo Fas-mediated apoptosis in vitro is still controversial (13,28,29,30). The combination of IFN-γ together with TNF-α or IL-1β facilitates Fas-mediated apoptosis in primary human thyrocytes in vitro, but any one of the cytokines alone is not effective in mediating these effects (28). In this study, few TUNEL+ cells were detected in cultured TECs from FLIP Tg− or Tg+ mice when TECs were stimulated by agonist anti-Fas or cytokines (IFN-γ and TNF-α) alone (Fig. 2, C–F), even at 3-fold higher concentrations (data not shown). When TECs that had been pretreated with IFN-γ and TNF-α for 4 d were stimulated by anti-Fas, many TUNEL+ cells were detected in TECs from FLIP Tg− mice (Fig. 2G), indicating that combinations of IFN-γ and TNF-α (Fig. 2G) or IFN-γ and IL-1β (data not shown) also facilitates Fas-mediated apoptosis in primary murine thyrocytes in vitro. Exposing murine TECs to IFN-γ and TNF-α results in changes in expression or activity of Fas and FasL, molecules involved in the Fas/FasL signaling pathway.
Consistent with our previous in vivo studies (14,15), Fas, but not FasL, was constitutively expressed by murine TECs, and expression of both molecules was up-regulated in response to proinflammatory cytokines (Fig. 5). Kawakami et al. (29) and Bretz et al. (28) also showed that Fas was constitutively expressed on TECs, and Giordano et al. showed that FasL was induced on TEC by proinflammatory cytokines (30). As shown previously for cultured TECs from humans (23), neither anti-Fas nor proinflammatory cytokines alone induced efficient apoptosis of TECs (Fig. 2), whereas the combination of anti-Fas and cytokines induced TEC to undergo apoptosis. Anti-Fas (Jo-2) is an agonist antibody that mimics the function of FasL to cross-link Fas. Fas and FasL mRNA were up-regulated by proinflammatory cytokines alone in both FLIP Tg+ and Tg− TECs (Fig. 5B), but this was apparently not sufficient to induce apoptosis of TECs unless anti-Fas was added to amplify the apoptotic signal (Fig. 2C). If the antiapoptotic molecule FLIP is overexpressed, the TECs are protected from apoptosis. Thus, proinflammatory cytokines apparently facilitate apoptosis of TECs by inducing up-regulation of Fas and FasL (Figs. 2 and 5).
In this study, the decreased susceptibility of TEC of FLIP Tg+ mice to Fas-mediated apoptosis correlated with overexpression of the antiapoptotic molecule FLIP on TECs. Although both Fas and FasL were up-regulated on FLIP Tg+ TEC to trigger the initial apoptosis signal, overexpression of transgenic FLIP inhibits activation of caspase-8, resulting in inactivation of caspase-3. The Fas/FasL pathway is effectively blocked, resulting in decreased apoptosis of TECs of FLIP Tg+ mice, consistent with decreased detection of TUNEL+ cells (Fig. 2) and decreased active caspase-8 and active caspase-3 on TECs of FLIP Tg+ mice (Fig. 3). When FLIP siRNA was transfected into TEC of FLIP Tg+ mice, the up-regulated Fas and FasL induced strong apoptosis of these TECs due to loss of expression of FLIP. The increased apoptosis of FLIP siRNA-transfected TECs of FLIP Tg+ mice is consistent with the higher expression of active caspases on FLIP siRNA-transfected TECs of FLIP Tg+ mice.
The observation that FLIP mRNA on TECs of FLIP Tg− mice is up-regulated to some extent after addition of cytokines (Fig. 5) may be an adaptation response to cell injury to prevent cells from damage. Bcl-xL and Bcl-2, important antiapoptotic molecules belonging to the Bcl-2 family, inhibit apoptosis by regulating mitochondrial membrane potential and cytochrome c release needed for activation of caspase-9 (31,32,33,34). In this study, Bcl-2 and Bcl-xL mRNA was constitutively expressed on TECs, and expression levels of these molecules did not change after treatment with cytokines or anti-Fas (Fig. 5). Because Bcl-2 and Bcl-xL are important apoptotic molecules involved in the mitochondrial signal pathway, they may not contribute to apoptosis induced by death receptor-mediated pathways such as the Fas/FasL pathway in mice as suggested by others (35,36,37).
