Abstract
The central melanocortin system is a critical contributor to energy balance control. Melanocortin receptors (MC-Rs) are widely distributed throughout forebrain and caudal brainstem nuclei. To assess the contribution of hindbrain MC-Rs to the control of energy expenditure, the MC3/4R agonist melanotan II (MTII) was delivered to either the fourth ventricle or medullary raphe of neurologically intact rats and chronic decerebrate (CD) rats, and interscapular brown adipose tissue (IBAT) temperature (TIBAT), core temperature (TC), heart rate (HR), and spontaneous activity were recorded. Fourth ventricular MTII (0.1, 1.0 nmol) significantly increased TIBAT, TC, and HR in intact rats (TC: +0.33 ± 0.08, +0.41 ± 0.09 C; HR: +40.84 ± 7.29, +69.04 ± 6.83 beats per minute) and in CDs (TC: +1.39 ± 0.67, +1.52 ± 0.37 C; HR: +83.21 ± 19.2, +107.38 ± 17.65 beats per minute). Response magnitude was greater in CD rats than in neurologically intact rats. TIBAT, TC, and HR were significantly increased after 10 pmol MTII delivery to the medullary raphe of intact rats, and here too, the response magnitude was greater in decerebrate rats. The hyperthermia, IBAT thermogenesis, and tachycardia observed in CD rats after fourth ventricular and hindbrain parenchymal MTII injections support the hypothesis that hindbrain MC-R stimulation engages endemic circuits that link sympathetic outflows to thermogenic and cardiac effectors, and that forebrain processing and forebrain-caudal brainstem communication are not required for response production.
MELANOCORTIN ligands and receptors are an essential component of the central nervous system control of energy balance. Mutations of the genes for the melanocortin 4 receptor (MC4-R) or the ligand precursor, proopiomelanocortin (POMC), are associated with severe obesity in humans (1,2,3). In rodent models, agonist stimulation (pharmacological or genetic) of MC4-R potently reduces food intake and body weight, whereas antagonism of the MC4-R results in hyperphagia and obesity (4,5,6). Although MC-R (MC4-R, MC3-R) treatment-induced changes in body weight are typically ascribed to changes in food intake, other data suggest that MC-R effects on body weight may also be mediated by alterations in energy expenditure. For example, increased energy expenditure follows peripheral or forebrain ventricular application of MC-R agonists (7,8,9), whereas reduced expenditure results from MC-R antagonist treatment or targeted deletion of the MC4-R (10,11). Despite a wide anatomical distribution of MC4-R (12,29), the field has emphasized hypothalamic contributions to MC4-R-mediated energetic effects (e.g. see Refs. 13 and 14) but has not thoroughly characterized the contribution of extra-hypothalamic MC4-Rs or the downstream neural circuitry mediating MC4-R-induced energy balance effects.
This paper addresses whether MC-R-bearing neurons in the caudal brainstem contribute to energy expenditure responses, and whether the sympathetic output circuits mediating the energetic effects involve processing by both caudal brainstem and forebrain structures. A useful strategy for highlighting the candidate MC-R-bearing neurons comes from studies that examine MC4-R expression in the sympathetic pre-motor neurons controlling interscapular brown adipose tissue (IBAT) temperature (TIBAT). Notable among the identified neurons are the MC4-R expressing neurons in the hypothalamic paraventricular nucleus (PVN), the dorsomedial hypothalamic nucleus, and lateral hypothalamic area that are retrogradely labeled with pseudorabies-virus injections into IBAT, a key thermogenic effector in rodents (11,15). Although MC-R-bearing hypothalamic neurons, especially those of the PVN, provide a focus for many studies addressing the mediation of MC-R effects on energy expenditure (13,14), MC4-Rs are also expressed extra-hypothalamically in several caudal brainstem nuclei that are linked to the control of IBAT and cardiac responses. Caudal brainstem neurons, including those of the nucleus tractus solitarius (NTS), medullary raphe [raphe pallidus (RPa), raphe obscurus, raphe magnus], parabrachial nucleus, and rostroventrolateral medulla (RVLM), express MC4-R and are retrogradely labeled by IBAT pseudorabies virus injection (11). A critical role for RPa neurons in the control of IBAT thermogenesis, heart rate (HR), and sympathetic outflows is well established (16,17,18,19,20,21,22,23). Neurons expressing MC4-R in the RPa and RVLM are also associated with cardiovascular efferent control by other investigators (24,25,26).
To examine the contribution of MC-R-bearing caudal brainstem neurons to energy expenditure control and to determine whether processing endemic to the hindbrain (in the absence of forebrain processing) is required for MC-R-mediated autonomic response production, MC-R agonist-induced energetic responses of neurologically intact rats were compared with those of rats whose caudal brainstem was neurally isolated from the forebrain via complete supracollicular transection. TIBAT, core temperature (TC), HR, and spontaneous activity were monitored in response to hindbrain (fourth) ventricular, medullary raphe parenchymal, and systemic (ip) injection of melanotan II (MTII), a ligand of the MC4-R and MC3-R. Results establish a role for the hindbrain MC-Rs in the control of energy expenditure, and show that endemic caudal brainstem circuits are sufficient for hindbrain-generated response production and that hypothalamic processing and hypothalamic-forebrain communication are not necessary.
Materials and Methods
Subjects
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 300–400 g at surgery and housed individually in plastic bins under a 12-h light, 12-h dark cycle (0800 h lights on), participated in the five experiments described below. Pelleted food (Purina 5001; St. Louis, MO) and water were available ad libitum unless otherwise noted. All procedures conformed to the institutional standards of animal care and use (University of Pennsylvania).
Surgery
Rats were anesthetized with ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) delivered im.
Fourth intracerebroventricular (icv) and medullary raphe cannula.
Rats in experiments 1 and 3–6 received a fourth icv guide cannula (22 gauge; Plastics One, Inc., Roanoke, VA) with its tip stereotaxically positioned 2.0 mm above the fourth ventricle (coordinates: on the midline, 2.5-mm anterior to the occipital suture, and 4.5-mm ventral to the dura, with injector aimed 6.5-mm ventral from dura). Rats in experiments 4–6 also underwent a decerebration surgery. Rats in experiment 3A received a guide cannula aimed at the medullary raphe (coordinates: on the midline, 3-mm posterior to lambda, and 7.4-mm ventral to the dura, with injector aimed 9.4-mm ventral to dura). Medullary raphe injections for the rats in experiments 3B and 6 used the fourth icv guide cannula (above) with injectors positioned 5 mm below the guide cannula aimed at the medullary raphe (injector aimed 9.5-mm ventral to dura). Cannulas were attached to the skull with dental acrylic and jeweler’s screws, and closed with an obturator.
