Abstract
Immunocytochemical studies have shown that nuclear and extranuclear estrogen receptors (ERs) are present in several extrahypothalamic brain regions. The goal of this study was to determine the subcellular location of functional ERs, particularly extranuclear ERs, by demonstrating 125I-estradiol binding in the rat forebrain and medullary sections prepared for light and electron microscopic autoradiography. Some sections were immunocytochemically labeled with the catecholamine-synthesizing enzyme, tyrosine hydroxylase (TH), prior to the autoradiographic procedure. By light microscopy, dense accumulations of silver grains denoting 125I-estradiol binding were observed over cells in the ventromedial and arcuate hypothalamic nuclei, amygdala, and nucleus of the solitary tract. In sections labeled for TH, large accumulations of silver grains were admixed with TH-labeled processes in the medial nucleus of the amygdala and over TH-labeled perikarya in the medial and commissural nucleus of the solitary tract. Electron microscopic analyses were focused on the rostral ventrolateral medulla and the hippocampal CA1 region, two regions previously shown to have extranuclear ERs. In the rostral ventrolateral medulla, silver grains indicative of 125I-estradiol binding were found within a few large terminals, affiliated with mitochondria. In the hippocampus, autoradiographic silver grains denoting 125I-estradiol binding were associated with mitochondria in dendritic shafts or were near synaptic specializations on dendritic spines. These patterns of silver grain labeling were not seen in sections from rats that received 125I-estradiol combined with cold estradiol. The association of 125I-estradiol binding with pre- and postsynaptic profiles supports a functional role for nonnuclear ERs in brain.
ESTROGENS CAN AFFECT the structure and function of many extrahypothalamic brain regions. In particular, estrogen can promote the formation of dendritic spines and alter neuronal excitability in the rat hippocampal CA1 region (for review see Ref. 1). In the nucleus of the solitary tract (NTS) and rostral ventrolateral medulla (RVLM), estrogens can modulate sympathetic tone and modulate calcium currents (2,3). Previously we demonstrated using immunocytochemical methods that estrogen receptors (ERs) are present in nuclear as well as extranuclear sites in the hippocampal formation and the RVLM (3,4,5). These immunocytochemical studies identified the total pool of ER subtypes that are present in these brain areas. However, only a subset of these receptors are likely to bind estrogens; other ERs are either in reserve pools or are in the process of transport to and from the cell nucleus and extranuclear sites and may be in conformations that do not recognize estrogen.
Early studies using 3H-estradiol showed a distribution of estrogen binding in the hippocampal formation that was limited to interneuron cell nuclei (6). However, the use of the 125I isotope allowed for the achievement of sensitive, discrete localization of estrogen binding in the rat hippocampal formation (7,8). Notably, additional 125I-estradiol binding was detected in pyramidal cell somata as well as in fields containing pyramidal cell dendrites. However, whether 125I-estradiol binding was localized in discrete extranuclear sites could not be resolved by light microscopy. Thus, the goal of this study was to determine the location of potentially functional ERs, particularly extranuclear ERs, by demonstrating 125I-estradiol binding in the rat forebrain and medullary sections prepared for light and electron microscopic autoradiography.
Materials and Methods
Experimental animals
Female (n = 15; 125–150 g) young, adult ovariectomized Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used. All rats were housed with 12-h light, 12-h dark cycles (lights on 0600–1800 h) and fed a diet reduced in estrogen content (diet no. TD96155l Harlan Teklabs, Madison, WI). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Weill-Cornell Medical College and Merck Research Laboratories Institutional Animal Care and Use Committees.
