Abstract
The human glycoprotein hormone α-subunit (αGSU) gene is transcriptionally regulated by glucocorticoids in a cell type-specific fashion. In direct contrast to repression of αGSU by glucocorticoids in placenta, glucocorticoid receptor (GR) modulation in the pituitary is little understood. We show that glucocorticoids stimulate the αGSU promoter in immortalized pituitary gonadotrope-derived LβT2 cells, whereas estrogens, androgens, and progestins have no significant effect. Moreover, GR acts in a dose-dependent manner at physiological concentrations of glucocorticoids. Transient transfection of GR with dexamethasone (Dex) treatment further stimulates the αGSU promoter, but this induction is severely diminished using a receptor mutated in the DNA-binding domain. Truncation and cis mutations demonstrate that glucocorticoid response element 2 (GRE2) and cAMP-response element 2 (CRE2) within −168 bp of the human αGSU promoter are critical for induction. Moreover, dominant-negative CRE-binding protein markedly inhibits basal but also Dex induction of αGSU promoter activity. Additionally, GR specifically binds to GRE2 in the human αGSU promoter in vitro and to the 5′ region of the endogenous mouse αGSU gene in vivo. Furthermore, overexpression of the homeobox factor, Distal-less 3 that regulates this gene in placental cells through a site partially overlapping GRE2, blocks Dex induction of αGSU in gonadotrope cells, indicating that placenta-specific expression of Dlx3 may interfere with GR, resulting in repression in placental cells vs. induction in gonadotrope cells. These results demonstrate the stimulatory role played by glucocorticoids in αGSU gene expression in the pituitary gonadotrope, in contrast to repression in placental cells, and highlight the tissue-specific nature of steroid hormone action.
THE GLYCOPROTEIN HORMONE α-subunit (αGSU) gene is expressed in pituitary gonadotropes and thyrotropes in all mammalian species as well as in primate and equine placenta. It heterodimerizes with separate β-subunits of the glycoprotein hormones, including those of LH, FSH, TSH, and human chorionic gonadotropin (hCG) to give the biologically active, heterodimeric hormones (reviewed in Ref. 1). The common α-subunit and the four unique β-subunits are each the product of an individual, single-copy gene (2). In the pituitary, LH and FSH are synthesized in the gonadotrope and TSH is synthesized in the thyrotrope, whereas hCG is a product of human placental trophoblast cells. These hormones play critical roles in reproduction (LH and FSH), growth and metabolism (TSH), and maintenance of pregnancy (hCG).
Steroid hormones regulate gonadotropin subunit gene expression either by acting at the hypothalamus to alter GnRH pulsatility or acting directly at the pituitary gonadotrope. The negative feedback effects of gonadal steroids include repression of the transcription of both the common αGSU and LHβ subunit genes (1). More specifically, estrogens suppress αGSU transcription in vivo largely by suppressing hypothalamic GnRH, because estradiol had no effect on α-subunit mRNA synthesis in rat pituitary cells in vitro (3). In addition, activated estrogen receptor (ER)-α failed to suppress expression of a chimeric human α-chloramphenicol acetyltransferase (α-CAT) vector in cotransfection studies in αT3-1 cells (4), a mouse cell line model for the developing pituitary gonadotrope. Little is known about progesterone actions on α-subunit transcription; it either reduces (5) or has no effect in rat pituitaries (6). Androgens, like estrogens, appear to suppress α-subunit expression. In vivo, testosterone suppressed α-subunit mRNA synthesis (7). The human α-CAT reporter gene was also repressed by testosterone when transiently transfected into αT3-1 cells along with androgen receptor (AR) (8). This suppressive effect of androgens on human α-subunit transcription was found to be mediated by protein-protein interactions with the two cAMP-response element (CRE)-binding transcription factors c-Jun and activating transcription factor 2 rather than direct DNA binding by AR (9) because a high-affinity AR-binding site located from −111 to −97 could be mutated without affecting the repression (10).
Unlike gonadal steroids, the effect of glucocorticoids on human αGSU gene expression at the level of gonadotropes has not yet been characterized. The effects of glucocorticoids in vivo on α-subunit gene expression are inconclusive. Several studies have demonstrated that corticosterone increased (11), decreased (12), or had no effect (13) on α-subunit mRNA in rat pituitary. In rat GH3 pituitary somatolactotropic cells, which do not express endogenous αGSU, it has been shown that coexpression of glucocorticoid receptor (GR) with the human αGSU promoter increased activity (14), but the mechanisms of action of glucocorticoid induction of α-subunit gene expression in GH3 cells have not been characterized. In contrast to GR modulation of α-subunit gene expression in the pituitary, the regulation of α-subunit gene expression by glucocorticoids in human placental cells has been studied at the molecular level. Several studies in the placental cell model, JEG-3 human choriocarcinoma cells, showed that glucocorticoids can inhibit expression of the human α-subunit gene by a mechanism involving mutual binding of ligand-activated GR and CRE-binding protein (CREB) (15,16) that is independent of specific DNA binding by GR.
Because the role of glucocorticoids in human αGSU gene expression in pituitary gonadotropes has not yet been studied, we examined the responsiveness of the human αGSU promoter to glucocorticoids at the level of the gonadotrope and characterized the mechanism of action. Accordingly, we used the LβT2 gonadotrope-derived immortalized cell line as a model system in which to study gonadotropin gene expression in a pure population of gonadotrope cells. The LβT2 cell line endogenously expresses many markers of a mature gonadotrope including αGSU, FSHβ, LHβ, GnRH receptor, activin, activin receptors, follistatin, and inhibin (17). It has also been shown to endogenously express GR and respond to glucocorticoids (18). These properties make the LβT2 cell line an excellent model system for directly studying the regulation of gonadotropin gene expression by glucocorticoids in a homologous model system. In the current study, we demonstrate that glucocorticoids stimulate activity of human αGSU promoter in pituitary gonadotropes through direct DNA binding of activated GR to a specific glucocorticoid-response element in the αGSU proximal promoter. This is in marked contrast to the mechanisms of suppression of the same human αGSU gene by androgens in αT3-1 gonadotrope cells and by glucocorticoids in placental cells, both of which involve protein-protein interaction rather than direct DNA binding. Thus, these studies illuminate the high degree of tissue specificity and nuclear receptor specificity of the mechanisms of action of steroid hormones on gene expression.
Materials and Methods
Hormones
Promegestone (R5020) and methyltrienolone (R1881) were purchased from NEN Life Science Products Life Sciences (Boston, MA), and 17β-estradiol, corticosterone, and dexamethasone (Dex) were purchased from Sigma-Aldrich (St. Louis, MO).