Decerebration surgery.
Supracollicular decerebration was performed in two hemi-transection stages separated by at least 1 wk, as previously described (27). Decerebrate rats received fourth icv cannulas during the second hemisection surgery. Pair-fed neurologically intact control rats were also anesthetized on two occasions and implanted with fourth icv cannulas during the second surgery. Rats recovered for at least 1 wk before the experiment started. The completeness of the intended transection was verified histologically after the experiment. Only rats with a histologically verified complete transection were included in the data analyses.
Telemetric transponder surgery.
Telemetric transponders (HRC 4000 VitalView; Mini Mitter/Respironics, Bend, OR) for recording TC, HR, and spontaneous physical activity (SPA) were inserted into the abdominal cavity, with the leads positioned sc and secured to the chest muscles on either side of the heart with sutures. In experiment 3, animals received a smaller telemetric transponder for recording TIBAT and SPA (G2, VitalView; Mini Mitter/Respironics). The skin overlying the IBAT pad was opened and the transponder positioned on the right side of IBAT, avoiding the midline vessels and nerves, and secured with sutures to the overlying muscle. In experiments 1, 4, and 6, separate groups of animals were implanted with IPTT-300 (Bio Medic Data Systems, Sealord, DE) transponders that measured only the TIBAT.
Experimental procedures
Cannula position verification.
At least 7 d after surgery, fourth icv cannula placement was assessed by measurement of the sympathoadrenal-mediated glycemic response to 5-thio-d-glucose [210 μg in 2 μl artificial cerebral spinal fluid (aCSF)] (28). A postinjection elevation of at least 100% of baseline plasma glucose level was required for subject inclusion. The medullary raphe placement was determined histologically after the experiment with injection of pontamine sky blue at the 100 nl volume used in the experiments.
Habituation training.
Before the start of experimental testing, rats were acclimated to handling and injections used in a given experiment (fourth icv, parenchymal, ip).
Food intake and body weight monitoring.
Food was removed at injection time (early in the light cycle, between 0930 and 1100 h) and returned 8 h later, late in the light phase. Thereby, food was not available during the period of energetic response measurement. Food intake and body weight measurements were performed 24 h after the injection of drug. Given this design, all noted differences in food intake reflect longer latency effects of MTII (from 8–24 h after injection). For ad libitum-feeding rats, food was always available during the dark cycle, and a minimum of 48 h was allotted between experimental testing for all animals.
Experiment 1: effects of stimulating caudal brainstem MC-Rs via fourth icv MTII injection on energy expenditure
Neurologically intact rats (n = 11) received fourth icv injections early in the light cycle. Three conditions were run in a counterbalanced fashion across separate days with at least 2 d between conditions. Responses were examined following a control condition with fourth icv vehicle (1 μl aCSF) and two doses of MTII: 0.1 and 1.0 nmol (dose selection based on Refs. 8 and 9). HR, TC, and SPA were continuously monitored for 8 h at 5-min intervals (TC and SPA) or 30-sec intervals (HR) in rats with implanted HRC-4000 transponders. TIBAT was monitored every hour for 7 h in experiment 1.
Experiment 2: effects of systemic MC-R ligand injection on energy expenditure
This experiment was designed to determine whether any of the energy balance effects seen with fourth icv MTII injection could be attributed to actions on peripheral MC-Rs via drug efflux from the brain. All other features of the design were identical to experiment 1 except that vehicle (0.2 ml saline) and MTII (1.0 nmol in 0.2 ml saline) were injected ip (n = 12).
Experiment 3: effects of stimulating medullary raphe MC4-Rs via intraparenchymal MTII injection on energy expenditure
All rats received two counterbalanced conditions (100 nl injections of aCSF or MTII 10 pmol) separated by at least 2 d. Pilot studies determined that 5 or 10 pmol MTII delivered fourth icv were without effect on TC (data not shown). In experiment 3A, in one set of rats (n = 8), TIBAT and SPA were monitored with G2 transponders every 5 min for 8 h. In experiment 3B, in a second set of rats (n = 12), HR, TC, and SPA were monitored with HRC 4000 transponders for 8 h at 5-min intervals (TC, SPA) and 30-sec intervals (HR).
Experiment 4: effects of stimulating caudal brainstem MC-Rs via fourth icv injection on TIBAT in chronic decerebrate (CD) rats and intact control rats
Diet maintenance.
CD rats do not spontaneously ingest food (27); therefore, they were maintained with four daily gastric intubations of 9 ml liquid diet (AIN 76A rodent diet; Research Diets, New Brunswick, NJ). This maintenance regime provides 79 kcal/d and adequate hydration; rats gain weight on this regime. Feedings were separated by intervals of at least 2 h. CD and gavage-fed (GF) intact control rats were maintained on this feeding paradigm except during experimental testing when animals were only GF three times: once 2 h before experiments commenced and twice after the experimental testing. TC of CD rats is more variable than that of GF control rats. Rectal temperature was measured at each gavage feeding, and rats were cooled or heated if TC was less than 34.0 or above 38.5 C (except during experimental testing).
Test days.
The experimental design was identical to that of experiment 1, with the exception of the dose. All rats (CDs: n = 10, GF: n = 9) were tested under control condition: fourth icv vehicle (1 μl aCSF) and 1.0 nmol MTII.
Experiment 5: effects of stimulating caudal brainstem MC-Rs via fourth icv MTII injection on TC, HR, and SPA in CD and intact rats with and without oral food access
Diet maintenance.
CD (n = 6) and GF control (n = 11) rats were maintained as in experiment 4. A third group, meal-fed intact rats (n = 11), was included. These rats had oral access to the same diet that was intubated in the other two groups. Meal-fed rats were presented with 9 ml liquid diet at the same times that the other groups received the diet by gavage. In all cases the 9-ml aliquot was consumed within 5–10 min. The feeding maintenance condition of the intact rats in the GF and meal-fed oral access groups were subsequently reversed to allow within-subject comparison. Rats were retested with MTII and vehicle injections after 2 wk on a given feeding maintenance regime.
