Abstract
Early studies reveal that testicular orphan nuclear receptor 4 (TR4) modulates signaling pathways that control various cell functions. However, how TR4 activity is regulated without the involvement of specific ligand(s) remains unclear. Here we identify a daf-16 family protein-binding element (DBE; 5′-TGTTTAC-3′) in the TR4 promoter that can be recognized by the forkhead transcriptional factor FOXO3a, a key stress-responsive factor, through which TR4 gene expression is activated. The interaction between DBE and FOXO3a was confirmed using EMSA and chromatin immunoprecipitation assays. Activation of FOXO3a by oxidative stress and phosphatidylinositol 3-kinase inhibitor induced TR4 expression; in contrast, suppression of FOXO3a by small interfering RNA can reduce oxidative stress-induced TR4 expression. The biological consequence of the FOXO3a-induced TR4 by oxidative stress is to protect against stress-induced cell death in which cells with reduced FOXO3a are less resistant to oxidative stress, and addition of functional TR4 can increase stress resistance. These results suggest that this new identified oxidative stress-FOXO3a-TR4 pathway is a fundamentally important mechanism regulating stress resistance and cell survival.
TESTICULAR ORPHAN nuclear receptor 4 (TR4) (also known as TAK1 and NR2C2) is a member of the nuclear receptor superfamily for which a ligand has not yet been found (1,2,3). TR4 is closely related to the retinoid X receptor, chicken ovalbumin upstream promoter-transcription factor, and hepatocyte nuclear factor 4 in sequence and structure (4) and binds to AGGTCA DNA sequence motifs in direct repeat orientation, with variable spacing, in the promoters of its target genes. As an orphan nuclear receptor with several known regulatory targets (5,6,7,8,9,10), TR4 regulates many signaling pathways such as apolipoprotein E (5,11), vitamin D receptor (10), retinoic acid receptor (9), androgen receptor (8), and estrogen receptor (6), indicating that TR4 is involved in many physiological functions. Recent studies with knockout of TR4 gene in mice revealed that TR4 is essential for normal spermatogenesis, motor coordination, and cerebellum development (12,13,14). However, the in vivo physiological roles of TR4 and how the biological activity of TR4 is triggered or regulated without involvement of specific ligands remains unclear (15).
DAF-16 is a forkhead-type transcription factor, functioning downstream of insulin-like signals and is known to be critical to regulation of the life span in Caenorhabditis elegans (16,17,18,19). Mammalian DAF-16 homologues include Forkhead box O (FOXO)-1 (FKHR), FOXO3a (FKHRL1), and FOXO4 (AFX) (hereafter collectively called FOXO), which share the same consensus binding sequence, TGTTTAC. The FOXO family are regulated by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Direct phosphorylation of FOXO proteins by Akt results in cytoplasmic retention and inactivation of FOXOs, inhibiting the expression of FOXO-regulated genes, which control diverse functions, including cell differentiation, proliferation, cell death, metabolism, and longevity (20,21,22,23,24,25,26). Recently several studies have shown that FOXO3a plays an important role in stress resistance and longevity in mammals. In response to stress, FOXO3a can promote cell cycle arrest at the G1/S and G2/M transitions, allowing time for repair of damaged DNA and for detoxification of cells (27,28). The expression of active forms of FOXO proteins up-regulates several genes involved in DNA repair such as growth arrest and DNA damage-inducible protein 45 (28,29). FOXO3a protein has also been reported to allow detoxification of reactive oxygen species (ROS) by up-regulating the free radical scavenging enzymes, including manganese superoxide dismutase (MnSOD) and catalase (23,28,29,30). Therefore, FOXO3a controls several aspects of the cellular resistance to oxidative stress challenge (21).
In this study, we found that FOXO3a can bind to the TR4 promoter region that contains the consensus DNA-binding sequence TGTTTAC and then induce TR4 gene expression. We also demonstrated that oxidative stress enhanced TR4 gene expression through activating the FOXO3a. Cells lacking TR4 displayed less tolerance to oxidative stress challenge, and introducing functional TR4 can restore cell survival in H2O2-treated FOXO3 small interfering RNA (siRNA) cells. These findings indicate that TR4 may be a key direct downstream target of FOXO3a for mediation of oxidative stress challenge, and biological activity of TR4 is important for cell survival.
Materials and Methods
Experimental animals and genotyping
TR4 knockout (KO) mice were obtained from Lexicon Genetics Inc. (The Woodlands, TX) and were generated and genotyped as described previously (13,14). Animals were maintained and experimental procedures on animals were conducted in accordance with guidelines outlined by the Guide for the Care and Use of Laboratory Animals and approved by the University Committee on Animal Resource.
Cell culture, treatment, and transfections
For primary culture of mouse dermal fibroblast (MDF) cells, skin was taken from 12-wk-old wild-type (WT) or TR4 KO mice and cut into 2-mm squares and placed in the center of wells of six-well plates with a sterile 22-mm glass coverslip over the skin specimens. DMEM supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) were used in the culturing of fibroblasts. Upon confluence, the coverslips were removed and cells were harvested using trypsin/EDTA. The cells were used for studies after the second passage. Mouse myoblast C2C12 and human lung carcinoma H1299 cells were purchased from the American Type Culture Collection (Manassas, VA) and maintained in standard culture medium as recommended. These two cell lines contain moderate amounts of TR4 with high transfection efficiency. H2O2 was added at 250 μm for 2 h, then medium was changed to fresh medium. LY294002 was purchased from Calbiochem (La Jolla, CA) and used with indicated doses. Transfections were performed by using SuperFect (QIAGEN, Valencia, CA) following the manufacturer’s instructions. In general, the transfection efficiency of H1299 and C2C12 cells is around 25–40%.
Plasmid constructs and site-directed mutagenesis
The expression vectors for hFOXO3a-WT, a constitutively active A3 FOXO3a mutant (FOXO3a-TM), and parental vector were kindly provided by Michael Greenberg (Harvard Medical School, Boston, MA). In the A3 mutant, three sites of Akt phosphorylation of FOXO3a (T32, S253, and S315) were mutated to alanine residues (31). pMT2, pMT2-HA-FOXO4, and pMT2-HA-FOXO4-T451E were generously provided by Dr. Boudewijn M. T. Burgering (University Medical Center Utrecht, Utrecht, The Netherlands). pGL-6 × daf-16 family protein-binding element (DBE) was kindly provided by Dr. Furuyama (Kumamoto University, Kumamoto, Japan) (32). The plasmid pCMX and pCMX-TR4 have been described previously (11). pRL-TK (TK Renilla luciferase) was purchased from Promega (Madison, WI).