Reporter plasmid construction
The reporter plasmid, αGSU-luc, contains a 1.8-kb fragment (−1760 to +45) of the human α-subunit gene of the glycoprotein hormones linked to the luciferase (luc) reporter gene in the vector pGL3 basic (Promega Corp., Madison, WI). Deletions of the 1.8-kb promoter linked to a CAT reporter gene were described previously (19). Some of these deletions (−845, −668, −391, and −224) were subcloned into the NheI and BglII sites of pGL3 basic. For smaller deletions (−168, −116, and −90), PCR was performed using the appropriate primers (Table 1) to create αGSU promoter truncations with HindIII and KpnI linkers in a total volume of 100 μl. PCR conditions were as follows: 35 cycles consisting of 1 min at 95 C, 1 min at 55 C, and 1 min at 72 C and an extension of 2 min at 72 C. These promoter truncations were then subcloned into the HindIII and KpnI sites of pGL3 basic. Sequences of all promoter fragments were confirmed with dideoxynucleotide sequencing by the DNA Sequencing Shared Resource, University of California, San Diego, Cancer Center. The receptor expression vectors all contained rat cDNAs and were as follows: AR, pSG5-rAR (20); progesterone receptor (PR), pCMV5-rPRB (provided by Benita Katzenellenbogen); GR, pSG5-rGR (provided by Keith Yamamoto); and ER, pcDNA3.1-rERα (21). The dominant-negative (DN) inhibitor of CREB, DN-CREB, originally called M1-CREB, and its empty vector PRSET5 were described by Stauber et al. (22). The GR-DBD and GRdim4 mutants were described by Thackray et al. (18). The pCI-Dlx3 plasmid was kindly provided by Maria Morasso.
Table 1.
Oligonucleotides (5′ to 3′ orientation)
| Primers/probes | Sequencea |
|---|---|
| Subcloning PCR oligonucleotide primers | |
| aGSU reverse | CCCAAGCTTAGTTAATGAAGTCCTCACCT |
| −90αGSU forward | CCGGGTACCTCATTGGATGGAATTTC |
| −116α GSU forward | CCGGGTACCTGGTAATTACACCAAGT |
| −168αGSU forward | CCGGGTACCAGGGTTGAAACAAGAT |
| ChIP PCR oligonucleotide primers | |
| Forward (−246 promoter) | GAAAATGGCCAAATGCTCTC |
| Reverse (−54 promoter) | TGTTCCCAGCTGCACATAAG |
| Forward (+932 coding region) | TGACTGGAGCTGGTGAGATG |
| Reverse (+1169 coding region) | GCTTCCAGGAGGCTAGGAGT |
| Mutagenesis oligonucleotides | |
| mGRE1 | GGGTTGAAACAAGATAACATGAAATTGACGTCATGGTAAAAATTG |
| mGRE2 | GTCATGGTAATTAGACCAACTAGGCTTCAATCATTGG |
| mGRE3 | CAATCATTGGATGCAATTTCCTCTTGATCCCAGGGC |
| mCRE1 | GAAACAAGATAAGATCAAATTGCTTGCATGGTAAAAATTGACG |
| mCRE2 | GACGTCATGGTAAAAATTGCTTGCATGGTAATTACACCAAGTAC |
| mCRE1 + 2 | GATAAGATCAAATTGCTTGCATGGTAAAAATTGCTTGCATGGTAATTAC |
| EMSA oligonucleotide probes | |
| −147αGSU WT | AAGATAAGATCAAATTGACGTCATGGTAAAAATTGACGTCATGGTA |
| −129αGSU WT | AAAATTGACGTCATGGTAATTACACCAAGTACCCTTCAATCA |
| −129mCRE2 | AAAATTGCTTGCATGGTAATTACACCAAGTACCCTTCAATCA |
| −129mGRE2 | AAAATTGACGTCATGGTAATTAGACCAACTAGGCTTCAATCA |
| −116αGSU WT | TGGTAATTACACCAAGTACCCTTCAATCATTGGATGGAATTTCCTGTTGATCCCAGGGC |
| −116mGRE2 | TGGTAATTAGACCAACTAGGCTTCAATCATTGGATGGAATTTCCTGTTGATCCCAGGGC |
| −116mGRE3 | TGGTAATTACACCAAGTACCCTTCAATCATTGGATCCAATTTCCTCTTCATCCCAGGGC |
| −116mGRE2 + 3 | TGGTAATTACACCAACTAGGCTTCAATCATTCCATGGAATTTCCTCTTCATCCCAGGGC |
Mutated sequences are underlined.
Mutagenesis
The QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was used to generate mutations in the CREs (CRE1 and CRE2) and in the putative glucocorticoid response elements (GREs: GRE1, GRE2, and GRE3). The mutagenesis was performed using the 1.8-kb αGSU-luc plasmid and the appropriate oligonucleotides according to the manufacturer’s protocol (Table 1). All mutations were confirmed by sequencing.
Cell culture and transient transfections
All transient transfections were performed in the LβT2 cell line. Cells were maintained in 10-cm plates in DMEM (Cellgro; Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (Omega Scientific Inc., Tarzana, CA) at 37 C in 5% CO2. Cells were split into 12-well plates at 3 × 105 cells per well and transfected 24 h later. The cells were transfected by FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer’s protocol. Each well received 400 ng of a luc reporter construct as well as 100 ng of β-galactosidase reporter plasmid driven by a herpes virus thymidine kinase promoter as a control for transfection efficiency. Cells were also transfected with 200 ng GR or empty vector, unless otherwise noted and then serum starved for 6 h after transfection. After 18 h of starvation, the cells were treated with one of the following treatments: 0.1% ethanol (vehicle control), 0.1% BSA (vehicle control), 0.1% ethanol with 0.1% BSA (vehicle control), 100 nm Dex (unless otherwise noted), 10 nm GnRH (unless otherwise noted), or both 100 nm Dex and 10 nm GnRH. Treatment with ethanol or Dex was for 24 h, and treatment with 0.1% BSA or GnRH was for 4 h, unless otherwise noted.
Luciferase and β-galactosidase assays
After hormonal or vehicle treatment, the cells were washed with 1× PBS and then lysed with 0.1 m K-phosphate buffer (pH 7.8) containing 0.2% Triton X-100. After lysis, 20 μl of the cell extracts was transferred to a 96-well Nunc luminometer plate. Luciferase activity was determined using a buffer containing the following: 100 mm Tris-HCl (pH 7.8), 15 mm MgSO4, 10 mm ATP, and 65 mm luciferin. The Galacto-light β-galactosidase assay (Tropix, Bedford, MA) was used to measure β-galactosidase activity according to the manufacturer’s protocol. To monitor the activity of these reporter genes, a Veritas Microplate Luminometer (Turner Biosystems) was used.
Chromatin immunoprecipitation (ChIP) assay
LβT2 cells were grown to confluency in 15-cm plates, and proteins were cross-linked to DNA by the addition of 1% formaldehyde directly to the cell medium. The nuclear fraction was obtained and chromatin was sonicated to an average length of 400 bp in sonication buffer (50 mm HEPES, 140 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% Na-deoxycholate, and 0.1% SDS). The lysate was diluted with ChIP dilution buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris (pH 8), 167 mm NaCl] to a total of 3.5 ml and precleared with 100 μl Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Protein-DNA complexes were incubated overnight with the N499 GR rabbit polyclonal antibody (provided by Keith Yamamoto) or a nonspecific mouse IgG control (Santa Cruz Biotechnology) and precipitated with Protein A/G beads (Santa Cruz Biotechnology). A fraction of the protein-DNA was not precipitated but set aside as the input. The agarose beads were washed in the following order: low-salt wash buffer [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris (pH 8), 150 mm NaCl], high-salt wash buffer [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris (pH 8), 500 mm NaCl], LiCl wash buffer [250 mm LiCl, 1% Nonidet P-40, 1% Na-deoxycholate, 1 mm EDTA, 10 mm Tris (pH 8)] and twice with Tris-EDTA buffer. The protein-DNA complexes were eluted with elution buffer (1% SDS, 0.1 m NaHCO3) and the cross-links were reversed with the addition of 200 mm NaCl and incubation at 65 C for 4 h. The DNA was phenol-chloroform extracted, precipitated, and then resuspended in 50 μl water. Primers used in PCR are listed in Table 1. PCR conditions used were the following: 4 min at 95 C, followed by 26 cycles consisting of 1 min at 95 C, 1 min at 60 C, and 1 min at 72 C and an extension of 10 min at 72 C. The PCR product was labeled by including [α-32P]dATP in the nucleotide mix and run on a 5% acrylamide gel in 0.5× Tris-borate-EDTA buffer. The gels were dried and subjected to autoradiography.