Test days.
The experimental design was identical to that of experiment 1.
Experiment 6: effects of stimulating medullary raphe MC-Rs via intraparenchymal MTII injection on energy expenditure in CD and GF intact rats
CD (n = 4) and GF intact controls (n = 5) rats received medullary raphe injections early in the light cycle. The design was identical to that of experiment 3. TIBAT was recorded every hour for 7 h.
Statistical analysis
All energy expenditure parameters were analyzed by ANOVAs on 5 or 6-h postinjection averages, and followed by post hoc t tests and Tukey’s honestly significant difference test as appropriate. Twenty-four-hour food intake and body weight were analyzed by ANOVA, followed by post hoc t tests and Tukey’s honestly significant difference test as appropriate. All statistical analysis was conducted using STATISTICA software (StatSoft, Inc., Tulsa, OK). Differences were considered significant at P < 0.05.
Results
Experiment 1
TC.
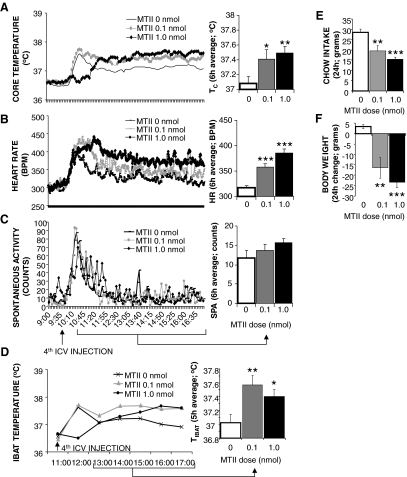
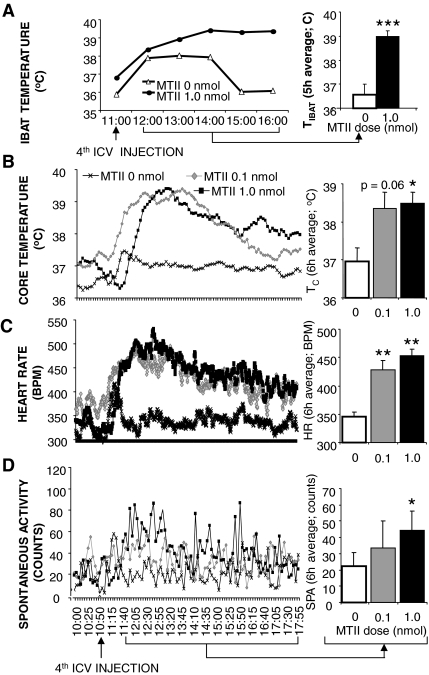
Figure 1A shows that fourth icv injection of each dose of MTII increased TC for the 6-h postinjection period in neurologically intact rats. A one-way ANOVA examining treatment effects on average postinjection TC values revealed a significant drug treatment effect [F (2,20) = 8.12; P < 0.005]. Post hoc analysis revealed a significant effect of both MTII doses on TC (0.1 nmol: P < 0.05; 1.0 nmol: P < 0.005).
Figure 1.
Effect of caudal brainstem MC-R stimulation with fourth ventricular MTII on TC (A), HR (B), spontaneous activity (C), and TIBAT (D) in chow-fed neurologically intact rats. Line graphs represent across-rat average parameter measurements through the recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. The histograms next to line graphs provide 6- or 5-h (TIBAT) postinjection averages + sem for each parameter at each dose. Effect of fourth ventricular MTII injection on 24-h food intake (E) and 24-h change in body weight (F) in chow-fed intact rats. Food was made available to animals 8 h after injections and through the 12-h period of the dark cycle. Histograms represent means + sem. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. BPM, Beats per minute.
TIBAT.
Figure 1D shows that MTII significantly increased TIBAT [F (2,16) = 8.39; P < 0.005]. Both MTII doses significantly elevated TIBAT (0.1 nmol: P < 0.005; 1.0 nmol: P < 0.05). A short latency increase in all measurements apparent in the vehicle condition reflects the animals’ arousal associated with the injection procedure. However, for the 1.0-nmol MTII dose, some rats did not show the transient elevation in TC or TIBAT, contributing to the impression that temperature parameters declined to this dose. This initial response was, however, variable and not statistically significant.
HR.
Figure 1B shows that MTII dose-dependently increased HR, compared with saline control. A one-way ANOVA of average HR values for the 6-h period after injection yielded a significant drug effect [F (2,20) = 33.33; P < 0.0001]; post hoc analysis showed a significant effect of both doses of MTII on HR (0.1 nmol: P < 0.0005; 1.0 nmol: P < 0.0001).
Spontaneous physical activity.
It was not significantly increased by fourth icv MTII [F (2,20) = 2.45; P = 0.11] (Fig. 1C).
Food intake and body weight.
Both MTII doses significantly decreased 24-h food intake [F (2,20) = 17.28; P < 0.0001] and body weight [F (2,20) = 17.75; P < 0.0001] (Fig. 1, E and F).
Experiment 2
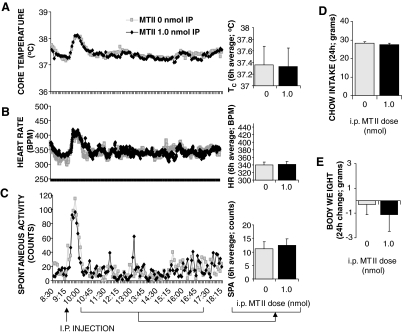
Peripheral delivery (ip) of the higher dose of MTII (1.0 nmol) was without effect on all of the measured energetic and food intake parameters (Fig. 2).
Figure 2.
Effect of peripheral (ip) MTII treatment on TC (A), HR (B), spontaneous activity (C), 24-h food intake (D), and 24-h change in body weight (E) in chow-fed intact rats. Line graphs represent across-rat average parameter measurements through the 8-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. Histograms represent means + sem. BPM, Beats per minute.
Experiment 3
Experiment 3A, TIBAT group.