Procedures for 125I-estradiol (17α-iodo-vinyl-11β-methoxyestradiol, a generous gift of Dr. R. Hanson, Northeastern University, Boston MA) synthesis and administration were similar to those described previously by Shughrue and Merchenthaler (8). In brief, 125I-estradiol (specific activity 2200 Ci/mmol; 2 μg/kg body weight; ∼0.5–1 mCi) in 50% dimethylsulfoxide, 40% saline, and 10% ethanol (200 μl) was administered sc to female rats that had been ovariectomized for approximately 2 wk. To assess nonspecific binding, six of the rats received injections of cold (nonradiolabeled) estradiol (250 μg/kg), and the other nine received sesame oil vehicle (100 μl) 30 min before the 125I-estradiol injection. One or 4 h after the 125I-estradiol injection, the rats were anesthetized with ketamine/xylazine (10 and 200 mg/kg, respectively, ip) and perfused through the ascending aorta sequentially with 10–15 ml saline (0.9%) containing 1000 U of heparin and then 250 ml of one of the following: 1) 4% paraformaldehyde in 0.1 m phosphate buffer (PB; pH 7.6) [the fixative used by others for 125I-estradiol binding at the light microscopic level (8)]; 2) 3.75% acrolein and 2% paraformaldehyde in PB [the fix we routinely use for immunocytochemistry and electron microscopy (5)]; or 3) 2% acrolein and 2% paraformaldehyde in PB (vehicle: n = 7; cold estradiol: n = 4). The brains were removed from the skull and postfixed in 2% paraformaldehyde overnight. Brains were blocked coronally into forebrain and hindbrain regions and sectioned 40 μm thick with a vibrating microtome (Vibratome; Leica, Wien, Austria) and collected into cold PB.
Autoradiographic procedures
For pilot studies determining the effect of fixation condition on 125I-estradiol binding, whole brains were frozen and forebrain and hindbrain sections (20 μm thick) were cut on a cryostat, and the sections were thaw mounted on slides. The slides were apposed to Kodak BioMax MR film (Fisher Scientific, Pittsburgh, PA) for 4–7 d. Radioactive microscale standards (catalog no. RPA504L, C14; Amersham, Arlington Heights, VA) were used to ensure that the signal was in the linear range of the film.
Light and electron microscopic autoradiographic procedures were a modification of those described previously (9). Sections for light microscopy were mounted out of cold 0.05 m PB on slides that had been cleaned in chromic acid and coated with 1% gelatin (J. T. Baker Chemicals, Philipsburg, NJ) and 0.05% chromium potassium sulfate. After drying, sections were dipped in cold 0.05% 175 bloom gelatin (catalog no. 16562; Electron Microscopy Sciences) to prevent radioligand leaching (8) and then air dried. Sections were not dehydrated before autoradiographic procedures because we found that this resulted in diffusion of the isotope. In a darkroom with more than 50% humidity, slides were dipped in Ilford L-4 photographic emulsion (Polysciences, Inc., Warrington, PA) diluted 1:1 with water. Slides were separated by lead foil and stored in light tight boxes containing desiccant at 4 C. After an experimentally determined interval (ranging from 1 to 6 months), the autoradiographs were developed in D-19 (Kodak, Rochester, NY) for 3.5 min at 17 C and fixed in Polymax T diluted 1:3 with water (Kodak) for 8 min. Sections were rinsed for 1 h in distilled water, dehydrated and coverslipped in DPX (Sigma-Aldrich, St. Louis, MO). Half of the tissue autoradiograms were counterstained with 0.25% Thionin (Sigma-Aldrich) before dehydration.
Sections destined for electron microscopic autoradiography were postfixed for 1 h in 2% osmium tetroxide, dehydrated through alcohols and propylene oxide, and embedded between two sheets of plastic in EMbed 812 (EMS, Old Saybrook, CT). Ultrathin sections (70 nm thick) from either the dorsal CA1 region of the hippocampus or the C1 area of the RVLM [approximately levels 32 and 61 of Swanson (10), respectively] were cut on an ultratome (Ultracut; Leica). The sections were collected on parlodion-coated slides and counterstained with uranyl acetate and Reynold’s lead citrate. Sections then were coated with eight 1-sec pulses of carbon on a carbon coater (WCMC Imaging Core, New York, NY). Slides were dipped in Ilford L-4 emulsion diluted 1:4 and stored as described above. After 12–24 months of exposure, the autoradiograms were developed in Microdol-X (Kodak) for 4 min at 17 C and fixed in 30% sodium thiosulfate for 8 min. The parlodion coating was floated onto a water bath, and the thin sections were picked up on nickel grids. Sections were examined by electron microscopy after thinning the parlodion membrane with amyl acetate.
Immunocytochemistry
Before the light microscopic autoradiographic procedures, some sections through select brain areas were processed for the immunocytochemical localization of tyrosine hydroxylase (TH). For this, sections were treated with 1% sodium borohydride in PB for 30 min, incubated in 0.5% BSA in 0.1 m Tris-saline (TS; pH 7.6) for 30 min. Sections were placed in TH antiserum [1:2000; Incstar, Stillwater, MN; characterized previously (3)] and 0.1% BSA in TS for 1 d at room temperature. The sections then were incubated in the following: 1) biotinylated goat antirabbit IgG in 0.1% BSA (1:400; Vector Labs, Burlingame, CA) for 30 min; 2) avidin-biotin complex for 30 min; and 3) diaminobenzidine (Aldrich, Milwaukee, WI) and H2O2 for 6 min. All incubations were separated by washes of TS.