Preparation of protein extracts
Full-length Flag-GR (kindly provided by Steve Nordeen) was overexpressed in Sf9 insect cells via a baculovirus expression system by the University of Colorado Cancer Center Tissue Culture Core Facility. The Sf9 cells were inoculated with virus at a multiplicity of infection of 1.0 and grown for an additional 48 h at 27 C. Cells containing GR were treated for the last 24 h before harvest with 500 nm triamcinolone acetonide (final concentration), respectively. The cells were harvested by centrifugation at 1500 rpm for 15 min, washed once in Tris-glycerol buffer [10 mm Tris-HCl (pH 8.0) and 10% glycerol] and frozen as a pellet at −80 C. Sf9 cells were lysed in a homogenization buffer [20 mm Tris-HCl (pH 7.5), 350 mm NaCl, 1 mm dithiothreitol, 10% glycerol, 0.5 μg/ml leupeptin, 10 μg/ml bacitracin, 2 μg/ml aprotinin, 1 μg/ml pepstatin]. All procedures were done at 0–4 C. The cell lysate was centrifuged at 40,000 rpm for 30 min, and the supernatant was taken as a soluble whole-cell extract.
EMSA
To determine whether GR could bind the proximal mouse αGSU promoter, whole-cell extracts containing GR were incubated with 1 fmol 32P-labeled oligonucleotide at 4 C for 30 min in a DNA-binding buffer [10 mm HEPES (pH 7.8), 50 mm KCl, 5 mm MgCl2, 0.1% Nonidet P-40, 1 mm dithiothreitol, 2 μg poly(deoxyinosine-deoxycytosine), and 10% glycerol]. The oligonucleotides were end-labeled with T4 DNA polymerase and [γ-32P]ATP. After 30 min, the DNA-binding reactions were run on a 5% polyacrylamide gel (30:1 acrylamide-bisacrylamide) containing 2.5% glycerol in 0.5× Tris-acetate-EDTA buffer. The N499 GR rabbit polyclonal antibody was used to supershift GR, and nonspecific rabbit IgG was used as a negative control for binding. A 1000-fold excess of the relevant oligonucleotide was used for competition. Oligonucleotides used for EMSA are listed in Table 1.
Data normalization and statistical analysis
All experiments were performed in triplicate and repeated a minimum of three times. Transfection efficiency was controlled for by dividing all luc values by the corresponding β-galactosidase values. This ratio was then expressed relative to the empty pGL3 plasmid to control for hormone effects on the vector DNA. Asterisks represent values that are significantly different from vehicle as determined by the Student’s t test for independent samples or one-way ANOVA followed by the Tukey-Kramer honestly significant difference post hoc test or by two-way ANOVA using the statistical package JMP 5.0 (SAS, Cary, NC). Significance was set at P ≤ 0.05. Daggers represent a synergistic relationship as determined by a two-way ANOVA (23). The results are presented as fold induction relative to the vehicle control.
Results
Glucocorticoids specifically regulate human αGSU gene expression in LβT2 gonadotrope cells
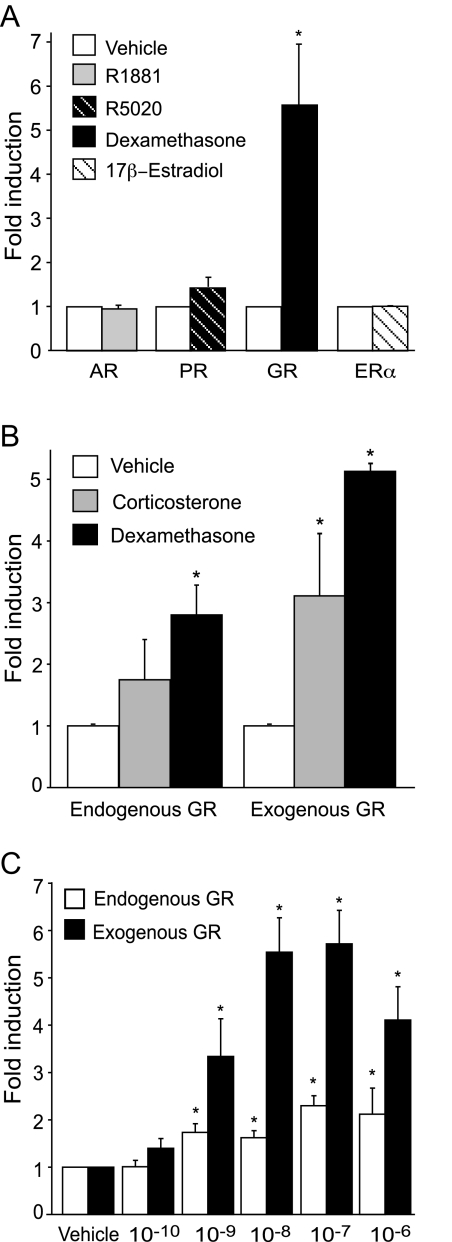
Understanding the molecular mechanisms of steroid modulation of human αGSU has been difficult due to heterogeneity of the anterior pituitary. Immortalized LβT2 cells are a model of a mature gonadotrope cell and express endogenous steroid receptors including AR, PR, ERα, and GR (18). To investigate the effects of steroid hormones on the expression of the human αGSU in pituitary gonadotropes, immortalized LβT2 cells were transiently transfected with the proximal 1.8 kb of the human αGSU promoter linked to a luc reporter gene (1.8αGSUluc) along with the appropriate steroid receptor expression vector. Four steroid receptors were tested: AR, PR, GR, and ERα. The cells were treated with the appropriate hormone, 10−7 m R5020 (synthetic progestin), 10−7 m R1881 (synthetic androgen), 10−7 m 17β-estradiol, or 10−7 m Dex (synthetic glucocorticoid), or with 0.1% ethanol (vehicle control) for 24 h before harvest. Treatment of the cells with Dex resulted in approximately 5-fold induction, whereas treatment with androgens and estrogens did not have an effect, and progestins stimulated a mere 1.4-fold induction (Fig. 1A).
Figure 1.
A, Glucocorticoids specifically induce human αGSU gene expression in LβT2 gonadotrope cells. LβT2 cells were transiently cotransfected with the 1.8-kb αGSU-luc reporter gene and with 200 ng of the respective receptor expression vectors indicated on the graph. The cells were serum starved overnight and then treated with 100 nm R1881 (synthetic androgen), R5020 (synthetic progesterone), Dex (synthetic glucocorticoid), or 17β-estradiol for 24 h. Luciferase activity was assayed and normalized to β-galactosidase activity and shown relative to the empty reporter vector. B, The 1.8-kb αGSUluc reporter gene was transiently transfected into LβT2 cells without (endogenous GR) or with (exogenous GR) the GR expression vector. The cells were serum starved overnight and then treated for 24 h with 100 nm corticosterone, a natural glucocorticoid, or Dex, a synthetic glucocorticoid. C, The 1.8-kb αGSUluc reporter gene was transiently transfected into LβT2 cells without (endogenous GR) or with (exogenous GR) the GR expression vector. The cells were serum starved overnight and then treated for 24 h with the indicated Dex concentrations (100 pm to 1 μm). Data represent the mean ± sem of at least three experiments performed in triplicate and are presented as fold induction relative to the vehicle control. *, Dex induction is significantly different from the vehicle-treated control, Student’s t test, P < 0.05.