For the TR4 promoter luciferase (Luc) reporter, a total of 2.0 kb of human genomic sequence spanning 1920 bp of the TR4 promoter and 95 bp of the native transcript were amplified by PCR from human genomic DNA using Pfx DNA polymerase (Invitrogen, Carlsbad, CA). The 5′ and 3′ amplification primers included KpnI and XhoI restriction sites, respectively. The amplified product was ligated into the KpnI and XhoI sites of pGL3 basic (Promega), and the sequence of the construct was confirmed. The plasmid was named pGL-TR4–1920.
5′-Serial deletion derivatives of TR4 promoter Luc reporter, including pGL-TR4–1420, pGL-TR4–1152, and pGL-TR4–605, were constructed by inserting different PCR-amplified DNA fragments containing 1420, 1152, and 605 bp of the human TR4 upstream region into the KpnI and XhoI sites of pGL3 basic using the plasmid pGL-TR4–1920 as the DNA template.
Site-directed mutagenesis of footprinted regions of TR4 promoter reporter pGL-TR4–1920 was performed with the QuikChange site- directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. The following oligonucleotide was used for mutagenesis: 804-CACAAGATCTGGATCGTATGCTTACTAAGAAACCAAAAATTATAGG-759, with the mutated nucleotide underlined.
Luciferase reporter assays
The cells were transfected with indicated reporter constructs or cotransfected with indicated constructs. Luc counts were normalized using TK Renilla luciferase. After washing with PBS and harvesting 48 h after transfection, cells were lysed in passive lysis buffer and luciferase activity was analyzed using a luminometer and dual-luciferase assay kit according to the manufacturer’s instructions (Promega). Results were obtained from at least three sets of transfection and are presented as the mean ± sd.
Immunofluorescence and microscopy
Briefly, C2C12 cells were plated on slide chambers, incubated overnight, and then treated with 20 μm LY294002 for 24 h or treated with 250 μm H2O2 for 2 h. The cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 100% methanol for 15 min at 4 C. The slides were rinsed with PBS twice and incubated in 5% BSA for 30 min. The primary antibody against FOXO3a was added for 1 h, and the secondary antibodies (antirabbit IgG) were added for 1 h, followed by application of the counter medium containing 4′,6′-diaminodino-2-phenylindole. Slides were examined by microscope. A RED-conjugating antirabbit antibody was used as secondary antibody.
RNA interference
The pSuperior.retro.puro vector (OligoEngine) was used for the expression of siRNA in C2C12 cells. FOXO3a-siRNA vector was generated by a gene-specific insert (GTGGAGCTGGACCCGGAGT) to target FOXO3a. A scrambled control vector was constructed using an insert (GTGTCTGTAGGAGTCATCC) with no significant homology to any mammalian gene sequence (33). To establish stable C2C12 cells, FOXO3a siRNA and scramble were transfected into C2C12 cells using SuperFect (QIAGEN). After 48 h of transfection, cells were selected by 4 μg/ml puromycin for 3 wk, and surviving cells were isolated and cultured for obtaining single colonies.
Chromatin immunoprecipitation (ChIP) assay
Cells (107) were fixed with 1% formaldehyde, pelleted, washed, and resuspended in 400 μl sodium dodecyl sulfate (SDS) lysis buffer [1% SDS, 10 mm EDTA, and 50 mm Tris-Cl (pH 8.0), with protease inhibitors]. After 10 min of incubation on ice, 600 μl ChIP dilution buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl (pH 8.0), and 16.7 mm NaCl, with protease inhibitors] was added, and genomic DNA was sheared by sonication to an average size of 500 bp. After removing 5% of the solution for evaluation of input complex, the lysates were precleared with 30 μl salmon sperm DNA-protein A agarose beads (Upstate Biotechnology, Lake Placid, NY) for 1 h at 4 C, divided into two equal parts, and immunoprecipitated overnight at 4 C using 4 μg antibody against either FOXO3a or control rabbit IgG. The immunocomplexes were collected using 30 μl salmon sperm DNA-protein A agarose beads; washed sequentially with ChIP wash buffer 1 [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.0), 150 mm NaCl], ChIP wash buffer 2 [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.0), 500 mm NaCl], and ChIP wash buffer 3 [0.25 m LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mm EDTA, 10 mm Tris (pH 8.0)]; and twice with 10 mm Tris-HCl (pH 8.0) and 1 mm EDTA (pH 8.0). DNA-protein complexes were collected in ChIP elution buffer (1% SDS, 0.1 m NaHCO3) and disrupted by incubation at 65 C for 5 h in the presence of 312 mm NaCl and 0.06 μg ribonuclease A per microliter. Proteins were removed by overnight digestion with proteinase K at 37 C, and DNA fragments were purified by phenol-chloroform extraction and ethanol precipitation and analyzed by quantitative PCR. The following primer pairs, which span the region −935 to −607 of the TR4 promoter, were used for the amplification of the FOXO3a-DBE site: forward primer, 5′-AGCCCAAGAGTTTAAGAGCAAG-3′; reverse primer, 5′-AGCGTCTGTAGGTCGGAAG-3′. The control internal TR4 primers were 5′-TTGCCTCTCAGGTACATCAC-3′ (−1441 bp) and 5′-TTTCCCACACAGTATCCATTTG-3′ (−1129 bp).
EMSA
The EMSA reaction was performed as described previously (33). Briefly, FOXO3a protein for EMSA was transcribed and translated in TNT reticulocyte lysates (Promega) with metabolical labeling with 35S-Met. The oligonucleotide probe for EMSA was end labeled with [α-32P]ATP, using T4 polynucleotide kinase (New England Biolabs, Beverly, MA). The EMSA reaction was performed with 2 μl TNT lysates in an EMSA buffer containing 10 mm HEPES (pH 7.9), 6% (vol/vol) glycerol, 2% (vol/vol) Ficoll, 100 mm KCl, 0.5 mm EDTA, 2.5 mm MgCl2, and 1 mm dithiothreitol. The reaction mixtures of proteins and DNA were incubated for 15 min at room temperature. For the antibody supershift assay, the mixtures were incubated for another 15 min in the presence of the anti-FOXO3a antibody. The protein DNA complexes were analyzed on a 5% native polyacrylamide gel. The results were visualized by autoradiography (Storm PhosphorImaging System; Amersham Pharmacia, Piscataway, NJ). The sequence of the probe used was 5′-GATCTGGATCGTATGTTTACTAAGAAACC-3′.