Analysis
For light microscopy, sections were photographed with a Nikon E800 light microscope equipped with bright field, dark field and DIC optics and a Micropublisher digital camera (Q-imaging, Barnaby, British Columbia, Canada). Final electron microscopic preparations were analyzed on a FEI Tecnai Biotwin transmission electron microscope equipped with a digital camera system (software version 3.2, Advanced Microscopy Techniques, Danvers, MA). To prepare figures, the levels, sharpness, brightness, and contrast were adjusted in Adobe Photoshop 7.0 (Adobe Systems, New York, NY) on an Apple Power Macintosh G5 computer (New York, NY). Final figures were assembled in Quark Xpress 6.1 (Denver, CO).
The subcellular location of 125I-estradiol binding was examined from one hippocampal and one RVLM block from four rats each. Criteria for defining labeled profiles was similar to that described previously (11). Briefly, profiles were considered labeled if they contained more than one silver grain that either was not in a straight line and/or verified in adjacent or semiserial (on same grid) sections. Profiles containing autoradiographic silver grains were classified according to the nomenclature of Peters et al. (12). Dendrite profiles contained regular microtubule arrays and usually were postsynaptic to axon terminal profiles. Terminal profiles had minimal diameters greater than 0.2 μm, contained numerous small synaptic vesicles, sometimes contained large dense-core vesicles, and often contacted other neuronal profiles.
Results
The degree of 125I-estradiol binding was sensitive to fixation conditions
Previous studies localized 125I-estradiol binding in tissue prepared for light microscopic autoradiography. Thus, the goal of the first portion of this study was to determine conditions for 125I-estradiol binding that were compatible with electron microscopy as well as immunocytochemistry. As described in more detail in Materials and Methods, 125I-estradiol (∼0.5–1 mCi) was administered in vivo to 15 ovariectomized rats. To control for specificity of the labeling, six of the rats received injections of cold (nonradiolabeled) estradiol (250 μg/kg) 30 min before the 125I-estradiol injection. Three fixation conditions initially were used: 1) 4% paraformaldehyde; 2) 3.75% acrolein and 2% paraformaldehyde; or 3) 2% acrolein and 2% paraformaldehyde.
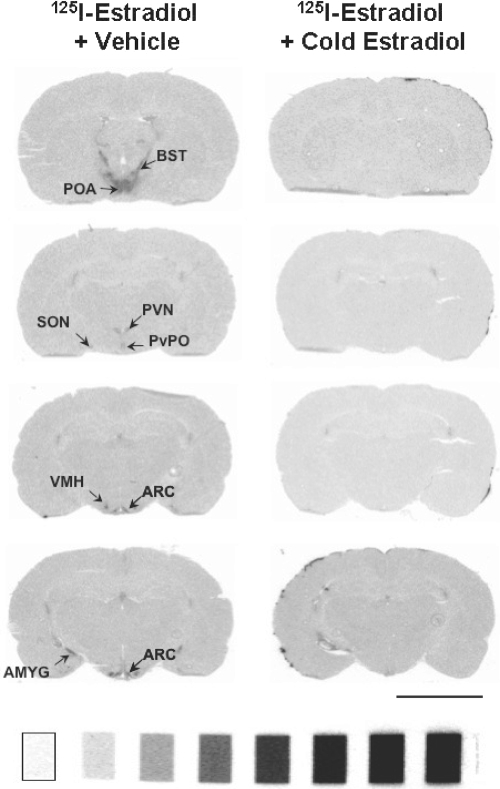
When assessed with film autoradiography, the most intense specific 125I-estradiol binding was observed in the hypothalamus of sections fixed with 4% paraformaldehyde (Fig. 1). Specific labeling was detected in the preoptic area, bed nucleus of stria terminalis, and the paraventricular, periventricular, arcuate, and ventromedial nuclei of the hypothalamus and in the amygdala. Labeling also was noted in the periaqueductal gray (data not shown). Tissue fixed with 3.75% acrolein and 2% paraformaldehyde yielded a less intense signal. Sections fixed with 2% acrolein and 2% paraformaldehyde yielded a signal that was nearly equivalent to the signal seen in the 4% paraformaldehyde fixed tissue. No specific binding was seen in the hypothalamus or amygdala of rats that received nonradioactive (i.e. cold) estradiol before the 125I-estradiol injection, regardless of fixation condition (Fig. 1). Because the 2% acrolein and 2% paraformaldehyde fixative is compatible with electron microscopy and immunocytochemistry, subsequent studies used this fixative.