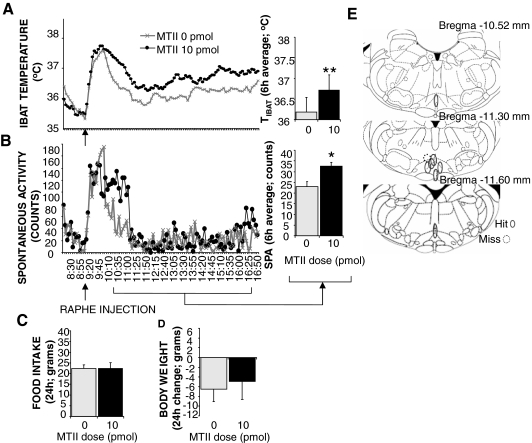
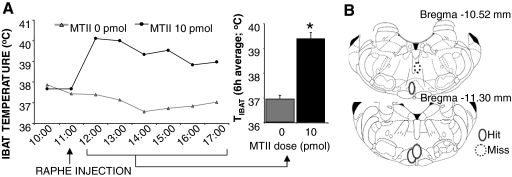
Figure 3E is a reconstruction of the injection sites for the eight animals tested. Microscopical analyses of the dye injection revealed that seven rats had placements within the medullary raphe. Figure 3A shows that average TIBAT was significantly increased (P < 0.005) after 10-pmol MTII injection. Average SPA was also increased (P < 0.05) (Fig. 3B). No significant changes were observed in the 24-h food intake or 24-h body weight of these rats (Fig. 3, C and D).
Figure 3.
Effect of stimulation of medullary raphe MC4-Rs via parenchymal injection of 10 pmol MTII on TIBAT (A), spontaneous activity (B), 24-h food intake (C), and change in body weight (D). Line graphs represent across-rat average parameter measurements through the 8-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. Histograms represent means + sem. *, P < 0.05; **, P < 0.005. E, Reconstruction of injection sites based on microscopical analysis of dye injection at the same volume (100 nl) as the melanocortin agonist. Microscopical analysis revealed that seven of eight rats had placements within the medullary raphe (RPa, raphe obscurus, raphe magnus). Solid line ovals indicate instances where the dye injection placement was within the medullary raphe (positive placements). Dotted line circles represent negative placements (injection sites that were judged to be outside of medullary raphe). Placements shown were between −10.52 and −11.60 mm from bregma (54).
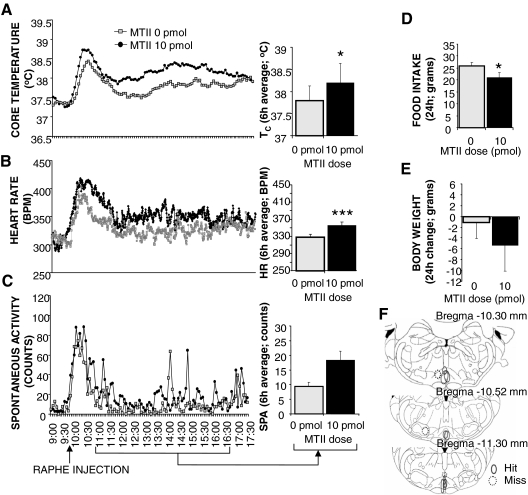
Experiment 3B, TC and HR group.
Figure 4F reconstructs the injection sites of a second group of animals and reveals that nine rats had medullary raphe placements. Figure 4, A–E, displays the physiological response of the nine rats with confirmed medullary raphe placements, and shows that MTII injection significantly increased TC and HR (P < 0.05 and P < 0.0001, respectively). These rats also showed a decrease in 24-h food intake after MTII, whereas SPA and 24-h body weight did not change significantly. Comparison of the injection sites in Figs. 3E and 4F revealed that on average, the medullary raphe placements of the first group were caudal to those of the second group. These placement differences may account for the between-group differences in SPA and food intake responses.
Figure 4.
Effect of 10 pmol MTII stimulation of medullary raphe MC4-Rs on TC (A), HR (B), spontaneous activity (C), 24-h food intake (D), and 24-h change in body weight (E). Line graphs represent across-rat average parameter measurements through the 8-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. Histograms in A–C represent 6-h means + sem. *, P < 0.05; ***, P < 0.0005. F, Reconstruction of injection sites based on microscopical analysis of dye injection at the same volume (100 nl) as the melanocortin agonist. Microscopical analysis revealed that nine of 12 rats had placements within the medullary raphe. Solid line ovals mark the area of the dye injection for each rat with confirmed medullary raphe placement. Dotted line circles represent injection sites that were judged to be outside of medullary raphe. Placements shown were between −10.30 and −11.30 mm from bregma (54). BPM, Beats per minute.
Experiment 4
Figure 5A shows that MTII (1.0 nmol) produced a large and long duration elevation in TIBAT in CD rats. Two-way ANOVA (neurological preparation and drug treatment as main variables) revealed a significant interaction of drug and neurological preparation [F (1,17) = 33.85; P < 0.0001]. A post hoc test showed that there was a significant effect of MTII on CD TIBAT (P ≤ 0.0001), but surprisingly, no effect on GF control rats (P = 0.212) (Fig. 7D). The absence of an energy expenditure effect in GF intact rats contrasted with the potent energetic effects of MTII observed in the chow-fed intact rats of experiment 1 and suggested that oral access to food in intact animals could be a relevant variable in the MTII driven sympathetic responses. This finding encouraged inclusions of a second dose of MTII (0.1 nmol) and the addition of an oral food access meal-fed control group that was in experiment 5.
Figure 5.
Effect of fourth ventricle MTII injection in CD rats on TIBAT (A), TC (B), HR (C), and spontaneous activity (D) in CD rats. Line graphs represent across-rat average parameter measurements through the recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. Histograms represent 6 or 5 h (TIBAT) means + sem. *, P < 0.05; **, P < 0.005; ***, P< 0.0005. BPM, Beats per minute.
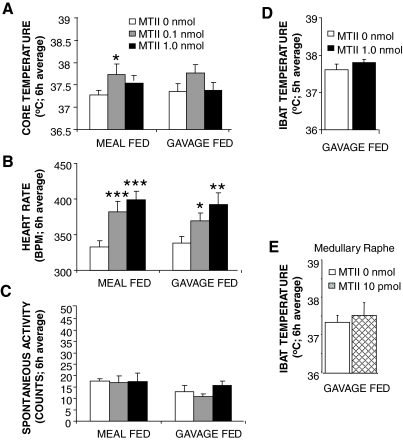
Figure 7.