To determine whether the effect of glucocorticoids on human αGSU gene expression was physiologically significant, LβT2 cells were transiently transfected with or without a GR expression vector and treated with either Dex or with corticosterone, the physiological form of circulating glucocorticoids in mice. The results show that endogenous levels of GR were sufficient for the stimulatory effect of Dex on human αGSU gene expression (Fig. 1B). Corticosterone had a stimulatory effect on human αGSU gene expression with exogenous expression of GR and a strong trend with endogenous GR that did not reach statistical significance. Because treatment of the cells with Dex and transfected exogenous GR showed a greater induction in comparison with endogenous GR, subsequent experiments used these conditions to maximize the effect.
Glucocorticoids stimulate human αGSU gene expression in a dose-dependent manner
LβT2 cells were transfected with or without the GR expression vector and then treated with increasing concentrations of Dex to determine whether the induction of αGSU-luc was dose dependent (Fig. 1C). For cells with both endogenous and exogenous transfected GR, a significant induction occurred with hormone concentrations as low as 10−9 m. The induction increased with higher concentrations of hormone and the maximal dose was achieved at 10−7 m for both endogenous and exogenous GR. These results showed that regulation of αGSU by glucocorticoids occurs in a dose-dependent manner and that physiological concentration of glucocorticoids, 10−8 m (24), can induce the αGSU promoter.
GR binds the endogenous αGSU promoter in vivo
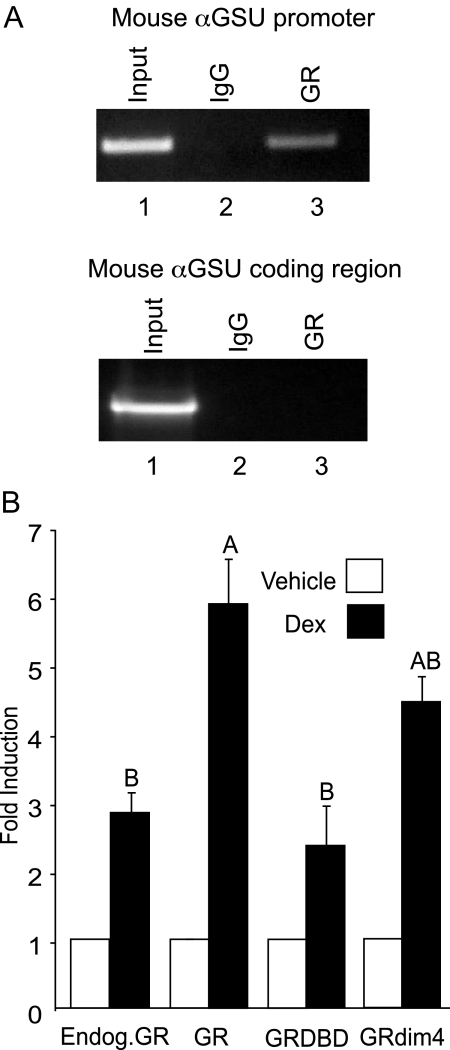
ChIP analysis was used to determine whether endogenous GR binds to the endogenous mouse αGSU gene in live LβT2 cells with the use of an antibody specific to the receptor. The ChIP assay (Fig. 2A) showed that the antibody against GR precipitated the mouse αGSU proximal promoter, demonstrating that GR specifically binds to the 5′ region of the endogenous mouse gene (upper panel, lane 3). The mouse αGSU promoter (primers −246 to −54) was also amplified from the input chromatin as a positive control for genomic DNA preparation and PCR conditions (upper panel, lane 1). Furthermore, the αGSU gene did not precipitate with the nonspecific mouse IgG, used as a negative control (upper panel, lane 2). As a control for specificity, primers encompassing part of the downstream coding region of the αGSU gene (+932 to +1169) were also used in PCR. Although these primers amplified αGSU from the input chromatin as expected (lower panel, lane 1), no bands were amplified from the precipitated DNA (lower panel, lanes 2 and 3), indicating that GR specifically binds to the 5′ region of the αGSU gene.
Figure 2.
A, GR binds to the αGSU promoter in vivo. ChIP was performed using the cross-linked protein/chromatin from LβT2 cells (treated with vehicle or 100 nm Dex) using antibodies directed against GR or nonspecific IgG as a negative control: upper panel, PCR primers (Table 1) encompassing the proximal promoter of αGSU (−246 to −54) were used to detect precipitation of genomic DNA; lower panel, PCR primers encompassing the downstream αGSU-coding region (+932 to +1169) were used as a control for specificity. PCR amplification was performed on 0.2% of chromatin input. B, DNA binding by GR is required to facilitate human αGSU gene expression. The 1.8-kb αGSUluc reporter gene was transiently cotransfected into LβT2 cells with the wild-type GR, GR-DBD mutant expression vector, or GRdim4 mutant expression vector as indicated. The cells were serum starved overnight and then treated with 100 nm Dex for 24 h. Data represent the mean ± sem of at least three experiments performed in triplicate and are presented as fold induction relative to the vehicle control. The GR-DBD mutant response was significantly different from the GR response. Levels of induction not connected by the same letter are significantly different, using one-way ANOVA followed by Tukey’s post hoc test, P < 0.05.
DNA binding by GR is necessary for transcriptional activation of the human αGSU promoter
Although the ChIP assay showed that there was binding of GR to the αGSU promoter in live LβT2 cells, this method cannot determine whether the receptor binds directly to DNA or indirectly, via another transcription factor. To determine whether direct DNA binding by GR plays a critical role in the transactivation of αGSU by Dex, we transfected LβT2 cells with GR mutants deficient in DNA binding and the ability to form homodimers. These mutant receptors included the GR-DNA-binding domain (DBD) mutant that has C476W and R479Q mutations in the DBD, causing it to lose the ability to bind DNA (25). The second mutant, GRdim4, contains a dim1 mutation, A458T, which has been previously described (26), as well as three other mutations (N454D, R460D, and D462C); these mutations cause the receptor to lose the ability to form dimers as well as interact with other transcription factors, such as activating protein-1 (AP-1) (27). After 24 h of 100 nm Dex treatment, we observed that the induction of the αGSU promoter with the GR-DBD mutant was reduced to levels of induction equivalent to those caused by endogenous levels of GR, indicating that induction activity of the transfected, overexpressed GR was abolished by this mutation. The activity of the endogenous GR did not appear to be reduced by the transfected GR-DBD mutant, but we expect that higher levels of the mutant GR might suppress the endogenous response. In addition, the mutant GRdim4 also appeared to decrease induction levels in comparison with the overexpressed wild-type receptor although not to a statistically significant degree (Fig. 2B). These data indicate that the DBD of GR is necessary to facilitate human αGSU gene induction by glucocorticoids in pituitary gonadotrope cells.