DNA pull-down assay
Oligonucleotides corresponding to FOXO DBE were synthesized, biotin labeled at the N terminus, and used in pull-down assays. Sequences of the oligonucleotides were: MnSOD-DBE (sense), 5′-biotin-CTTCTGACGTCTGTAAACAAGCCCAGCCC-3′; MnSOD-DBE (antisense), 5′-GGGCTGGGCTTGTTTACAGACGT-CAGAAG-3′; TR4-DBE (sense), 5′-biotin-GATCTGGAT-CGTATGTTTACTAAGAAACC-3′; TR4-DBE (antisense), 5′-GGTTTCTTAGTAAACATACGATCCAGATC-3′; TR4-DBE-m (sense), 5′-biotin-GATCTGGATCGTATGCTTACTAAGAAACC-3′; TR4-DBE-m (antisense), 5′-GGTTTCTTAGTAAGCATACGATCCAGATC-3′.
The double-stranded probes were made by annealing a 50-μm mixture of complementary oligonucleotides in 10 mm Tris-Cl, 50 mm NaCl, and 1 mm EDTA (pH 8.0) by heating to 95 C for 5 min followed by slowly cooling to room temperature. FOXO3a protein for DNA pull-down assay was transcribed and translated in TNT reticulocyte lysates (Promega) and was labeled with 35S-MET.
For pull-down assays, 2 μl TNT lysates were incubated in a 25-μl reaction mixture consisting of 10 μm probe and 1× binding buffer, which is the same as the EMSA buffer, and 0.05 mg/ml poly(deoxyinosine-deoxycytosine) (Promega). Incubations were for 30 min at room temperature, after which the reaction volume was increased to 0.5 ml with modified binding buffer. To capture the complexes, streptavidin-agarose (Sigma, St. Louis, MO) was added, and the incubation continued for 6 h at 4 C. The complexes were collected by pulse centrifugation in a microcentrifuge, washed three times with modified binding buffer, and eluted from the beads by addition of 2× gel loading buffer and heating to 95 C for 5 min. Proteins were then separated by 8% SDS-PAGE and visualized by autoradiography.
Western blot analysis
Whole-cell extracts were isolated in radioimmunoprecipitation assay buffer supplemented with phosphatase inhibitors. Protein samples were separated by electrophoresis in 8 or 10% polyacrylamide-SDS gel and subjected to immunoblotting. Antibodies used included those for TR4, anti-FOXO3a, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin. The anti-TR4 antibody, N15, was produced as described previously (8). The anti-FKHRL1 (FOXO3a) (no. 06951) was from the Upstate Biotechnology. The alkaline phosphatase-conjugated or horseradish peroxidase-conjugated secondary antimouse or antirabbit antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Real-time PCR analysis
Total RNAs were isolated by using TRIzol following the manufacturer’s protocol. Five micrograms of RNA were reverse transcribed by SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Oligo-deoxythymidine was used as the primer for first-strand synthesis. cDNA was subjected to real-time PCR using the SYBR Green PCR reagents kit (Bio-Rad Laboratories, Hercules, CA). Experiments were performed in triplicate for each data point. β-Actin was used as a control for normalization. Quantitative analysis was performed using iCycler analysis software (Bio-Rad Laboratories). The quantification of the each sample relative to the control was calculated using the 2-ΔΔCT method. PCR primers were as follows: mouse TR4, forward primer, 5′-CATATTCACCACCTCGGACAAC-3′; reverse primer, 5′-TGACGCCACAGACCACAC-3′; and human TR4, forward primer, 5′-GGCYCTGAACCTGCCTCTG-3′; reverse primer, 5′-AGGATGAACTGCTG-TTTGGG-3′.
Results
FOXO3a induces TR4 promoter activity
To date, no ligand has been identified for TR4, and how the biological activity of TR4 is regulated without the involvement of specific ligand(s) still remains largely unknown. To explore how upstream signal/factor(s), such as transcriptional factor(s), can regulate TR4, we analyzed the sequence of the TR4 promoter, and found a consensus FOXOs binding site (DBE, 5′-TGTTTAC-3′) (32,34,35), located at −781 to −787 bp and a DBE cobinding site (5′-GAGATCAA-3′) located at −648 to −656 bp (35) (Fig. 1A). Early reports illustrated that FOXOs function as key transcription factors to regulate several important genes that control diverse biological activities, including cell differentiation, proliferation, cell death, metabolism, and longevity (20,21,22,23,24,25,26). Therefore, we were interested in determining whether FOXOs can regulate TR4 genes and consequently affect cellular behavior.
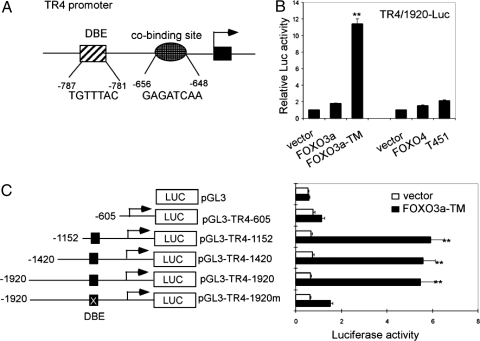
Figure 1.
FOXO induces TR4 promoter activity. A, Schematic representation of TR4 promoter. Putative FOXO consensus binding site and potential cobinding site locations and sequences are shown. B, H1299 cells were transfected with 500 ng pGL-TR4–1920 and 200 ng vector, FOXO3a, FOXO3a-TM, FOXO4, or FOXO4-T451E. After 48 h, cells were harvested and Luc activities were measured. **, P < 0.01 (ANOVA), as compared with vector or FOXO3a WT. C, Schematic diagram of 5′ serial deletions and site-directed mutagenesis of TR4 promoter reporter constructs (left panel); 500 ng pGL3 and indicated TR4 promoter reporter constructs were cotransfected with 200 ng FOXO3a-TM or vector into H1299 cells. After 48 h, cells were harvested and Luc activities were measured. **, P < 0.01 (ANOVA), as compared with vector or FOXO3a-TM upon pGL3, pGL-TR4–605 or pGL-TR4–1920m.
We first constructed a Luc reporter linked to the TR4 5′ promoter with the sequence from −1920 to +95 bp and examined the influence of FOXOs on the TR4 promoter activity. As shown in Fig. 1B, in H1299 cells, overexpression of FOXO3a-TM, a constitutive activated FOXO3a, significantly increased the TR4 promoter activity, and the activation of TR4-Luc was induced to a lesser extent by WT FOXO3a. The effect on TR4-Luc by another FOXO factor, FOXO4, was examined, and we found that both FOXO4 and FOXO4-T451E, a constitutive activated FOXO4, can slightly induce TR4 promoter activity (Fig. 1B); therefore, we focused only on the FOXO3a in the following studies.