Effect of caudal brainstem MC-R stimulation with fourth icv delivered MTII in GF and meal-fed neurologically intact control rats on TC (A), HR (B), spontaneous activity (C), and TIBAT (D). E, Effect of medullary raphe MTII delivery to GF rats on TIBAT. Histograms represent means + sem. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. BPM, Beats per minute.
Experiment 5
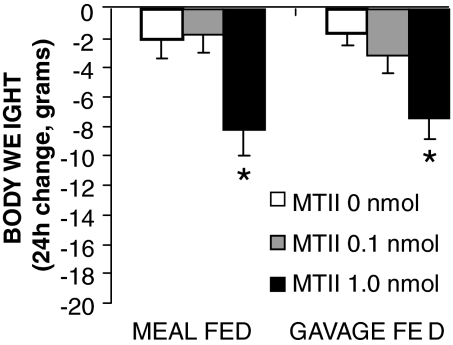
For CD rats, fourth icv administration of MTII significantly increased TC, HR, and SPA [TC: F (2,10) = 4.97, P < 0.05; HR: F (2,10) = 19.99, P < 0.0005; and SPA: F (2,10) = 5.81, P < 0.05] (Fig. 5, B–D). In meal-fed control rats with oral access to liquid diet, MTII treatment also significantly increased TC and HR [TC: F (2,20) = 5.10, P < 0.05; HR: F (2,20) = 22.26, P < 0.0001] (Fig. 7, A and B). Consistent with the TIBAT results for GF intact rats in experiment 4, there was no overall significant effect of MTII on TC or SPA in GF controls (Fig. 7, A and C). Although TIBAT was increased under baseline conditions in GF rats compared with intact chow-fed controls, which could potentially contribute to the lack of effect of MTII, the TC of GF and chow-fed rats after vehicle treatment was not significantly different, yet MTII effect was still not present in GF rats, pointing to other mechanisms behind the lack of effect then increased baseline temperature. Although significant tachycardia was noted in these MTII-treated GF rats [GF: F (2,20) = 11.16; P < 0.0005], it was attenuated compared with the CDs or the meal-fed oral access controls (Fig. 7B). Twenty-four hour body weight was significantly decreased in the meal-fed rats with oral food access [F (2,20) = 5.3; P < 0.05] after MTII delivery. Interestingly, body weight was also reduced by MTII in GF controls [F (2,20) = 6.3; P < 0.05] (Fig. 8). Post hoc tests revealed that this effect is produced by the highest dose of MTII in both groups. CD body weight declined after both MTII doses; however, that effect was not statistically significant. All groups had identical energy intake, indicating that the MTII effect on energy expenditure provided the basis for the observed suppression in body weight. It should be noted that the values for TC, TIBAT, and HR measured at baseline were the same for decerebrate and intact control rats.
Figure 8.
Effect of fourth icv MTII treatment on 24-h body weight change of GF and meal-fed neurologically intact rats. Note that all animals had identical food intake (either delivered by gavage or consumed in entirety during a meal), therefore, all noted changes in body weight are a result of treatment-induced changes in energy expenditure. Histograms represent means + sem. *, P < 0.05.
Experiment 6
Reconstruction of the parenchymal placements revealed that the injection sites in three rats were located within the medullary raphe (Fig. 6B). The average TIBAT in CD rats with the confirmed medullary raphe placements was significantly increased (P < 0.05) after 10-pmol MTII injection (Fig. 6A). No MTII-driven TIBAT effect was observed in GF controls (Fig. 7E).
Figure 6.
A, Effect of medullary raphe MC4-R stimulation with 10 pmol MTII in CD rats on TIBAT. Line graph represents across-rat average parameter measurements through the 8-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. Histogram represents 6-h means + sem. *, P < 0.05. B, Reconstruction of injection sites based on microscopical analysis of dye injection at the same volume (100 nl) as the melanocortin agonist. Microscopical analysis revealed that three of four rats had placements within the medullary raphe. The dye injection placement for each animal is represented by solid line ovals. Dotted line circles represent negative placements. Placements shown were between −10.52 and −11.30 mm from bregma (54).
Discussion
We show that MC-R-bearing caudal brainstem neurons and their local caudal brainstem projections to sympathetic effectors play a critical and previously unrecognized role in the central melanocortin system’s contribution to energy expenditure control. Long-lasting and robust increases in thermogenesis and cardiovascular activity were observed after hindbrain ventricular delivery of MTII to neurologically intact control and to CD rats. Further support for the caudal brainstem MC-R site of action comes from the parenchymal injection results where IBAT thermogenesis and tachycardia were triggered by picomolar MTII stimulation of the medullary raphe MC4-Rs. These data are the first to demonstrate a functional effect of a ventricle subthreshold MC-R agonist dose delivered to the MC4-R-bearing neurons of the medullary raphe. The hyperthermia, IBAT thermogenesis, and tachycardia observed in CD rats after fourth icv and parenchymal MTII injections support the hypothesis that hindbrain MC-R stimulation engages endemic circuits that link autonomic outflows to thermogenic and cardiac effectors in the absence of forebrain processing or forebrain-caudal brainstem communication.