Figure 1.
Specific 125I-estradiol binding can be detected in the rat hypothalamus and amygdala. Film autoradiograms of coronal forebrain sections from ovariectomized rats treated with 125I-estradiol (1 mCi) after pretreatment with vehicle (left) or unlabeled (cold) estradiol (250 μg/kg; right). 125I-estradiol binding was apparent in several hypothalamic nuclei and the amygdala. Rats treated with cold estradiol before 125I-estradiol had no detectable binding. 14C microscales: range 1.15–32.7 kBq/g, 31–883 nCi/g. AMYG, Amygdala; ARC, arcuate nucleus; BST, bed nucleus of the stria terminalis; POA, preoptic area; PVN, paraventricular nucleus of the hypothalamus; PvPO, periventricular hypothalamus; SON, supraoptic nucleus; VMH, ventromedial hypothalamus. Bar, 5 mm.
By light microscopy, 125I-estradiol binding was in brain regions known to contain ERs
Free-floating sections from animals injected with 125I-estradiol (with or without cold estradiol) were mounted on slides and prepared for light microscopic autoradiography. Some sections were immunocytochemically labeled with the catecholamine-synthesizing enzyme, TH, before the autoradiographic procedure.
Consistent with previous studies (7), exposure periods of 6 wk yielded dense accumulations of silver grains denoting 125I-estradiol binding over cells in the ventromedial and arcuate hypothalamic nuclei (Fig. 2A). At high magnifications distinct clusters of silver grains could be distinguished over Nissl-stained nuclei (Fig. 2B). This pattern of labeling was absent in sections through the arcuate from rats administered cold estradiol before the 125I-estradiol injection (supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://endo.endojournals.org).
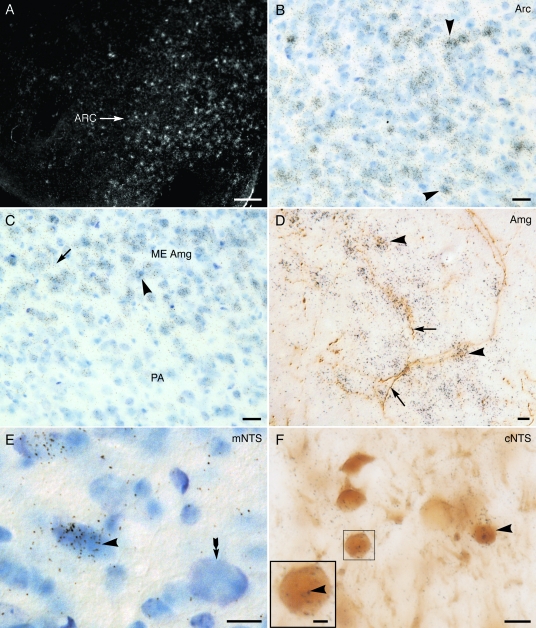
Figure 2.
Light microscopic localization of 125I-estradiol binding in select brain areas. A, Low-magnification, dark-field photomicrograph showing autoradiographic silver grains (white) indicative of 125I-estradiol binding over the arcuate nucleus (ARC). B, Higher-magnification, bright-field photomicrograph of ARC region in A shows dense accumulations of autoradiographic silver grains over Nissl stained nuclei (examples, arrowheads). C, Bright-field photomicrograph showing dense accumulation of silver grains over (example, arrowhead) and between (example, arrow) Nissl stained nuclei in the medial (ME), but not the posterior (PA), nucleus of the amygdala (Amg). D, In this bright-field photomicrograph from the medial nucleus of the amygdala, autoradiographic silver grains (examples, arrowhead) indicative of 125I-estradiol binding overlap TH-labeled processes (brown; examples, arrow). E, In the medial NTS (mNTS), autoradiographic silver grains (black) are concentrated over a Nissl-stained cell (blue; example, arrowhead). A Nissl-stained cell lacking 125I-estradiol binding (arrow) is shown for comparison. F, In a dual-labeled section of the cNTS, silver grains indicative of 125I-estradiol binding are over TH-labeled neuronal perikarya (arrowhead). Boxed region is enlarged to show silver grains (example, arrowhead) over a representative TH-labeled (brown) neuron. Autoradiographic exposure time (A–C), 2.5; (D–F), 5 months. Bar (A), 1 mm; (B–F), 25 μm; (inset, F), 12 μm.