To verify whether FOXO3a-induced TR4 promoter activity is mediated though the DBE, we constructed a series of deletions of the TR4 promoter reporter either including or excluding FOXO-DBE and its cobinding site (Fig. 1C, left panel). We then tested the effect of FOXO3a-TM on these different constructs. As shown in Fig. 1C (right panel), the TR4 promoter reporter constructs that contain FOXO3a-DBE (pGL-TR4–1920, pGL-TR4–1420, and pGL-TR4–1152) can be induced by FOXO3a-TM to much higher degree than those constructs that exclude FOXO3a-DBE (pGL-TR4–605 and pGL-3 basic vector), suggesting that FOXO3a-DBE is responsible for the FOXO3a-induced TR4 gene expression.
FOXOs are transcriptional factors that bind to the consensus binding sequence, TGTTTAC and transactivate downstream targeted genes. The change of any one base in TGTTTAC was reported previously to dissociate the FOXO from the DBE (23,32). To further prove that FOXO3a directly binds to FOXO3a-DBE to induce TR4 gene expression, site-directed mutagenesis was performed in which a mutation at −785 bp of the TR4 promoter, from T to C, was introduced. As shown in Fig. 1C, introducing FOXO3a-TM to pGL-TR4–1920m, a single T-to-C substitution in the TGTTTAC sequence, almost eliminated the FOXO3a-induced TR4 expression. These data strongly suggest that FOXO3a can induce TR4 promoter activity through binding to the FOXO3a-DBE located at −781 to −787 bp in the TR4 promoter.
FOXO3a directly binds to the TR4 promoter
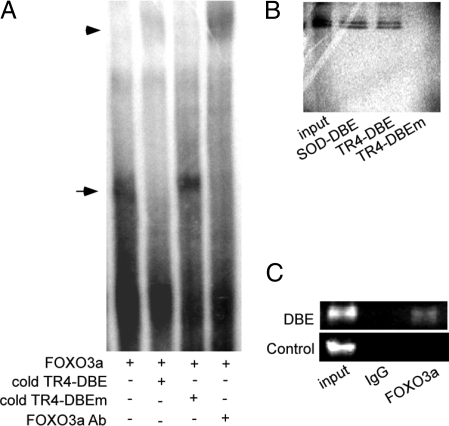
EMSA was used to confirm the binding of FOXO3a to the TR4-DBE in the TR4 promoter. As shown in Fig. 2A, a specific band was formed when in vitro-translated FOXO3a was incubated with [32P]-labeled TR4-DBE (lane 1), and this specific band can be competed out with TR4-DBE cold competition (lane 2) but not mutated TR4-DBE cold competition (lane 3). Furthermore, the specific band can be supershifted by FOXO3a antibody (lane 4). The direct binding of FOXO3a to TR4-DBE was further tested by DNA pull-down assays in which [35S]-labeled FOXO3a protein was incubated with biotinylated double-stranded FOXO-DBE oligonucleotides that are from MnSOD promoter (as a positive control) and TR4 promoter. As shown in Fig. 2B, DNA-protein complexes were found in FOXO3a with both DBEs from the promoters of MnSOD as well as TR4 (lanes 2 and 3). However, the DBE mutant oligonucleotide (TR4-DBEm) failed to form a DNA-protein complex (lane 4). Data from Fig. 2, A and B, suggest that FOXO3a binds to DBE in TR4 promoter specifically in vitro. ChIP assays further prove the binding between FOXO3a and TR4/DBE in intact cells in vivo. Using FOXO3a-specific antibody vs. normal IgG control, to pull down the FOXO3a-DNA complex from nontransfected cells, we found a specific PCR product can be amplified only from the FOXO3a antibody pull-down complex, not IgG control (Fig. 2C). Together, Fig. 2 results demonstrate that FOXO3a can bind directly to the DBE in vitro and in vivo.
Figure 2.
FOXO3a directly binds to the TR4 promoter. A, [32P]-labeled TR4-DBE probe and in vitro-translated FOXO3a protein were used in EMSA. FOXO3a-specific antibody, the double-stranded DNA oligonucleotide of TR4-DBE, or its single point-mutant TR4-DBEm were added in EMSA (lanes 4, 2, and 3, respectively). B, DNA pull-down assays used [35S]-labeled in vitro-translated FOXO3a protein with biotinylated, double-stranded DNA oligonucleotides encompassing FOXO DBE of the MnSOD promoter (SOD-DBE), FOXO DBE of the TR4 promoter (TR4-DBE), and its point mutant (TR4-DBEm) in lanes 2, 3, and 4, respectively. Lane 1, input control protein alone. C, ChIP assay showed FOXO3a bound to the TR4 promoter in H1299 cells. The DNA protein complexes were cross-linked with formaldehyde and subjected to ChIP assay using an anti-FOXO3a antibody or rabbit IgG (negative control) and the primers to amplified DBE and control primers. The primer sequences and location were indicated in Materials and Methods.
FOXO3a induces TR4 gene expression and transactivation
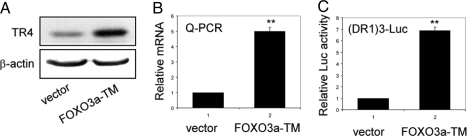
To examine whether the binding of FOXO3a to the TR4 5′ promoter region would result in induction of TR4 expression and transactivation, we applied both Western blot and quantitative PCR analyses to determine TR4 expression after transfection of FOXO3a into H1299 cells. As shown in Fig. 3, A and B, overexpression of FOXO3a-TM resulted in increased TR4 protein and mRNA expression 3 d after transfection. The FOXO3a-induced TR4 expression was also found after 2 d transfection with less degree of induction (data not shown). To assay TR4 activity, the synthetic TR4-responsive element, which is composed of AGGTCA with direct repeat motif with one nucleotide space (DR1), was constructed into a reporter gene pGL3p-Luc. As shown in Fig. 3C, overexpression of constitutive FOXO3a-TM significantly induced the (DR-1)3-Luc activity. Together, results from Figs. 1–3 demonstrate that FOXO3a transactivates TR4 gene activity via binding directly to the DBE in the TR4 5′ promoter.
Figure 3.
FOXO3a enhances TR4 expression and transactivation. A, H1299 (5 × 105) cells were seeded into 100-mm dishes and transfected with 10 μg FOXO3a-TM or 10 μg empty vector by Superfect. After transfection for 72 h, the cells were harvested, and protein was isolated for Western blot by using antibody against FOXO3a with β-actin as a loading control. B, H1299 (5 × 105) cells were transfected with 10 μg of FOXO3a-TM or 10 μg empty vector by Superfect. After 48 h, the cells were harvested, and total RNA was isolated to perform real-time PCR. **, P < 0.01 (Student’s t analysis) as compared with vector. C, pGL-DR13 (500 ng) were cotransfected with 200 ng FOXO3a-TM or empty vector into H1299 cells. After 48 h, the Luc activity was measured. **, P < 0.01 (Student’s t analysis) as compared with vector.