The qualitative profile of results for CD and for chow-fed control rats was similar; increases in energetic and cardiovascular parameters were observed in both groups after hindbrain MC-R stimulation. However, quantitatively, the magnitudes of the mean energetic responses were 2- (HR) to 4-fold (temperature) greater in CD rats than intact rats after fourth icv MTII application (Table 1). Quantitative differences between neurological groups were also observed with application of 10 pmol MTII to the medullary raphe. Here, intact chow-fed rats increased TIBAT by approximately 0.5 C on average. By contrast, the same dose of MTII increased TIBAT of a CD rat by approximately 3.0 C (Figs. 3A and 6A). There are several interpretations for these quantitative differences between decerebrate and intact rats. The endogenous agonist for the caudal brainstem MC-Rs originates from two anatomically disparate sources: one in the hypothalamic arcuate (ARC) nucleus (30) and the other in the hindbrain NTS commissural nucleus (31). Transection of the descending projections from ARC POMC neurons could eliminate a significant percentage of the endogenous agonist for a given caudal brainstem nucleus. This could result in a compensatory increase in expression of MC-Rs in hindbrain nuclei. Up-regulation of MC-Rs in response to decreased agonist availability has been reported (13). Although projections from both ARC POMC and NTS POMC neurons terminate in the caudal brainstem (32,33), it is not clear what the source(s) of the endogenous agonist is for each of the individual MC-R-expressing hindbrain nuclei. For each nucleus it is possible that the agonist is supplied entirely by NTS POMC neurons, ARC POMC neurons, or some combination of the two (32). Sim and Joseph (33) showed that ARC POMC projections innervate midline caudal brainstem nuclei, whereas lateral caudal brainstem regions receive projections from NTS POMC neurons; in some cases, structures received terminal fields from both sources. In collaboration with H. R. Berthoud (34), we have begun to examine tissues from decerebrated rats to quantify the percentage of α-MSH fibers that originate in ARC POMC neurons and project to various hindbrain MC-R-bearing nuclei. For the NTS, we recently showed that approximately 70% of the MSH fibers terminating in all subregions of the NTS originate in ARC POMC neurons (34). Additional work is needed to determine the source of MSH ligand for RPa, RVLM, and other relevant hindbrain nuclei, and whether eliminating a major source of endogenous ligand (ARC POMC neurons) increases exogenous agonist binding and receptor expression in caudal brainstem nuclei.
Table 1.
Comparisons between treatment effect of decerebrates and three groups of intact rats with different feeding maintenance
| MTII dose (nmol) | CD | Intact ad libitum fed | Intact meal fed | Intact GF | |
|---|---|---|---|---|---|
| TC (C) | 0.1 | 1.39 ± 0.67 | 0.33 ± 0.08 | 0.46 ± 0.17 | 0.34 ± 0.2 |
| 1.0 | 1.52 ± 0.37 | 0.41 ± 0.09 | 0.28 ± 0.12 | 0.03 ± 0.13 | |
| HR (BPM) | 0.1 | 83.21 ± 19.2 | 40.84 ± 7.29 | 48.22 ± 6.69 | 28.74 ± 6.44 |
| 1.0 | 107.38 ± 17.65 | 69.04 ± 6.83 | 73.46 ± 14.03 | 57.18 ± 11.83 | |
| Spontaneous activity (counts) | 0.1 | 11.16 ± 6.36 | 2.11 ± 2.34 | −1.71 ± 3.2 | −2.72 ± 2.13 |
| 1.0 | 21.92 ± 8.01 | 4.05 ± 1.19 | −0.44 ± 3.08 | −0.07 ± 3.60 | |
| Interscapular brown fat temperature (C) | 1.0 | 2.43 ± 1.07 | 0.38 ± 0.14 | 0.20 ± 0.15 |
Fourth icv MTII treatment induced effect sizes for TC, TIBAT, HR, and spontaneous activity expressed as changes in average postinjection values. All values represent average differences in MTII and vehicle values ± sem Four groups of rats are compared that differ by feeding maintenance (ad libitum fed, meal-fed, GF) or surgical status (decerebrate; intact). BPM, Beats per minute.
The activation of MC-Rs is also influenced by the endogenous antagonist, agouti-related protein (AgRP), made in ARC neurons (35). It is generally thought that antagonist-containing neurons project most heavily to forebrain areas (36,37). However, hindbrain projections have also been reported (38). The transection of descending AgRP projections may provide another explanation for the greater response to MTII observed in CD. The elimination of AgRP projections to hindbrain neurons that also receive MSH fibers (regardless of their origin) may result in greater MC-R activation and potentially contribute to the observed effects. A different type of interpretation for the greater response magnitude in CD rats relates to the role of forebrain-hypothalamic nuclei in mediating fine-grained response control. For example, HR is increased in response to skin cooling and contributes to the dispersion of the heat produced by sympathetic activation of brown adipose tissue. For intact rats there is a significant correlation between HR and ambient temperature over the 4–23 C range. By contrast, for decerebrate rats, the HR response to an intermediate cold temperature is as robust as that observed with the coldest temperature, indicating a loss of fine-grained control in the absence of connections with the forebrain (39). A similar explanation for the greater MTII-stimulated responses of the CD rats could apply in the current data.
The responses of GF intact rats differed qualitatively from those of chow-fed intact rats and oral-fed rats (Table 1). However, the variability of these responses was great, yielding trends, but no significant differences between the intact rat subgroups. Nonetheless, the pattern of these between-group differences is clear, with lesser response magnitude for the GF intact group than for the two intact groups with oral access to food. This outcome suggests a role for oral stimulation in the observed responses. Saito et al. (40) demonstrated that oropharyngeal stimulation contributes to IBAT thermogenesis. Gavage feeding reduced oropharyngeal stimulation and decreased IBAT norepinephrine turnover relative to that seen in oral-fed rats. Our studies are consistent with the view that gavage feeding decreases the magnitude of sympathetically mediated energy expenditure output. Our data place components of the melanocortin system within the sympathetic nervous system output circuitry that is altered by oral exposure to food. Two provisos are worth noting. First, CD rats responded robustly to the MC-R agonist, yet they were gavage fed. This suggests that the inhibitory effect of bypassing the mouth on MTII-driven energetic responses is forebrain mediated. Second, even though the energetic response magnitude of GF intact rats was attenuated, the animals still exhibited significant weight loss after the drug treatment (Fig. 8). This result underscores the contribution of MC-R-induced energy expenditure to body weight control because the food intake of these rats was matched to that in the vehicle condition such that weight loss could not be attributed to the anorectic effect of the treatment.