Longer exposure periods (3–6 months) revealed 125I-estradiol binding in medial nucleus of the amygdala (Fig. 2, C and D), the hippocampal formation (supplemental Fig. 1E), and the NTS (Fig. 2, E and F and supplemental Fig. 1G). In Nissl-stained sections, silver grains were clustered over cell nuclei as well as interspersed between cells in the medial nucleus of the amygdala (Fig. 2C; supplemental Fig. 1C). Both types of labeling were not apparent in the adjacent posterior nucleus of the amygdala (Fig. 2C) and in sections from rats administered cold estradiol before the 125I-estradiol injection (supplemental Fig. 1B). In sections labeled for TH, 125I-estradiol silver grains had an overlapping distribution with TH-labeled processes in the medial nucleus of the amygdala (Fig. 2D). However, 125I-estradiol silver grains did not overlap TH-labeled fibers in the medial amygdala from rats administered cold estradiol before the 125I-estradiol injection (supplemental Fig. 1D) or in the nearby piriform cortex from 125I-estradiol-injected rats (supplemental Fig. 1H). Consistent with previous reports in the hippocampal formation (7), silver grains were found over principal cell layers and in strata radiatum and oriens, particularly the CA3 region (supplemental Fig. 1E). Accumulations of silver grains over pyramidal cell bodies in the hippocampal formation were not apparent in rats that had been injected with cold estradiol before 125I-estradiol (supplemental Fig. 1F). In the NTS, particularly the commissural and medial regions, clusters of silver grains could be detected over the nuclei of Nissl-stained cells (Fig. 2E). Dual-labeling studies revealed that many of the 125I-estradiol-labeled nuclei in the commissural region were in TH-labeled neurons (Fig. 2F). Fewer TH-labeled cells in the medial NTS contained autoradiographic silver grains (supplemental Fig. 1G). In the amygdala and NTS, the intensity of the autoradiographic signal in dually labeled sections was noticeably less than in the sections processed for autoradiographic labeling only.
By electron microscopy, 125I-estradiol binding additionally was detected at extranuclear sites
Previously we demonstrated ER immunoreactivity in extranuclear sites in the RVLM and hippocampal formation (3,4,5). However, anatomical evidence for estrogen binding to these nongenomic ERs is lacking. Thus, to determine whether 125I-estradiol binding is also at extranuclear sites, sections from rats injected with 125I-estradiol were prepared for electron microscopic autoradiography (described in detail in Materials and Methods). Because the light microscopic studies showed that 125I-estradiol binding was diminished in sections processed for TH, sections for electron microscopy were labeled only for 125I-estradiol binding. Optimal exposure times for the autoradiographic preparations ranged from 12 to 14 months.
At the electron microscopic level, silver grains indicative of 125I-estradiol binding were found within large terminals in the RVLM (Fig. 3A; confirmed in semiserial section, see supplemental Fig. 2A). Within terminals, the silver grains were over clusters of mitochondria. The labeled terminals were usually large (>1.0 μm) and did not form synaptic contacts in the plane of the section analyzed. Silver grains were not detected in postsynaptic profiles in the RVLM sections examined.
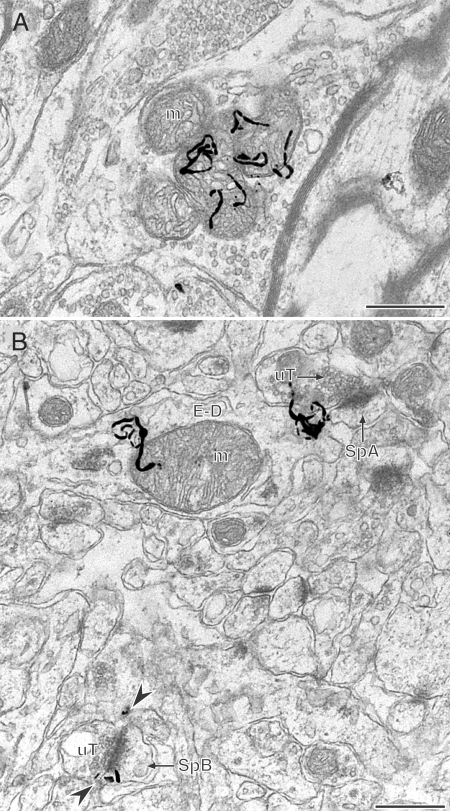
Figure 3.