Modulation of FOXO3a activity by LY294002 increases TR4 gene expression
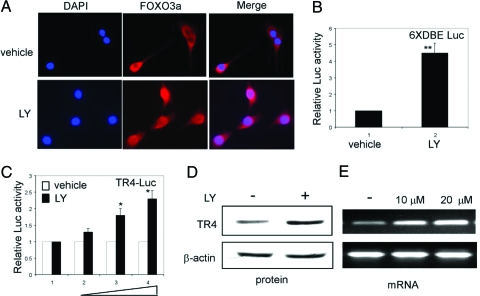
FOXOs are regulated by the PI3K/Akt pathway. Direct phosphorylation of FOXO proteins by Akt results in cytoplasmic retention and the inactivation of FOXOs, which inhibit expression of FOXO-regulated genes. To test whether modulation of FOXO3a activity can alter the TR4 gene expression, we added 20 μm LY294002, a selective PI3K inhibitor that could activate FOXO3a, into C2C12 cells and found that FOXO3a translocated into the nucleus resulting in the activation of its target 6×DBE Luc reporter (Fig. 4, A and B). Similarly, the activation of FOXO3a by LY294002 treatment could promote the TR4 promoter activity, protein, and mRNA expression (Fig. 4, C–E). Together results from Fig. 4 clearly demonstrate that modulation of FOXO3a activity might result in the increased TR4 gene expression.
Figure 4.
LY294002 increases TR4 gene expression through activating FOXO3a. A, C2C12 cells (1 × 104) were plated into two-well chamber sliders for 24 h and then treated with 20 μm LY294002 or the same amount of vehicle (ethanol) for 16 h. Cells were fixed and FOXO3a was stained as described in Materials and Methods. B, pGL-DBE × 6 (500 ng) were transfected into C2C12 cells, which were treated with 20 μm LY294002 or vehicle for 16 h before the Luc activity was measured. **, P < 0.01 (Student’s t test analysis) as compared with vehicle. C, LY294002 enhanced TR4 promoter activity. pGL-TR4-1920 (500 ng) were transfected into C2C12 cells, which were treated with 0, 10, 20, or 30 μm LY294002 or vehicle for 16 h before the Luc activity was measured. *, P < 0.05 (ANOVA) as compared with vehicle or 10 μm LY294002. D, C2C12 cells were treated with 20 μm LY294002 or vehicle for 24 h and then harvested for Western blot analysis. E, C2C12 cells were treated with 10 or 20 μm LY294002 or vehicle for 16 h, and then total RNA was isolated to perform PCR.
H2O2 induces TR4 gene expression through activating FOXO3a
Early reports documented well that FOXO3a might function as a stress-responsive transcription factor. In response to oxidative stress, the cytosol FOXO3a can be activated and translocated into the nucleus via phosphorylation mechanisms (21). We were interested to see whether oxidative stress might go through FOXO3a to induce TR4 gene expression. As expected, we found that FOXO3a translocated into the nucleus, resulting in the activation of its target 6 × DBE Luc reporter after cells were challenged with oxidative stress inducer H2O2 (Fig. 5, A and B). We also found that H2O2 could induce the TR4 promoter activity in C2C12 and H1299 cells (Fig. 5C). TR4 protein and mRNA expression were enhanced after treatment with H2O2 in C2C12 and H1299 cells as well as primary MDF cells (Fig. 5, D and E). TR4 transactivation determined by pGL-(DR1)3 Luc reporter assay was also enhanced in C2C12 and H1299 cells after being challenged by H2O2 (data not shown). Together these data show that H2O2 might induce TR4 gene expression and transactivation through activating the FOXO3a.
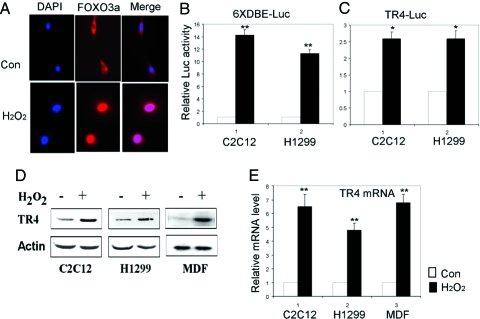
Figure 5.
H2O2 induces TR4 gene expression through activating FOXO3a. A, C2C12 cells (1 × 104) were plated into two-well chamber slides for 24 h and then untreated or treated with 250 μm H2O2 for 2 h. Cells were fixed and stained as described in Materials and Methods. Con, Control; DAPI, 4′,6′-diamidino-2-phenylindole. B, pGL-DBE × 6 (500 ng) were transfected into H1299 and C2C12 cells for 40 h and then left untreated or treated with 250 μm H2O2 for 2 h; the Luc activity was measured 6 h after H2O2 treatment. **, P < 0.01 (Student’s t test analysis) as compared with control. C, pGL-TR4-1920 (500 ng) were transfected into H1299 and C2C12 cells for 40 h and then left untreated or treated with 250 μm H2O2 for 2 h; the Luc activity was measured 6 h after H2O2 treatment. *, P < 0.05 (Student’s t test analysis) as compared with control. D, Total cell lysates of C2C12, H1299, and primary MDF cells untreated or treated with 250 μm H2O2 for 2 h were analyzed for expression of TR4 with actin as a loading control. E, Total RNA isolated from C2C12, H1299 and primary MDF cells untreated or treated with 250 μm H2O2 for 2 h were subjected to real-time PCR. **, P < 0.01 (Student’s t test analysis) as compared with control.
Suppression of FOXO3a via FOXO3a-siRNA interrupts the H2O2-induced TR4 expression and transactivation
To further prove FOXO3a plays essential roles for the mediation of induced TR4 expression and transactivation upon H2O2, we constructed FOXO3a-siRNA, to knock down FOXO3a mRNA expression, and examined its influence on the TR4 expression in C2C12 cells. As shown in Fig. 6A, FOXO3a-siRNA significantly inhibited endogenous FOXO3a expression, resulting in the suppression of TR4 protein expression (Fig. 6B). As expected, the H2O2-induced TR4 expression and transactivation were suppressed after transfecting FOXO3a-siRNA into C2C12 cells (Fig. 6, C–E). These data demonstrated that knockdown of FOXO3a expression could suppress H2O2-induced TR4 expression and transactivation, suggesting that TR4 is a direct target of FOXO3a that mediates cellular stress response.