The energetic effects obtained from selective stimulation of the MC4-R-bearing neurons of the medullary raphe and with fourth ventricular agonist delivery highlight the role of hindbrain MC-R-bearing neurons in the control of sympathetic, thermic, and cardiac responses. That said, there is also a role for hypothalamic-forebrain processing in melanocortin-mediated energetic effects. Many reciprocal neural projections exist between hypothalamic (especially PVN, ARC, and the lateral hypothalamic area) and hindbrain nuclei. In fact, the literature on sympathetic outflows and energy expenditure control emphasizes a role for these hypothalamic nuclei (for review, see Ref. 41). The neural circuitry underlying the expression of thermic and cardiac responses to hindbrain MC-R stimulation may well involve descending hypothalamic projections. We have already discussed that ARC POMC neurons project to a variety of sympathetic pre-motor targets in the hindbrain, and, therefore, it is appropriate to consider that the application of the MC-R agonist to MC4-R hindbrain neurons mimics, at least in part, the effects of endogenous ligand input from ARC. In addition, nonmelanocortinergic projections from hypothalamic nuclei may also play a role in the control of MC-R-driven energetic response. MC-R-bearing PVN neurons receive ligand from ARC and project to the caudal brainstem (42). At the same time, the results from the decerebrated rats make clear that forebrain processing and forebrain-brainstem communication are not required for caudal brainstem stimulated responses and that downstream circuits endemic to the caudal brainstem mediate the observed responses. The current data are consistent with those from previous studies from our laboratory (43) that show that the increase in uncoupling protein-1 gene expression driven by fourth ventricular MTII can be mediated by circuitry intrinsic to the caudal brainstem and spinal cord.
The increased energetic response to fourth ventricular application of MTII in chow-fed intact rats reported here is consistent with results from an earlier report by Zheng et al. (44). However, responses in that study were of lesser magnitude and duration than those observed here with MTII doses within a similar range. In the Zheng et al. (44) study, rats had access to food during energetic response recordings, and recordings took place during the dark/active phase. In our study, rats were tested in the light phase in the absence of food. These paradigmatic differences appear to explain the observed differences in responses. We observed that rats stimulated with MTII in the light phase without access to food have greater response magnitude and duration than when the same rats are examined in the dark phase with access to food (unpublished observations).
We showed that hindbrain MC-Rs contribute to the energy expenditure observed with central melanocortinergic stimulation. This result would seem consistent with the recent perspective of Balthasar et al. (45). These investigators selectively expressed MC4-Rs in neurons of the PVN and amygdala in mice otherwise lacking the receptor. They found that this selective MC4-R expression reversed the hyperphagia seen in the MC4-R knockout mouse but did not increase the reduced energy expenditure profile of the knockout. Balthasar et al. (45) suggest that the energy intake and energy expenditure effects of the central melanocortin system are controlled by anatomically distinct portions of the system. Although we would agree that the melanocortin contribution to energy balance control is distributed across different brain regions, our data and those of others (e.g. see Refs. 46 and 47) do not support the view that feeding function is uniquely associated with basal forebrain elements of the melanocortin system and that energetic function is controlled uniquely by more caudal elements of the system. In our view, stimulation of MC-Rs in a variety of central sites, including the hypothalamus and caudal brainstem, reduces feeding and increases energetic response. For example, the present findings show that in addition to the energetic effects of fourth ventricular MC-R agonist delivery, food intake was also significantly reduced. Previous work shows that hindbrain parenchymal application of pmol doses of MTII or the synthetic MC3/4R antagonist SHU-9119 results in a respective decrease or increase in food intake (48). However, the idea that stimulation of anatomically disparate receptors can drive the same functional effect is not new. We and others have already shown that the feeding effect of central injection of leptin, urocortin, ghrelin, neuropeptide Y, fenfluramine, and norepinephrine is observed with basal forebrain, as well as caudal brainstem ligand application (46,47,49). It seems then that such a similarity in the functional output resulting from stimulation of anatomically distinct sites represents a degree of redundancy in the melanocortin system. Although this suggests common outputs, it is likely that there are differences in the pattern of input received by MC-R-bearing nuclei in different regions of the brain. Both energy intake and expenditure change in response to long-term exposure to calorie-dense diets (50,51) and also in response to pathogens. The melanocortin system plays a role in mediating the energetic effects triggered by these diverse physiological conditions (11,52). It is possible that responses resulting from these distinct challenges are processed at different levels of the brain, making the receptors divergent based on input but still producing the same functional output: decrease in intake and increase in expenditure.
MC-Rs are expressed on peripheral organs, like the heart (24) and adrenal medulla (53), implicated in metabolic and cardiovascular activity. Peripheral application of high doses of the MC-4R agonist increases HR, blood pressure, TIBAT, as well as locomotor activity (7). Therefore, some part of the energetic effects obtained after central application of MC-R agonists to the brain may occur from an action on peripheral receptors. To evaluate this possibility, we applied the highest dose of the agonist used centrally to the periphery and showed no effect on any measured energy expenditure parameter. Although this result does not eliminate the potential role of the peripheral MC-Rs in energy expenditure, it confirms that energetic effects observed in our study are induced by stimulation of central and not peripheral receptors.
We showed that hindbrain targeted MC-R stimulation and medullary raphe MC4-R agonist injection increases energetic responses, including hyperthermia, IBAT thermogenesis, and tachycardia. The source of the endogenous agonist for caudal brainstem MC-Rs occurs from both local (NTS) and forebrain (ARC) sources. It is still unclear to what extent each nucleus contributes to the endogenous agonist supply of the specific MC-R-bearing nuclei of the caudal brainstem mediated energetic responses under normal conditions. Therefore, the neural circuitry underlying the expression of thermic and cardiac responses to hindbrain MC-R stimulation may well involve descending hypothalamic projections. However, that the same pattern of response was observed in decerebrate and intact rats shows that the output circuitry responsible for the observed effects lies within the hindbrain and does not require forebrain processing or forebrain-caudal brainstem communication. The results of this study suggest future investigations designed to determine the range of factors and environmental conditions (e.g. diet, pathogens, cold exposure) that engage the previously underappreciated caudal brainstem portion of melanocortin system as it participates in the control of energy expenditure.
Acknowledgments
We thank Matt Hayes, Ph.D. (University of Pennsylvania), for his editorial comments and Lisa Maeng for her technical assistance.
Footnotes
These studies were supported by the National Institutes of Health Research Grant DK-21397 (to H.J.G.) and training Grant T32-GM-07517 (to K.P.S.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 27, 2008
Abbreviations: aCSF, Artificial cerebral spinal fluid; AgRP, agouti-related protein; ARC, arcuate; CD, chronic decerebrate; GF, gavage-fed; HR, heart rate; IBAT, interscapular brown adipose tissue; icv, intracerebroventricular; MC-R, melanocortin receptor; MTII, melanotan II; NTS, nucleus tractus solitarius; POMC, proopiomelanocortin; PVN, paraventricular nucleus; RPa, raphe pallidus; RVLM, rostroventrolateral medulla; SPA, spontaneous physical activity; TC, core temperature; TIBAT, interscapular brown adipose tissue temperature.