By electron microscopy, 125I-estradiol binding is detected at extranuclear sites. A, In the rostral ventrolateral medulla autoradiographic silver grains (black squiggly lines) denoting 125I-estradiol binding are found in a large terminal containing numerous mitochondria (m). B, In stratum radiatum of the CA1 region of the hippocampus, autoradiographic silver grains denoting 125I-estradiol binding are in a dendrite (E-D) overlying a mitochondrion (m) and near the neck of a dendritic spine (SpA) that is contacted by an unlabeled terminal (uT). In the same field, four autoradiographic silver grains (arrowheads) are adjacent to the perisynaptic regions of a small dendritic spine (SpB) that is contacted by a uT. Semiserial sections confirming labeling are shown in supplemental Fig. 2, A and B. Autoradiographic exposure time was 1 yr. Bars, 500 nm.
Autoradiographic silver grains denoting 125I-estradiol binding were found in dendritic profiles in the CA1 region of the hippocampus. In dendritic shafts, the silver grains were associated with mitochondria (Fig. 3B; confirmed in semiserial section, supplemental Fig. 2B). In dendritic spines, silver grains were in the neck region (Fig. 3B, spine A) or near the perisynaptic regions of the synaptic specializations (Fig. 3B, spine B). Whenever possible, the labeling was confirmed in serial sections (an example of confirmation of spine labeling in a semiserial section is shown in supplemental Fig. 2, C and D). However, when labeling could not be confirmed in serial sections, the presence or two or more silver grains over a profile was considered specific labeling (e.g. Fig. 3B, spine B). Labeled spines usually were contacted by terminals that formed asymmetric synapses.
The labeling in the RVLM and hippocampus appeared to be specific because control sections examined by electron microscopy had almost no silver grains.
Discussion
The present study provides the first evidence that extranuclear ERs can bind ligand in vivo and thus supports a functional role for these receptors in estrogen-mediated plasticity within neuronal populations that express them, including the RVLM and hippocampus.
Technical considerations
The 125I isotope has a much higher specific radioactivity than 3H, thus permitting the detection of low levels of binding sites (8,13). The estrogen binding that we observed was specific because it was not observed in rats that were preadministered nonradioactive estradiol.
Several factors affected the detectable signal of 125I-estradiol binding. First, addition of 3.75% acrolein to the paraformaldehyde fixative severely diminished the amount of 125I-estradiol binding detected in the hypothalamus. However, the reduction of the acrolein to 2% appeared to yield comparable 125I-estradiol binding as that seen with 4% paraformaldehyde. Second, dehydration of the sections mounted on slides resulted in diffusion of the isotope. Thus, the dehydration step was omitted, allowing for optimal detection of the isotope but likely increasing nonspecific background (11). Third, the immunocytochemical processing appeared to diminish the detectability of 125I-estradiol binding in brain areas that had low levels of nuclear estrogen receptors (e.g. NTS). All of these factors likely contributed to the long autoradiographic exposure times necessary for visualizing the 125I signal at both the light (2–6 months) and electron (12–14 months) microscope level.
At the ultrastructural level, the appearance of 125I-estrogen binding resembled that of 125I-labeled secondary antibodies (14,15) and 125I-neurotensin receptor binding (13). The autoradiographic silver grains usually diffusely radiated from the binding source. Thus, the profiles were considered labeled only if they contained two or more silver grains either within the boundaries of their plasmalemma or on adjacent (or semiserial) sections. Fortunately, the nonspecific binding was low as assessed from the control autoradiograms, allowing for discrimination of specific labeling.
125I-estradiol binds nuclei at hypothalamic and extrahypothalamic sites
Consistent with previous studies (8), 125I-estradiol binding was dense in hypothalamic nuclei as assessed by both ultrafilm and in emulsion dipped slides. The present studies also revealed binding of 125I-estradiol over nuclei in the amygdala and NTS. Because 125I-estradiol binds with equal affinity to both the ERα and ERβ (7,8), the localization of estrogen binding in these regions does not discriminate between ER subtype. Both ERα and ERβ protein and mRNA have been reported in nuclei in the amygdala (16,17,18) as well as the NTS in rat and mouse (19,20,21).