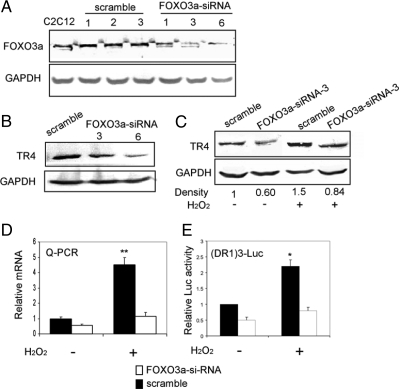
Figure 6.
siRNA-mediated knockdown of FOXO3a inhibits TR4 induction by oxidative stress. A, Knockdown of FOXO3a using siRNA. Total cell lysates of C2C12 parental cells, C2C12 FOXO3a scrambled stable clones -1, -2, -3 cells, and C2C12 FOXO3a siRNA stable clones -1, -3, and -6 cells were analyzed for expression of FOXO3a with GAPDH as a loading control. B, TR4 protein expression decreased after FOXO3a knockdown. Total cell lysates of C2C12 FOXO3a-scramble 1 cells, and C2C12 FOXO3a-siRNA-3 and -6 cells were analyzed for expression of TR4 with GAPDH as a loading control. C, Total cell lysates of C2C12 FOXO3a-scramble 1 cells and C2C12 FOXO3a-siRNA-3 cells untreated or treated with 250 μm H2O2 for 2 h were analyzed for expression of TR4 with GAPDH as a loading control. At least two independent experiments were done for each result. The image was scanned for determination of the density. The relative density, compared with GAPDH, was shown. D, Total RNA isolated from C2C12 FOXO3a-scramble 1 cells and C2C12 FOXO3a-siRNA-3 cells untreated or treated with 250 μm H2O2 for 2 h were subjected to real-time PCR. **, P < 0.01 (ANOVA) as compared with the other three groups. E, pGL-DR1 × 3 (500 ng) were cotransfected with 200 ng FOXO3a-scramble, or FOXO3a-siRNA into C2C12 cells untreated or treated with 250 μm H2O2 for 2 h; the Luc activity was measured 6 h after H2O2 treatment. *, P < 0.05 (ANOVA) as compared with the other three groups.
Restoring TR4 back to FOXO3a knockdown cells increases cell tolerance to oxidative stress
If FOXO3a is the TR4 upstream transcriptional factor that mediates cellular oxidative stress, then the reduction of cellular tolerance to oxidative stress in FOXO3a-siRNA cells should recover if we restore the downstream target TR4 back into cells. As shown in Fig. 7A, the TR4 and FOXO3a mRNA levels were determined by quantitative PCR in C2C12 stable cells. FOXO3a and TR4 mRNA were reduced in C2C12-FOXO3a-siRNA stable clones, compared with scramble control, whereas adding TR4 increased TR4 mRNA levels. As shown in Fig. 7B, the response to oxidative stress was then compared among three clones, and we found that C2C12 cells stably transfected with FOXO3a-siRNA were more sensitive to oxidative stress challenge, and fewer cells survived, compared with those FOXO3a-scramble transfected cells. Transfection of functional TR4 back into the FOXO3a-siRNA C2C12 cells then increased cell survival under the H2O2 treatment. These data indicated that TR4 may play an important role in cellular survival upon the oxidative stress challenge.
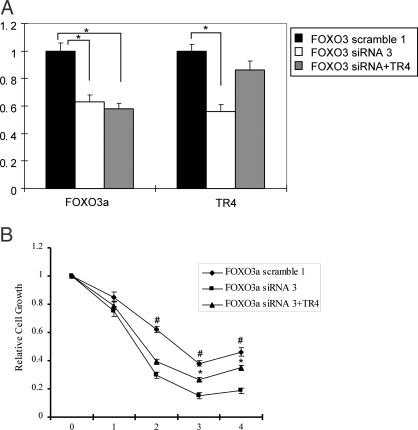
Figure 7.
FOXO3a knockdown cells display intolerance to H2O2 challenge and TR4 can rescue cell growth. A, Expression of FOXO3a and TR4 mRNA in FOXO3a-scramble 1, FOXO3a-siRNA 3, and FOXO3a-siRNA 3+TR4 C2C12 cells. *, P < 0.05 (ANOVA) as compared with the other two groups. B, Cells (1 × 103) of three C2C12 clones were seeded into 96-well dishes for 24 h and then untreated or treated with 250 μm H2O2 for 2 h. Dimetylthiazol-diphenyltetra-zoliumbromide (MTT) assay was performed at 0, 1, 2, 3, and 4 d after H2O2 treatment. Relative cell growth rates are presented as fold induction (means ± sd) relative to that of untreated cells on the same day set as 1, from three independent experiments. The relative cell growth rates at the same day were compared. *, P < 0.05 (ANOVA) when comparing FOXO3a-siRNA3 C2C12 cells with FOXO3a-siRNA3+TR4 cells at d 3 and 4. #, P < 0.05 (ANOVA) when comparing FOXO3a-scramble1 C2C12 cells with FOXO3a-siRNA3 cells at d 2, 3, and 4.
TR4 KO cells are less tolerant to oxidative stress challenge
To confirm the role of TR4 in the oxidative stress resistance, we compared primary MDFs from TR4 WT or TR4 KO mice. The genotype of MDFs was confirmed at both gene and mRNA levels (Fig. 8, A and B). We then treated TR4 WT vs. TR4 KO MDFs with 250 μm H2O2 and measured their survival on 0, 1, 2, and 3 d after treatment. As shown in Fig. 8C, TR4 KO MDFs were less tolerant to H2O2 challenge with less cell survival, compared with TR4 WT MDFs. Taken together, our results further confirmed TR4 roles in the response to oxidative stress challenge. These results strongly suggest that TR4 is a direct downstream target of FOXO3a that plays important roles in the cellular stress resistance.
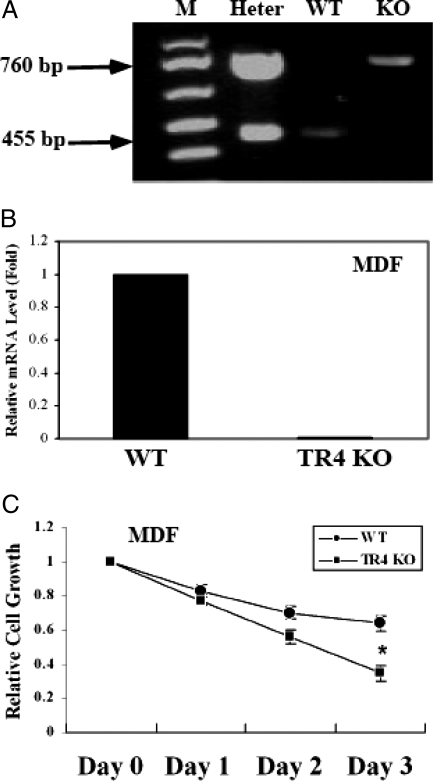
Figure 8.