In summary, the results of this study suggest that proapoptotic molecules such as FasL and Fas are involved in proinflammatory cytokine-sensitized apoptosis of murine cultured TECs. Highly expressed antiapoptotic molecules such as transgenic overexpression of FLIP can protect TECs from apoptosis by blocking apoptotic signal transduction. These in vitro results are consistent with our previous in vivo study on FLIP Tg+ mice (20,22).
Acknowledgments
We thank Dr. Gordon Sharp, Dr. Vincent DeMarco, Dr. Robert Ortmann, and Dr. Shiguang Yu for their helpful discussions.
Footnotes
This work was supported by National Institutes of Health Grant DK35527 and the Arthritis Foundation Eastern Chapter.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 20, 2008
Abbreviations: Bcl-2, B cell leukemia/lymphoma-2; Bcl-xL, B cell leukemia/lymphoma extra long; FADD, Fas-associated death domain; FasL, Fas ligand; FLAG, Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys; FLIP, Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein; G-EAT, granulomatous experimental autoimmune thyroiditis; HPRT, hypoxanthine phosphoribosyltransferase; IFN, interferon; IHC, immunohistochemistry; PCK, pan-cytokeratin; siRNA, small interfering RNA; TEC, thyroid epithelial cell; Tg, transgene or transgenic; TG, thyroglobulin; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick-end labeling.
References
- Wajant H 2002 The Fas signaling pathway: more than a paradigm. Science 296:1635–1636 [DOI] [PubMed] [Google Scholar]
- Thompson CB 1995 Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462 [DOI] [PubMed] [Google Scholar]
- Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A 1995 Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444–448 [DOI] [PubMed] [Google Scholar]
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA 1995 Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270:1189–1192 [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima SI, Sameshima M, Hase A, Seto Y, Nagata S 1991 The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66:233–243 [DOI] [PubMed] [Google Scholar]
- Stassi G, De Maria R 2002 Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol 2:195–204 [DOI] [PubMed] [Google Scholar]
- Stassi G, Di Liberto D, Todaro M, Zeuner A, Ricci-Vitiani L, Stoppacciaro A, Ruco L, Farina F, Zummo G, De Maria R 2000 Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol 1:483–488 [DOI] [PubMed] [Google Scholar]
- Suvannavejh GC, Dal Canto MC, Matis LA, Miller SD 2000 Fas-mediated apoptosis in clinical remissions of relapsing experimental autoimmune encephalomyelitis. J Clin Invest 105:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelko-Downes KA, Cross AH, Russell JH 1999 Dual role for Fas ligand in the initiation of and recovery from experimental allergic encephalomyelitis. J Exp Med 189:1195–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Imagawa A, Hanafusa T, Waguri M, Yamamoto K, Iwahashi H, Moriwaki M, Nakajima H, Miyagawa J, Namba M, Makino S, Nagata S, Kono N, Matsuzawa Y 1997 Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med 186:613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S 1997 Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med 3:738–743 [DOI] [PubMed] [Google Scholar]
- Batteux F, Lores P, Bucchini D, Chiocchia G 2000 Transgenic expression of Fas ligand on thyroid follicular cells prevents autoimmune thyroiditis. J Immunol 164:1681–1688 [DOI] [PubMed] [Google Scholar]
- Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzzo A 1997 Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science 275:960–963 [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen K, Sharp GC, Yagita H, Braley-Mullen H 2001 Expression and regulation of Fas and Fas ligand on thyrocytes and infiltrating cells during induction and resolution of granulomatous experimental autoimmune thyroiditis. J Immunol 167:6678–6686 [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen K, Sharp GC, Braley-Mullen H 2003 FLIP and FasL expression by inflammatory cells vs. thyrocytes can be predictive of chronic inflammation or resolution of autoimmune thyroiditis. Clin Immunol 108:221–233 [DOI] [PubMed] [Google Scholar]