In addition to localization of 125I-estradiol binding over cell nuclei in the medial nucleus of the amygdala, binding also was observed over neuronal processes labeled for TH. To our knowledge, this is the first report of possible extranuclear ERs in the amygdala. These data suggest that estradiol may act in this region to acutely regulate function including catecholaminergic afferents. Interestingly, this region of the amygdala is crucial in the expression of sex-specific social behaviors and has morphological differences in male and female rats (22,23).
In the NTS, particularly the commissural portion, some 125I-estradiol binding was located over nuclei identified as in catecholaminergic neurons by the presence of TH. This observation is consistent with our recent immunocytochemical studies showing ERα immunoreactive nuclei are mostly found in the commissural NTS (cNTS) of Sprague Dawley rats and that more than half are found in catecholaminergic neurons (our unpublished observations). Neurons in the cNTS, including catecholaminergic neurons, receive primarily chemoreceptive afferents, important for modulating responses to hypoxia (24), and project directly to the RVLM, which is critical for regulating baroreceptor output to the spinal cord (25). Moreover, catecholaminergic neurons in the NTS are connected with several autonomic-endocrine areas including the pontine parabrachial nucleus, hypothalamus, and amygdala (26). The present findings lend support to the idea that catecholaminergic neurons in the NTS are functionally activated by estrogens to affect cardiovascular-endocrine coordinating responses.
125I-estradiol binding is found at extranuclear sites
Electron microscopic autoradiography further revealed estrogen binding sites in extranuclear sites in the RVLM and hippocampus.
In the RVLM, 125I-estradiol binding was detected in presynaptic but not postsynaptic profiles. The lack of detection of binding in postsynaptic profiles could be due to technical factors, including the lack of dual labeling with TH to discriminate C1 catecholaminergic neuronal profiles or relatively lower levels of ERs in the RVLM, compared with the NTS (Milner, T. A., unpublished observations). Alternatively, it could suggest that 125I-estradiol is less able to detect extranuclear ERβ because our previous studies showed that this ER subtype predominates at postsynaptic sites in the RVLM (3). However, 125I-estradiol binding was detected readily in the paraventricular nucleus of the hypothalamus that, in the rat, expresses nuclear ERβ almost exclusively indicating that nuclear binding to that ER subtype was not impaired. Alternatively, an ERβ isoform reported to have reduced estrogen binding capacity (27) may predominate within postsynaptic sites in the RVLM as the antibodies used in our previous studies may not distinguish between isoforms. The detection of 125I-estradiol binding in axon terminals is congruent with immunocytochemical detection of ERs in the RVLM (3). Notably, 125I-estradiol binding was concentrated over mitochondria structures important in regulating cell metabolism and Ca2+ signaling (28). The affiliation of 125I-estradiol with mitochondria suggests that ERs may influence mitochondrial function (29). Overall, these findings support the notion that estrogens could rapidly regulate presynaptic profiles in this important cardiovascular region.
In the hippocampus, 125I-estradiol binding was detected in dendritic profiles, particularly in association with dendritic spines. This finding in consistent with our previous studies demonstrating that both ERα and ERβ immunoreactivities are found in dendritic spines in the hippocampal CA1 region (4,5,30). Notably, 125I-estradiol binding was found in the perisynaptic zone, similar to what we have observed previously for ERα immunoreactivity with postembedding electron microscopy (30). As reviewed by Woolley (1), estradiol can rapidly alter hippocampal neuronal firing rates and/or K+ currents to control the resting membrane potential or limit action potentials. These findings suggest that at least some of the extranuclear ERs are functional lending support to the notion that estradiol can act directly at postsynaptic sites to alter neuronal physiology.
In summary, these studies have confirmed the presence of nuclear estrogen binding in hypothalamus and extrahypothalamic regions as well as a newly documented area for extranuclear ERs, the medial amygdala. Moreover, the detection of 125I-estradiol labeling in discrete pre- and postsynaptic profiles in extrahypothalamic regions well established to be estradiol sensitive supports the estrogen binding capability and functionality of ERs expressed within discrete subcellular regions outside of the nucleus.
Acknowledgments
We thank Ms. Nora Tabori, Ms. Lee Cohen-Gould, Ms. Katie Mitterling, and Mr. Peter Zafian for technical assistance and Dr. Elizabeth Waters for her helpful suggestions. We are grateful to Dr. R. Hanson (Northeastern University, Boston, MA) for supplying the 17α-iodo-vinyl-11β-methoxyestradiol.