TR4 KO MDF cells are less tolerant to oxidative stress challenge. A, Genotyping of experimental animals. M, Marker; Heter, heterozygous; WT, wild type; KO, knockout. B, Expression of TR4 mRNA in primary MDF cells derived from TR4 WT and TR4 KO mice. **, P < 0.01 (Student’s t test analysis) as compared with WT. C, MDF cells (2 × 103) were seeded into 96-well dishes for 24 h and then untreated or treated with 250 μm H2O2 for 2 h. Dimetylthiazoldiphenyltetra-zoliumbromide assay was performed at 0, 1, 2, and 3 d after H2O2 treatment. Cell growth rates are presented as fold induction (means ± sd) relative to that of untreated cells on the same day set as 1 from three independent experiments. The difference of relative cell growth rates at the same day between primary MDF cells derived from TR4 WT and TR4 KO mice was analyzed. *, P < 0.05 (Student’s t test analysis) at d 3.
Discussion
Early studies reveal that TR4 modulates many signaling pathways (5,6,7,8,9,10). However, as an orphan receptor, there is no ligand identified for TR4, and how the biological activity of TR4 is triggered or regulated without the involvement of specific ligands still remains largely unknown. A recent study showed that phosphorylation of TR4 by MAPK could affect TR4 activity (15). In this present study, we identify a TR4 upstream regulator, FOXO3a, through which TR4 activity is regulated. Many approaches that modulate FOXO3a activities, such as gain or loss of FOXO3a expression, were used to verify this FOXO3a-TR4 signal pathway. Our findings demonstrated, for the first time, that FOXO3a directly regulates TR4 gene activity at the transcriptional level during the oxidative stress.
FOXO3a has been proven to be involved in determining the longevity-cell survival vs. death via regulating stress responses (21,36). In the presence of insulin or growth factors, the PI3K-Akt/SGK pathway is activated to inhibit FOXO3a-dependent transcription through the phosphorylation of FOXO3a and subsequent retention of FOXO3a in the cytoplasm. In contrast, FOXO3a can translocate into the nucleus in response to stress stimuli and then induce transcription activity (21). In this scenario, FOXO3a promotes cell cycle arrest at the G1/S and G2/M transitions that allow cells to detoxify and repair damaged DNA (27,28).
Several FOXO3a downstream targeted genes have been identified, such as DNA repair and growth arrest DNA damage-inducible protein 45 gene (28,29), ROS detoxification genes MnSOD and catalase (23,28,29,30) as well as cell cycle regulating genes, cyclin D1 and cyclin-dependent kinase inhibitor p27KIP1. Our data found that oxidative stress induced TR4 expression via a transcriptional regulation of FOXO3a to promote cell survival. Suppression of FOXO3a via siRNA resulted in less oxidative stress resistance with less cell survival after H2O2 treatment and addition of functional TR4 can increase the oxidative stress resistance with more cell survival after H2O2 treatment. Together our data demonstrated that TR4 is a direct key downstream target of FOXO3a for the mediation of oxidative stress challenge and overall cell survival.
Decreased tolerance to stress is thought to be the hallmark of aging (37), and accumulating evidence also suggests that oxidative cellular damage is the driving force to aging in species ranging from C. elegans to Drosophila to humans (38,39,40,41). Free radical-derived ROS are constantly generated in most living tissues and can potentially damage cellular macromolecules (lipids, nucleic acids, carbohydrates, and proteins), and excessive amounts of ROS accumulating in the cells would lead to organism deterioration (42) and eventually to death. Oxidative damage products such as protein carbonyls, 8-oxo-guanine, and lipid peroxidation products have been found to accumulate exponentially during aging in a variety of tissues in different species (41,43,44,45). Therefore, how well cells respond to oxidative stress could be a determining factor of the life span in different species of organisms.
Data in our study show that oxidative stress induced TR4 gene expression and transactivation activity. Cells lacking TR4 were more sensitive to oxidative stress. In addition, we also found that the 8-oxoguanine level was increased in TR4 KO MEF cells, which indicated a higher oxidative damage in TR4 KO MEF cells (data not shown). These results are consistent with the premature aging phenotypes of TR4 knockout mice including reduced life span, early loss of female fertility, osteoporosis, and kyphosis (Lee, Y.-F., manuscript in preparation). Using the pathway focus array to identify genes that are responsible for the anti-ROS effects mediated by TR4, we found that TR4 might be involved in the oxygen transport function, and the detailed molecular mechanisms are now under investigation.
All these evidences raise the possibility that TR4 plays important roles in mediating cellular response to oxidative stress and therefore, its activity might be also critical for organism longevity. Because TR4 belongs to the steroid nuclear receptor superfamily, it is very tempting to hypothesize that, like other receptors in this family, TR4 activity might also be regulated by small molecules/antagonists. These molecules might therefore be able to control cell survival by turning on/off TR4 in response to genotoxic stress. Identification of the oxidative stress-FOXO3a-TR4 axis not only adds a new player, TR4, into this complex cellular defense system but also enlightens the importance of the biological activity of TR4 for the cell survival.
Acknowledgments
The authors are grateful to Dr. M. Greenberg (Harvard Medical School, Boston, MA), Dr. B. M. T. Burgering (University Medical Center, Utrecht, The Netherlands), and Dr. Furuyama (Kumamoto University, Kumamoto, Japan) for their reagents. We thank Karen Wolf (University of Rochester Medical Center, Rochester, NY) for helpful reading of the manuscript.
Footnotes
This work was supported by American Cancer Society Grant RSG-06-123 (to Y.-F. L.); National Institutes of Health Grant RO1 DK 60948 (to C.C.); and the Pao Yu-Kong and Pao Zhao-Long scholarship for Chinese students studying abroad (to G.L.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 3, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; DBE, daf-16 family protein-binding element; FOXO, Forkhead box O; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KO, knockout; Luc, luciferase; MDF, mouse dermal fibroblast; MnSOD, manganese superoxide dismutase; PI3K, phosphatidylinositol 3-kinase; ROS, reactive oxygen species; SDS, sodium dodecyl sulfate; siRNA, small interfering RNA; TR4, testicular orphan nuclear receptor 4; WT, wild type.