- Tang H, Chen K, Sharp GC, McKee L, Braley-Mullen H 2000 Apoptosis of thyrocytes and effector cells during induction and resolution of granulomatous experimental autoimmune thyroiditis. Int Immunol 12:1629–1639 [DOI] [PubMed] [Google Scholar]
- Thome M, Tschopp J 2001 Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol 1:50–58 [DOI] [PubMed] [Google Scholar]
- Budd RC, Yeh WC, Tschopp J 2006 cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol 6:196–204 [DOI] [PubMed] [Google Scholar]
- Semra YK, Seidi OA, Sharief MK 2001 Overexpression of the apoptosis inhibitor FLIP in T cells correlates with disease activity in multiple sclerosis. J Neuroimmunol 113:268–274 [DOI] [PubMed] [Google Scholar]
- Fang Y, Wei Y, DeMarco V, Chen K, Sharp GC, Braley-Mullen H 2007 Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis. Am J Pathol 170:875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Hu Q, Kristan JM, Costa C, Shen Y, Gero D, Matis LA, Wang Y 2000 Significant role for Fas in the pathogenesis of autoimmune diabetes. J Immunol 164:2523–2532 [DOI] [PubMed] [Google Scholar]
- Fang Y, DeMarco VG, Sharp GC, Braley-Mullen H 2007 Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice. Endocrinology 148:5734–5745 [DOI] [PubMed] [Google Scholar]
- Mezosi E, Wang SH, Utsugi S, Bajnok L, Bretz JD, Gauger PG, Thompson NW, Baker Jr JR 2005 Induction and regulation of Fas-mediated apoptosis in human thyroid epithelial cells. Mol Endocrinol 19:804–811 [DOI] [PubMed] [Google Scholar]
- Jeker LT, Hejazi M, Burek CL, Rose NR, Caturegli P 1999 Mouse thyroid primary culture. Biochem Biophys Res Commun 257:511–515 [DOI] [PubMed] [Google Scholar]
- Li HS, Verginis P, Carayanniotis G 2006 Maturation of dendritic cells by necrotic thyrocytes facilitates induction of experimental autoimmune thyroiditis. Clin Exp Immunol 144:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Irmler M, Thome M 1998 Inhibition of Fas death signals by FLIPs. Curr Opin Immunol 10:552–558 [DOI] [PubMed] [Google Scholar]
- Wang SH, Arscott P, Wu P, Baker Jr JR 2006 No apparent damage in the thyroid of transgenic mice expressing antiapoptotic FLIP. Thyroid 16:1–8 [DOI] [PubMed] [Google Scholar]
- Bretz JD, Arscott PL, Myc A, Baker Jr JR 1999 Inflammatory cytokine regulation of Fas-mediated apoptosis in thyroid follicular cells. J Biol Chem 274:25433–25438 [DOI] [PubMed] [Google Scholar]
- Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Kawabe Y, Ishikawa N, Ito K, Nagataki S 1996 Thyroid-stimulating hormone inhibits Fas antigen-mediated apoptosis of human thyrocytes in vitro. Endocrinology 137:3163–3169 [DOI] [PubMed] [Google Scholar]
- Stassi G, Todaro M, Bucchieri F, Stoppacciaro A, Farina F, Zummo G, Testi R, De Maria R 1999 Fas/Fas ligand-driven T cell apoptosis as a consequence of ineffective thyroid immunoprivilege in Hashimoto’s thyroiditis. J Immunol 162:263–267 [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB 1997 Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91:627–637 [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X 1997 Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129–1132 [DOI] [PubMed] [Google Scholar]
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB 1993 Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597–608 [DOI] [PubMed] [Google Scholar]
- Gottschalk AR, Boise LH, Thompson CB, Quintans J 1994 Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc Natl Acad Sci USA 91:7350–7354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steemans M, Goossens V, Van de Craen M, Van Herreweghe F, Vancompernolle K, De Vos K, Vandenabeele P, Grooten J 1998 A caspase-activated factor (CAF) induces mitochondrial membrane depolarization and cytochrome c release by a nonproteolytic mechanism. J Exp Med 188:2193–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME 1998 Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G 1997 The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med 186:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]