Multiple sites in the human αGSU promoter contribute to glucocorticoid induction of the human αGSU
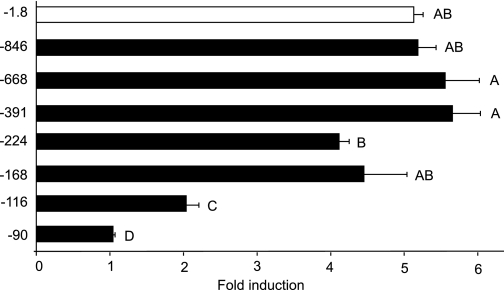
To map the promoter elements required for glucocorticoid responsiveness in the human αGSU promoter, truncation analysis and transient transfections were used. Truncations of the αGSU promoter were created to define the sequences of importance. LβT2 cells were transiently transfected with the following truncations: −845αGSU, −668αGSU, −391αGSU, −224αGSU, −168αGSU, −116αGSU, and −90αGSU (Fig. 3).
Figure 3.
Mapping the regions involved in induction of human αGSU by GR. The 1.8-kb αGSUluc, −845αGSUluc, −668αGSUluc, −391αGSUluc, −224αGSUluc, −168αGSUluc, −116αGSUluc, or −90αGSUluc reporter genes were transiently transfected into LβT2 cells along with the GR expression vector. The cells were serum starved overnight and then treated for 24 h with 100 nm Dex. Data represent the mean ± sem of at least three experiments performed in triplicate and are presented as fold induction relative to the control. The response with −1.8 kb and with the truncations −846, −668, −391, −224, and −168 bp are significantly different from the response with −116 and −90 truncations. Levels not connected by the same letter are significantly different, using one-way ANOVA followed by Tukey’s post hoc test, P < 0.05.
Upon treatment with 100 nm Dex, the ability of glucocorticoids to induce αGSU expression was assayed. Levels of expression induced by truncations −846αGSU, −668αGSU, −391αGSU, and −168αGSU were not significantly different from those induced by the wild-type 1.8-kb αGSU promoter (Fig. 3). Truncation of the promoter to −90 bp resulted in a loss of responsiveness to Dex. For truncation −224αGSU, which showed about a 4-fold induction, there was a significant decrease in induction compared with −668αGSU and −391αGSU, but it was not significantly different from wild type. Transfection of these truncations showed that there was a significant loss of induction between −168αGSU and −116αGSU (4-fold decreased to 2-fold). These results suggest that GR may act directly within the first 168 bp of the human αGSU at the level of the gonadotrope.
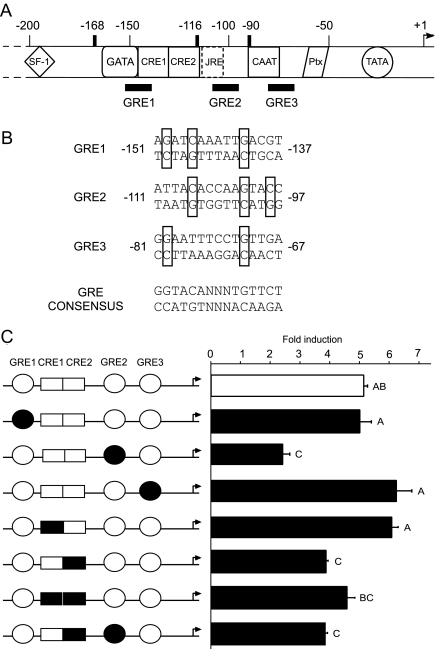
We have previously identified GRE-binding sites in this region of the human αGSU gene by deoxyribonclease I footprinting analysis with purified rat liver GR protein (15). This analysis revealed three GR-binding sites (GRE1 through -3; Fig. 4A), each of which exhibits partial homology with the sequence of the consensus GRE (Fig. 4B) (28). To investigate the importance of these GRE sites in GR-mediated induction of human αGSU transcription, we created mutations by site-directed mutagenesis in the context of the 1.8αGSUluc reporter gene. Mutations were designed to destroy the homology of these sites to the consensus GRE in the G/C residues critical for high-affinity binding while avoiding the creation of new potential GREs (Fig. 4B and Table 1); these mutants were designated mGRE1, mGRE2, and mGRE3.
Figure 4.
The cis elements involved in glucocorticoid regulation of human αGSU. A, Schematic representation of the 5′ proximal promoter of the human αGSU gene. The 220 bp of 5′ flanking sequences of the human αGSU promoter are shown encompassing the steroidogenic factor-1 (SF-1) binding site, the CAAT element, the CREs (CRE1 and CRE2), the JRE that is occupied in placental cells, the CAAT box, the pituitary homeobox (Ptx) element, and the TATA element. Overlapping with some of these elements are the putative GREs (GREs 1, 2, and 3). B, The sequences of three GREs and a GRE consensus are shown. Boxes indicate conservation of the key G and C residues with the consensus sequence. C, GRE2 and CRE2 have a significant role in the induction of human αGSU by GR. The wild-type 1.8-kb αGSUluc reporter gene or one of the three GRE/CRE cis mutants was transiently transfected into LβT2 along with GR expression vector. The cells were serum starved overnight and then treated for 24 h with 100 nm Dex. Data represent the mean ± sem of at least three experiments performed in triplicate and are presented as fold induction relative to the vehicle control. Induction with the GRE2 mutant, CRE2 mutant, and/or double GRE2/CRE2 mutant are significantly different from induction with wild-type GR. Levels not connected by the same letter are significantly different, using one-way ANOVA followed by Tukey’s post hoc test, P < 0.05.
Surprisingly, each of the three mutations decreased basal expression of the αGSU gene individually (data not shown). Because these GRE sites reside in the proximal region of the promoter where transcriptional activity is high and binding sites for known and unknown proteins are crowded together, it is probable that mutating these sites disturbed the binding of basal transcription factors. Mutations of GRE1 and GRE3 did not prevent the response to Dex, whereas the Dex responsiveness with mutation of GRE2 showed a significant decrease compared with the wild-type αGSU reporter gene (by 52%) (Fig. 4C). These results show that the region from −111 to −97, designated as GRE2, is necessary for glucocorticoid responsiveness. In addition to the three putative GRE sites, the two tandem CRE sites, located between positions −142 and −117, were also identified in the human αGSU promoter region within the larger region shown to be important by truncation analysis: −168 and −90. GR is known to interact with transcription factors CREB, activating transcription factor 3, and AP-1, which bind to these CRE sites (29,30,31). Thus, we investigated whether these sites are implicated in the ability of GR to regulate transcription of human αGSU. Site-directed mutagenesis was used to introduce mutations in these CRE sites, individually and simultaneously in the context of the 1.8αGSUluc reporter gene (Table 1). Results from cotransfection of these mutated promoters with the GR expression plasmid showed that these CRE sites do have a partial role in αGSU induction mediated by GR. As was shown by Schoderbek et al. (32), basal levels of luc activity remained the same when CRE1 and CRE2 were individually mutated, but levels decreased by 49.8% when both CRE sites were mutated (data not shown). Induction by Dex was inhibited by the mutation of CRE2 to approximately 73%, but no significant inhibition was seen with mutation of the CRE1 or with both mutations: CRE1 + 2 (Fig. 4C). Mutation of both GRE2 and CRE2 did not show further significant decreases in the induction by Dex compared with mutations of each site individually (Fig. 4C). These results indicated that CRE2 and GRE2 have functional activity important for glucocorticoid responsiveness of the human αGSU promoter.