References
- Beato M, Herrlich P, Schutz G 1995 Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Da Silva SL, Ideta R, Lee Y, Yeh S, Burbach JP 1994 Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily. Proc Natl Acad Sci USA 91:6040–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V 1997 Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J Mol Endocrinol 19:207–226 [DOI] [PubMed] [Google Scholar]
- Kim E, Xie S, Yeh SD, Lee YF, Collins LL, Hu YC, Shyr CR, Mu XM, Liu NC, Chen YT, Wang PH, Chang C 2003 Disruption of TR4 orphan nuclear receptor reduces the expression of liver apolipoprotein E/C-I/C-II gene cluster. J Biol Chem 278:46919–46926 [DOI] [PubMed] [Google Scholar]
- Lee YF, Lee HJ, Chang C 2002 Recent advances in the TR2 and TR4 orphan receptors of the nuclear receptor superfamily. J Steroid Biochem Mol Biol 81:291–308 [DOI] [PubMed] [Google Scholar]
- Lee YF, Pan HJ, Burbach JP, Morkin E, Chang C 1997 Identification of direct repeat 4 as a positive regulatory element for the human TR4 orphan receptor. A modulator for the thyroid hormone target genes. J Biol Chem 272:12215–12220 [DOI] [PubMed] [Google Scholar]
- Lee YF, Shyr CR, Thin TH, Lin WJ, Chang C 1999 Convergence of two repressors through heterodimer formation of androgen receptor and testicular orphan receptor-4: a unique signaling pathway in the steroid receptor superfamily. Proc Natl Acad Sci USA 96:14724–14729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YF, Young WJ, Burbach JP, Chang C 1998 Negative feedback control of the retinoid-retinoic acid/retinoid X receptor pathway by the human TR4 orphan receptor, a member of the steroid receptor superfamily. J Biol Chem 273:13437–13443 [DOI] [PubMed] [Google Scholar]
- Lee YF, Young WJ, Lin WJ, Shyr CR, Chang C 1999 Differential regulation of direct repeat 3 vitamin D3 and direct repeat 4 thyroid hormone signaling pathways by the human TR4 orphan receptor. J Biol Chem 274:16198–16205 [DOI] [PubMed] [Google Scholar]
- Kim E, Yang Z, Liu NC, Chang C 2005 Induction of apolipoprotein E expression by TR4 orphan nuclear receptor via 5′ proximal promoter region. Biochem Biophys Res Commun 328:85–90 [DOI] [PubMed] [Google Scholar]
- Mu X, Lee YF, Liu NC, Chen YT, Kim E, Shyr CR, Chang C 2004 Targeted inactivation of testicular nuclear orphan receptor 4 delays and disrupts late meiotic prophase and subsequent meiotic divisions of spermatogenesis. Mol Cell Biol 24:5887–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LL, Lee YF, Heinlein CA, Liu NC, Chen YT, Shyr CR, Meshul CK, Uno H, Platt KA, Chang C 2004 Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci USA 101:15058–15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Collins LL, Uno H, Chang C 2005 Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4. Mol Cell Biol 25:2722–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq MM, Gupta P, Tsai NP, Wei LN 2006 Modulation of testicular receptor 4 (TR4) activity by MAP-kinase mediated phosphorylation. Mol Cell Proteomics 5:2072–2082 [DOI] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G 2001 Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol 11:1950–1957 [DOI] [PubMed] [Google Scholar]
- Murakami S, Tedesco PM, Cypser JR, Johnson TE 2000 Molecular genetic mechanisms of life span manipulation in Caenorhabditis elegans. Ann NY Acad Sci 908:40–49 [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R 1993 A C. elegans mutant that lives twice as long as wild type. Nature 366:461–464 [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C 1997 daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278:1319–1322 [DOI] [PubMed] [Google Scholar]
- Accili D, Arden KC 2004 FOXOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426 [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A 2005 FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24:7410–7425 [DOI] [PubMed] [Google Scholar]
- Burgering BM, Kops GJ 2002 Cell cycle and death control: long live Forkheads. Trends Biochem Sci 27:352–360 [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM 2002 Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–321 [DOI] [PubMed] [Google Scholar]
- Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, Lam EW, Burgering BM 2002 Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol 22:2025–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH 2002 Cell cycle inhibition by FOXO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol 22:7842–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH 2002 The forkhead transcription factor FOXO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol 168:5024–5031 [DOI] [PubMed] [Google Scholar]
- Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N 2002 FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem 277:26729–26732 [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Grenier JM, Datta SR, Fornace Jr AJ, DiStefano PS, Chiang LW, Greenberg ME 2002 DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296:530–534 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR 2002 A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2:81–91 [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T 2002 Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295:2450–2452 [DOI] [PubMed] [Google Scholar]
- Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, Dalal SN, DeCaprio JA, Greenberg ME, Yaffe MB 2002 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol 156:817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Nakazawa T, Nakano I, Mori N 2000 Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J 349:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Xie S, Jamaluddin MS, Altuwaijri S, Ni J, Kim E, Chen YT, Hu YC, Wang L, Chuang KH, Wu CT, Chang C 2005 Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem 280:33558–33565 [DOI] [PubMed] [Google Scholar]
- Biggs 3rd WH, Cavenee WK, Arden KC 2001 Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome 12:416–425 [DOI] [PubMed] [Google Scholar]
- Xuan Z, Zhang MQ 2005 From worm to human: bioinformatics approaches to identify FOXO target genes. Mech Ageing Dev 126:209–215 [DOI] [PubMed] [Google Scholar]
- Birkenkamp KU, Coffer PJ 2003 Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans 31:292–297 [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN 2000 Why do we age? Nature 408:233–238 [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ 2000 Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247 [DOI] [PubMed] [Google Scholar]
- Hekimi S, Guarente L 2003 Genetics and the specificity of the aging process. Science 299:1351–1354 [DOI] [PubMed] [Google Scholar]
- Melov S, Coskun PE, Wallace DC 1999 Mouse models of mitochondrial disease, oxidative stress, and senescence. Mutat Res 434:233–242 [DOI] [PubMed] [Google Scholar]
- Landis GN, Tower J 2005 Superoxide dismutase evolution and life span regulation. Mech Ageing Dev 126:365–379 [DOI] [PubMed] [Google Scholar]
- Droge W 2002 Free radicals in the physiological control of cell function. Physiol Rev 82:47–95 [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R 1996 Oxidative stress, caloric restriction, and aging. Science 273:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Weindruch R, Aiken JM 1998 Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic Biol Med 25:740–747 [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS 1994 Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263:1128–1130 [DOI] [PubMed] [Google Scholar]