Footnotes
This work was supported by National Institutes of Health Grants NS07080 (to B.S.M.), DA08259 (to T.A.M.), and HL18974 (to T.A.M.).
Disclosure Summary: T.A.M., L.S.L., and S.E.A. have nothing to declare. Both L.S.L. and S.E.A. are employed by Merck & Co., where some of the work was performed. B.S.M. consults for Merck (less than $10,000).
First Published Online March 20, 2008
Abbreviations: cNTS, Commissural NTS; ER, estrogen receptor; NTS, nucleus of the solitary tract; PB, phosphate buffer; RVLM, rostral ventrolateral medulla; TH, tyrosine hydroxylase; TS, Tris-saline.
References
- Woolley CS 2007 Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47:657–680 [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ 2007 Role of oestrogen in the central regulation of autonomic function. Clin Exp Pharmacol Physiol 34:1–6 [DOI] [PubMed] [Google Scholar]
- Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA 2006 Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res 1094:163–178 [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE 2005 Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J Comp Neurol 491:81–95 [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE 2001 Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol 429:355–371 [PubMed] [Google Scholar]
- Loy R, Gerlach JL, McEwen BS 1989 Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Dev Brain Res 39:245–251 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I 2000 Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol 21:95–101 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I 2000 Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience 99:605–612 [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Milner TA 1986 Autoradiographic detection of 125I-secondary antiserum: a sensitive light and electron microscopic method compatible with peroxidase immunocytochemistry for dual localization of neuronal antigens. J Histochem Cytochem 34:707–718 [DOI] [PubMed] [Google Scholar]
- Swanson LW 1992 Brain maps: structure of the rat brain. 1st ed. Amsterdam: Elsevier [Google Scholar]
- Pickel VM, Milner TA 1989 Interchangeable uses of autoradiographic and peroxidase markers for electron microscopic dual labeling of axonal transport and immunocytochemistry. In: Heimer L, Zaborszky L, eds. Neuroanatomical tract-tracing methods II: recent progress. New York: Plenum; 97–127 [Google Scholar]
- Peters A, Palay SL, Webster Hd 1991 The fine structure of the nervous system. 3rd ed. New York: Oxford University Press [Google Scholar]
- Boudin H, Pélaprat D, Rostène W, Pickel VM, Beaudet A 1998 Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. J Neurosci 18:8473–8484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Bacon CE 1989 GABA-ergic neurons in the rat hippocampal formation: ultrastructure and synaptic relationships with catecholaminergic terminals. J Neurosci 9:3410–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Pickel VM, Reis DJ 1989 Ultrastructural basis for interactions between central opioids and catecholamines: I. Rostral ventrolateral medulla. J Neurosci 9:2114–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ 2003 Distribution of estrogen receptor α and β immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res 145:117–139 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I 2001 Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol 436:64–81 [PubMed] [Google Scholar]
- Haywood SA, Simonian SX, Van der Beek EM, Bicknell RJ, Herbison AE 1999 Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology 140:3255–3263 [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE 2003 Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology 144:2055–2067 [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B 2005 Estrogen receptor-α and -β immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol 488:152–179 [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS 2007 Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol 501:904–915 [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS 2005 Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci 25:10759–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero DA, Pickel VM, Milner TA, Anwar M, Otake K, Mtui EP, Park D 1994 Viscerosensory processing in nucleus tractus solitari: structural and neurochemical substrates. In: Barraco RA, ed. Nucleus of the solitary tract. Boca Raton, FL: CRC Press; 3–34 [Google Scholar]
- Aicher SA, Milner TA, Pickel VM, Reis DJ 2000 Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull 51:107–110 [DOI] [PubMed] [Google Scholar]
- Saper CB 2002 The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25:433–469 [DOI] [PubMed] [Google Scholar]
- Price Jr RH, Lorenzon N, Handa RJ 2000 Differential expression of estrogen receptor β splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Mol Brain Res 80:260–268 [DOI] [PubMed] [Google Scholar]
- Mironov SL, Ivannikov MV, Johansson M 2005 [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules: from mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J Biol Chem 280:715–721 [DOI] [PubMed] [Google Scholar]
- Yager JD, Chen JQ 2007 Mitochondrial estrogen receptors—new insights into specific functions. Trends Endocrinol Metab 18:89–91 [DOI] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH 2002 Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci 22:3608–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]