The phosphorylation state of CREB affects the transcriptional activation of the human αGSU promoter by glucocorticoids
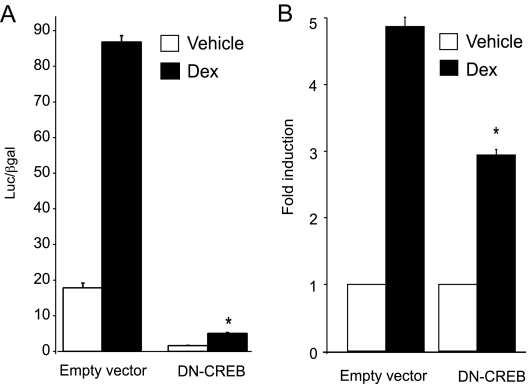
As a complimentary experiment to the involvement of CRE2 in GR signaling, we introduced a DN-CREB into LβT2 cells. This DN-CREB was designed to have an inactive kinase A phosphorylation site by replacing serine at position 133 with alanine (previously termed M1-CREB), which completely abolishes CREB transcriptional activity (33), although it does not affect DNA binding. We therefore compared the DN-CREB with the empty vector for interference with GR-activated transcription of human αGSU in LβT2 cells. We found that DN-CREB affected basal, as well as GR-induced, expression of human αGSU gene (Fig. 5A). Basal expression was reduced to 10% of control, and Dex induction was reduced significantly by 44% (Fig. 5B). This observation supports the results of the CRE2 cis mutation and further indicates that this CRE site plays a role in mediating the induction of αGSU by glucocorticoids. Moreover, our data indicate that activation of CREB (by phosphorylation) is required for basal expression and glucocorticoid induction of the human αGSU gene.
Figure 5.
DN-CREB decreases GR-induced expression of the human αGSU promoter. DN-CREB was overexpressed in LβT2 cells that had been transiently transfected with 1.8-kb αGSUluc and the GR expression vector. The cells were serum starved overnight and then treated for 24 h with 100 nm Dex. Data represent the mean ± sem of at least three experiments performed in triplicate. A, Bar graph depicting luc values normalized to β-galactosidase values; B, bar graph presented as fold induction relative to the control for empty vector vs. DN-CREB to show decrease in fold induction by Dex.
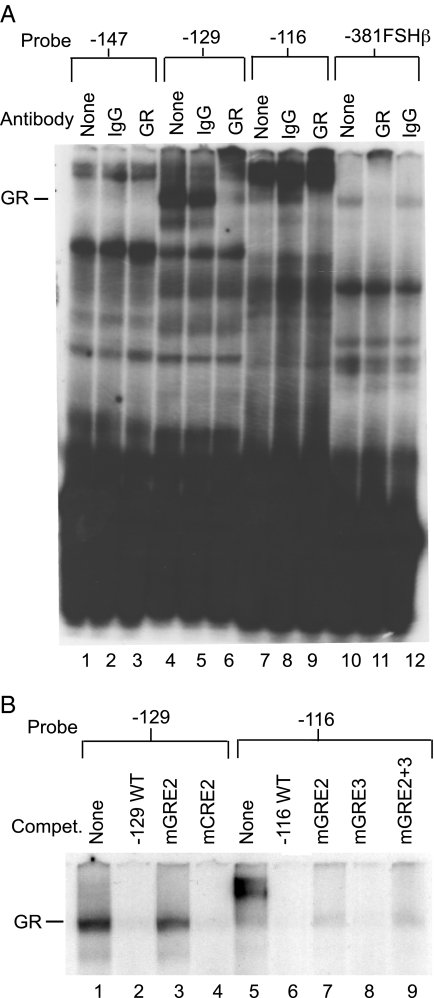
GR binds to the −111 GRE2 of the human αGSU promoter
To evaluate whether GR could bind directly to the GREs in the proximal human αGSU promoter in vitro, an EMSA was performed (Fig. 6). The probes were constructed from the 5′ region of the proximal human αGSU promoter and were designated −147, −129, and −116 (Table 1). Because the sites are located very close together, probe −147 encompasses the GRE1, CRE1, and CRE2 sites; probe −129 encompasses the CRE2 and GRE2 sites; and probe −116 encompasses the GRE2 and GRE3 sites. We have previously shown that GR binds to the −381 hormone response element in the mouse FSHβ promoter (18), and this probe was used as a positive control for GR binding (Fig. 6A, lanes 10–12). Although we did not detect GR binding using LβT2 nuclear extracts (data not shown), we did observe binding of GR overexpressed in Sf9 cells to the −129 wild-type oligonucleotide probe (Fig. 6A, lanes 4–6) and the −116 wild-type oligonucleotide probe (Fig. 6A, lanes 7–9), whereas no binding to the −147 wild-type oligonucleotide probe was observed. The resulting complexes in these lanes were supershifted by a GR-specific antibody (Fig. 6A, lanes 6, 9, and 12) but not by IgG (Fig. 6A, lanes 5, 8, and 11). GR binding to these elements was further examined by competition EMSA (Fig. 6B), and results showed that complexes displayed self-competition (Fig. 6B, lanes 2 and 6) and failed to compete with a probe with a GRE2 mutation (Fig. 6B, lanes 3 and 7) or a probe with GRE2 and GRE3 mutations (Fig. 6B, lane 9). The complexes successfully competed with the CRE2 mutant probe (Fig. 6B, lane 4) and the GRE3 mutant probe (Fig. 6B, lane 8). These results lead to the conclusion that GR can bind directly to GRE2 in the human αGSU gene.
Figure 6.
GR binds to the −111 GRE2 of the human αGSU promoter. Whole-cell extracts containing overexpressed GR from baculovirus-infected insect cells were incubated with 32P-labeled oligonucleotides representing the three GREs as indicated (see Table 1) and compared with a positive control probe from the mouse FSHβ gene that has been previously shown to bind GR. A, The N499 GR rabbit polyclonal antibody was used to supershift GR, and nonspecific rabbit IgG was used as a negative control for binding; B, 1000-fold excess of the relevant oligonucleotide probes, either wild-type or mutant (Table 1), was used for competition.
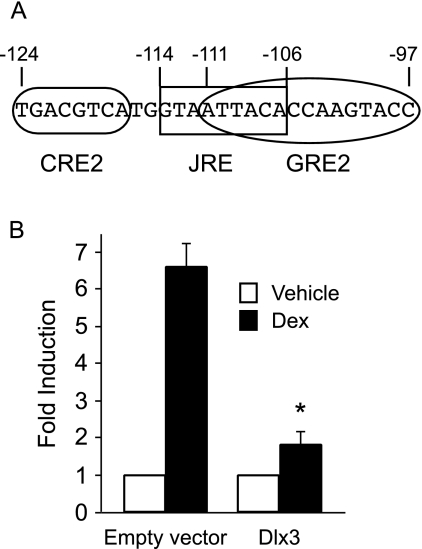
Overexpression of Dlx3 in LβT2 cells inhibits the transcriptional activation of the human αGSU promoter by glucocorticoids
The homeobox factor, Distal-less 3 (Dlx3), is expressed in the human choriocarcinoma cell line, JEG3, and in trophoblast cells, but not in the αT3-1 gonadotrope cell line. Dlx3 transactivates the human α-GSU gene in placental cells via binding at the junctional regulatory element (JRE) located from −114 to −106 (34). This element overlaps the GRE2 sequence by six nucleotides, covering the whole consensus half-site on the 5′ side and sits between GRE2 and CRE2 (Fig. 7A). This overlap creates the potential for interference in placental cells; Dlx-3 could interfere with GR binding to GRE2 as well as its interaction with the CRE2 binding proteins. To address the mechanism of glucocorticoid induction in gonadotrope cells vs. repression in placental cells, we determined whether ectopic expression of Dlx3 in gonadotrope cells would alter glucocorticoid responsiveness on the αGSU gene. LβT2 cells were transiently cotransfected with GR, 100 ng of either Dlx3 expression vector or pCI empty vector and with the 1.8-kb αGSU-luc reporter gene. As expected, the human αGSU gene was induced 6.6-fold after Dex treatment (Fig. 7B). This induction was reduced 72% to 1.8-fold in the presence of Dlx3. There was no effect on basal expression of human αGSU gene. These results indicate that Dlx3 has an inhibitory effect on GR-induced expression of human αGSU gene and provide a potential mechanism for the differential effects of glucocorticoids in gonadotropes vs. placental cells.