References
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S 1998 A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20:111–112 [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Gruters A 2003 Mutations in the human proopiomelanocortin gene. Ann NY Acad Sci 994:233–239 [DOI] [PubMed] [Google Scholar]
- Dubern B, Bisbis S, Talbaoui H, Le Beyec J, Tounian P, Lacorte JM, Clement K 2007 Homozygous null mutation of the melanocortin-4 receptor and severe early-onset obesity. J Pediatr 150:613–617 [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F 1997 Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U 1999 Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5:1066–1070 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM 1998 Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci 18:10128–10135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim U, Nicholson JR, Dokladny K, Dunant P, Hofbauer KG 2006 Cardiovascular responses to melanocortin 4-receptor stimulation in conscious unrestrained normotensive rats. Peptides 27:438–443 [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL 1999 Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33:542–547 [DOI] [PubMed] [Google Scholar]
- Murphy B, Nunes CN, Ronan JJ, Hanaway M, Fairhurst AM, Mellin TN 2000 Centrally administered MTII affects feeding, drinking, temperature, and activity in the Sprague-Dawley rat. J Appl Physiol 89:273–282 [DOI] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD 2000 A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 97:12339–12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W 2007 Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148:1550–1560 [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK 2003 Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457:213–235 [DOI] [PubMed] [Google Scholar]
- Harrold JA, Widdowson PS, Williams G 1999 Altered energy balance causes selective changes in melanocortin-4(MC4-R), but not melanocortin-3 (MC3-R), receptors in specific hypothalamic regions: further evidence that activation of MC4-R is a physiological inhibitor of feeding. Diabetes 48:267–271 [DOI] [PubMed] [Google Scholar]
- Williams G, Harrold JA, Cutler DJ 2000 The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc 59:385–396 [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF 2003 Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460:303–326 [DOI] [PubMed] [Google Scholar]
- Morrison SF 2003 Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience 121:17–24 [DOI] [PubMed] [Google Scholar]
- Morrison SF 2004 Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 19:67–74 [DOI] [PubMed] [Google Scholar]
- Fan W, Morrison SF, Cao WH, Yu P 2007 Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRPa) area. Brain Res 1179:61–69 [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF 2003 Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res 980:1–10 [DOI] [PubMed] [Google Scholar]
- McAllen RM, Farrell M, Johnson JM, Trevaks D, Cole L, McKinley MJ, Jackson G, Denton DA, Egan GF 2006 Human medullary responses to cooling and rewarming the skin: a functional MRI study. Proc Natl Acad Sci USA 103:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM 2001 Cold-activated raphe-spinal neurons in rats. J Physiol 535(Pt 3):841–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MA, Dampney RA 2004 Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol 287:R824–R832 [DOI] [PubMed] [Google Scholar]
- Blessing WW 2003 Lower brainstem pathways regulating sympathetically mediated changes in cutaneous blood flow. Cell Mol Neurobiol 23:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Jenny Wu CS, Dumont LM, Wild JM 2003 Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology 144:5488–5496 [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Dampney RA 1995 Powerful depressor and sympathoinhibitory effects evoked from neurons in the caudal raphe pallidus and obscurus. Am J Physiol 268(5 Pt 2):R1295–R1302 [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA 2002 Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol 538(Pt 3):941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R 1978 Chronically decerebrate rats demonstrate satiation but not bait shyness. Science 201:267–269 [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S 1981 Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD 1994 Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8:1298–1308 [DOI] [PubMed] [Google Scholar]
- Knigge KM, Joseph SA, Nocton J 1981 Topography of the ACTH-immunoreactive neurons in the basal hypothalamus of the rat brain. Brain Res 216:333–341 [DOI] [PubMed] [Google Scholar]
- Joseph SA, Pilcher WH, Bennett-Clarke C 1983 Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci Lett 38:221–225 [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, O'Donohue TL 1978 α-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci USA 75:6300–6304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA 1994 Connectivity between POMC systems and brainstem nuclei. Regul Pept 53:S135–S136 [Google Scholar]
- Berthoud HR, Wan S, Patterson LM, Babic T, Townsend RL, Sutton G, Skibicka KP, Butler A, Grill HJ, Travagli RA, Zheng H, Brainstem melanocortin signaling in the control of food intake and energy balance. Keystone Symposia, Neuronal Mechanisms Controlling Food Intake, Glucose Metabolism and Body Weight (X4), Program No. 110, Banff, Canada, 2008 (Abstract) [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS 1997 Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD 1999 Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140:1408–1415 [DOI] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ 1999 Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci 19:RC26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T 1998 The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA 95:15043–15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Nautiyal KM, Kaplan JM, Thermoregulatory responses to cold on chronic decerebrate. Society for Neuroscience, Washington, DC, 2005, Program No. 183.23 (Abstract) [Google Scholar]
- Saito M, Minokoshi Y, Shimazu T 1989 Metabolic and sympathetic nerve activities of brown adipose tissue in tube-fed rats. Am J Physiol 257(3 Pt 1):E374–E378 [DOI] [PubMed] [Google Scholar]
- Richard D 2007 Energy expenditure: a critical determinant of energy balance with key hypothalamic controls. Minerva Endocrinol 32:173–183 [PubMed] [Google Scholar]
- Horvath TL, Diano S 2004 The floating blueprint of hypothalamic feeding circuits. Nat Rev Neurosci 5:662–667 [DOI] [PubMed] [Google Scholar]
- Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ 2003 Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144:4692–4697 [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud HR 2005 Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289:R247–R258 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB 2005 Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM 2002 The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23:2–40 [DOI] [PubMed] [Google Scholar]
- Taylor K, Lester E, Hudson B, Ritter S 2007 Hypothalamic and hindbrain NPY, AGRP and NE increase consummatory feeding responses. Physiol Behav 90:744–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ 2000 The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141:1332–1337 [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Grill HJ, Kaplan JM 2005 Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes 54:1985–1993 [DOI] [PubMed] [Google Scholar]
- Landsberg L 2006 Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol 26:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB 2002 βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297:843–845 [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD 2001 Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Wild JM 1998 Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res 107:309–314 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998 The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press [Google Scholar]