Figure 7.
Overexpression of Dlx3 inhibits GR-induced human αGSU promoter in LβT2 cells. A, Sequence overlap of the JRE and the GRE2. Sequence is shown from −124 to −97 of the human αGSU gene. The rounded box indicates the sequence of CRE2, squared box indicates the JRE sequence, and the oval indicates the full GRE2 with both 6-bp half-sites and the 3-bp spacer. B, Either pCI empty vector or Dlx3 expression vector (100 ng) was overexpressed in LβT2 cells that had been transiently transfected with 1.8-kb αGSUluc and the GR expression vector. The cells were serum starved overnight and then treated for 24 h with 100 nm Dex. Data represent the mean ± sem of at least three experiments performed in triplicate and are presented as fold induction relative to the vehicle control.
GnRH and activin synergistically modulate Dex-induced human αGSU gene expression
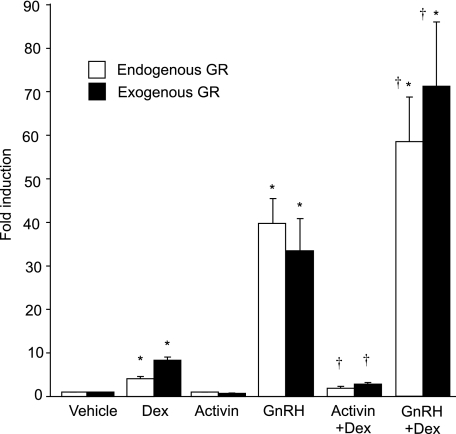
Gonadotropes are known to be responsive to the regulation of GnRH and activin on the reproductive axis. For example, we have previously shown that activin regulates the transcription of LHβ and FSHβ subunit genes in LβT2 cells (35,36). In contrast, the αGSU gene is repressed by activin in αT3-1 gonadotrope cells (37). Additionally, GnRH has been shown to induce transcription of FSHβ, LHβ, and αGSU (38,39,40). More recently, we have shown that activin and glucocorticoids synergistically regulate FSHβ transcription (41). Understanding how different hormones interact with each other in the regulation of gonadotropin synthesis can further elucidate the mechanisms of hormonal regulation of gonadotropins. Therefore, we examined whether there was cross-talk between the GnRH and glucocorticoid response as well as activin and glucocorticoid response of the human αGSU gene in the gonadotrope cell.
LβT2 cells were transiently transfected with the 1.8-kb αGSU-luc reporter plasmid and the GR expression vector. Cells were treated with Dex, GnRH, or activin individually or as cotreatments, and changes in luc activity were monitored (Fig. 8). Under these conditions, Dex alone induced human αGSU gene expression by 8.4-fold over vehicle treatment. There was no statistically significant effect by activin alone, although the trend supported the previous findings of repression (37) (Fig. 8). However, the effect of cotreatment with activin and Dex resulted in reduction of Dex induction by 65%, displaying an interactive repression as determined by two-way ANOVA (23). In response to GnRH stimulation, human αGSU expression was induced significantly to 33.5-fold induction over vehicle treatment. Cotreatment of GnRH and Dex resulted in a significantly greater 71.2-fold induction. This interaction between GnRH and Dex on the αGSU promoter was also determined to be synergistic by two-way ANOVA. To test these effects with endogenous GR, LβT2 cells were transiently transfected with 1.8-kb αGSU, but without the addition of exogenous GR, and were subject to the same hormone treatments. With the treatment of Dex alone, human αGSU was induced 4.1-fold, activin had no effect, and GnRH alone produced a 39.8-fold induction. Cotreatment with Dex and activin resulted in reduction of the Dex induction of human αGSU gene expression by 53%, also showing an interactive repression. Synergy was again observed with cotreatment of GnRH and Dex, resulting in a significantly increased fold induction of 58.4 (Fig. 8). These results indicate that GnRH and glucocorticoids synergistically interact to induce expression of the αGSU promoter at the level of the gonadotrope, whereas activin interacts with glucocorticoids to cause synergistic repression. Moreover, these interactions can occur with the endogenous GR present in the LβT2 cells, although a more dramatic response is seen upon addition of exogenous GR.
Figure 8.
GnRH and activin modulate Dex-induced human αGSU gene expression synergistically. The 1.8-kb αGSUluc reporter gene was transiently transfected into LβT2 cells with or without overexpression of GR (200 ng). Cells were serum starved overnight and then treated for 24 h with vehicle, 100 nm Dex, 10 nm GnRH, 10 ng/ml activin, 100 nm Dex with 10 nm GnRH, or 100 nm Dex with 10 ng/ml activin. Data represent the mean ± sem of at least three experiments performed in triplicate and are presented as fold induction relative to the control. Daggers (†) represent synergy between hormone treatments by two-way ANOVA, and asterisks (*) represent significant induction by hormone by one-way ANOVA, P < 0.05.
Discussion
Hormonal regulation of gonadotropin synthesis and secretion is exceptionally complex, involving, at the minimum, pulsatile hypothalamic input of GnRH and gonadal input of steroids and regulatory peptides (1). In addition, mounting evidence suggests that glucocorticoids also have an impact on gonadotropin gene expression (11,12,13,14,15,16,22,42,43,44). Our study adds to this body of evidence by demonstrating a direct transcriptional enhancement of the human αGSU gene by glucocorticoids at the level of gonadotropes.
Although in vivo studies have shown that glucocorticoids inhibit α-subunit secretion in humans (45,46,47,48), rats (49,50), and mice (51), in the present study we, as well as others (11,12,13,14,18,41), have shown that glucocorticoids play a positive role in gonadotropin subunit gene activation at the level of the gonadotrope cell. Furthermore, because both progestin and glucocorticoid levels peak during proestrus (52,53), and appear to be necessary for the secondary FSH surge (54,55), glucocorticoids may play a physiological role in the regulation of gonadotropin gene expression.
In the current study, we characterized the molecular mechanisms of glucocorticoid regulation of the human αGSU gene in gonadotropes. Our results show that glucocorticoids activate the proximal promoter of human αGSU in immortalized mouse gonadotrope-derived LβT2 cells in a dose-dependent manner with or without the overexpression of GR (Fig. 1). The effect of glucocorticoids was hormone specific and could not be demonstrated with other steroid hormones, including estrogens, progestins, or androgens (Fig. 1). We also provide evidence, using ChIP analysis, that the endogenous GR specifically binds to the 5′ region of the mouse αGSU gene in live LβT2 cells. Moreover, the use of mutant steroid receptors lacking the ability to bind DNA demonstrated that the DBD of GR is necessary to modulate human αGSU gene expression (Fig. 2). Additionally, we show that GR binds to a GRE within the αGSU promoter in vitro using gel shift analysis (Fig. 6). Furthermore, mutation of GRE2 or CRE2 in the context of the −1.8-kb αGSU-luc reporter gene inhibited the responsiveness of αGSU to glucocorticoids (Fig. 4). Collectively, these results are the first to demonstrate that GR can directly induce human αGSU gene expression within the gonadotrope.
Once we established that the DBD of GR was necessary for αGSU gene induction, it was then crucial to map the regions of the promoter to which GR binds. The responsiveness to glucocorticoids mapped to the −168-bp proximal region of the promoter. Our data concur with the studies of Gurr and Kourides (14) that demonstrated that α-subunit expression in rat somatolactotrope GH3 cells was induced by Dex when cotransfected with a glucocorticoid receptor expression plasmid. However, in contrast to our results in LβT2 gonadotrope cells, in GH3 cells, sequences upstream of −172αGSU were shown to be required for full Dex induction of human αGSU transcription (14). This suggests that in pituitary somatolactotrope cells, which do not normally express αGSU, there are additional factors not present in gonadotrope cells that interact with αGSU gene sequences upstream of −172 to enhance αGSU glucocorticoid-responsive transcription.
The human αGSU promoter has several regions that are known to be involved in basal and regulated expression in the pituitary; the pituitary glycoprotein hormone basal element (PGBE), which includes the α-basal elements (αBE1 and -2), the gonadotrope-specific element (GSE) that binds steroidogenic factor 1 (SF-1), a consensus GATA site that binds GATA-3, and tandem CREs that bind CREB and AP-1 (56,57,58). We had previously identified three putative GREs (15) in the region of the human αGSU promoter. Our cis-mutation analysis now shows that GRE2 and CRE2 (Fig. 4) are involved in the responsiveness of αGSU to glucocorticoids in pituitary gonadotrope cells. Additionally, gel-shift analysis demonstrated that GR binds to this specific GRE in the human αGSU gene in vitro (Fig. 6). In a previous study of repression of αGSU by GR in placental cells, GR-DBD protein had been shown to bind GRE2 in vitro, either as a dimer or monomer. However, these studies did not detect functional activity for this GRE in glucocorticoid repression of the αGSU promoter (22). Additionally, of the three putative GREs, GRE2 bears the highest degrees of identity to the known consensus for GR and evolutionary conservation. In the human αGSU promoter, GRE2 retains nine matches to the 12 defined nucleotides of the consensus GRE, whereas GRE1 has seven and GRE3 has six. In the mouse αGSU promoter, GRE2 retains six matches to the 12 defined nucleotides of the consensus GRE, whereas GRE1 has five and GRE3 has three. Thus, our current studies demonstrate that GRE2, a fairly conserved GRE, is a novel functional site for regulation of αGSU by glucocorticoids. Moreover, it was surprising to find that mutations in CRE2 reduced the responsiveness to glucocorticoids, yet mutations in CRE1, which comprises an identical sequence, did not have any effect. We propose that the reason for this is a matter of proximity between regulatory elements within the promoter. There are only 5 bp between CRE2 and GRE2, and the configuration of the αGSU promoter is such that the center of CRE2 is 10 bp from the center of the 5′ half-site of the GRE2 and 10 additional base pairs from the center of the 3′ half-site of GRE2 (Fig. 7A), placing the binding proteins on the same side of the helix. Therefore, we hypothesize that the transcription factors that bind CRE2 might directly interact with GR to increase the stabilization of binding to GRE2. This proposition is also supported by the fact that GR can bind to other transcription factors including AP-1 and CREB (25,59), both of which bind to CRE sites. Coactivator proteins that are known to interact with both CREB/AP-1 family members and nuclear receptors may act as bridge proteins across such a small distance of DNA. For example, four-and-a-half-LIM-only protein 2 (60) is a multifunctional coactivator that interacts with both CREB and steroid hormone receptors. Such proteins are thought to function as adaptors or scaffolds to support the assembly of multimeric protein complexes. Additionally, the nonphosphorylated DN-CREB reduced the induction of αGSU mediated by GR (Fig. 5), indicating that the activation of CREB by phosphorylation may have a role in GR-induced human αGSU gene expression. Interestingly, double cis mutations of GRE2 and CRE2 did not interfere with the stimulatory effect of GR on human αGSU gene expression any further than each mutation individually.
The effect of glucocorticoids on αGSU transcription is dependent on the cell type in which the gene is expressed. Induction by GR in the pituitary gonadotrope model is in contrast with the inhibitory effect of GR on human αGSU gene expression in JEG-3 placental cells (15,16,22). The placental studies have shown that glucocorticoid-dependent repression of human αGSU gene expression does not require binding of GR to the GRE but is dependent on a mechanism involving protein-protein interactions of GR with CREB. It is suggested that GR and CREB may interact directly in vivo possibly through a third protein or, more likely, may sequester a mutually required target protein in placental cells. In light of these data, we propose that the inability of glucocorticoids to activate human αGSU transcription in placental cells may result from a masking of the GR-binding sites on the αGSU gene by regulatory proteins present in placental cells that are not expressed in gonadotrope cells (61). For example, Dlx3, a homeodomain protein expressed in JEG-3 choriocarcinoma cells, but not in αT3-1 gonadotrope cells, binds to a site termed the JRE (34) located just 3′ to CRE2 that is important for placental expression and is directly overlapping with the 5′ half of the GRE2 sequence in the human αGSU gene (bases shared are −111 ATTACA −106; see Fig. 7A). This places the JRE between the CRE2 and GRE2 while overlapping the 5′ half-site of GRE2, perhaps interfering not only with GR binding but also with GR interaction with proteins bound to CRE2, or with bridging coactivators binding both CREB and GR. In fact, mutation of the JRE reduces basal expression in placental but not αT3-1 pituitary immature gonadotrope cells (62). Furthermore, mutation of the JRE reduces cAMP induction of human αGSU transcription in placental JEG-3 cells (34), but its effect on glucocorticoid regulation has not been tested in JEG-3 cells. In fact, introduction of Dlx3 into LβT2 cells does interfere with GR induction of human αGSU expression, dramatically reducing Dex induction. Therefore, Dlx3 may play a role in the cell-type-specific effect of glucocorticoids on αGSU transcription.
On the other hand, the differences between the actions of GR on αGSU in placental vs. pituitary gonadotrope cells may lie in a different complement of proteins binding to the CREs. Heckert et al. (63) showed that the identities of the proteins binding to the CREs in the human and rat αGSU genes are similar but differ in concentration in the αGSU-expressing placental vs. pituitary gonadotrope cell models. Alternatively, the lack of necessity for DNA binding of GR to the αGSU GRE2 in placental cells may be a result of a lack of tissue-specific cofactors necessary for the actions of GR or bridging to CRE-binding proteins. Additionally, it can also be hypothesized that in the absence of DNA binding of GR to the GRE in the αGSU promoter in placental cells, the GR acts as negative regulator of α-subunit transcription whereby GR and CREB or AP-1 compete for a coactivator protein such as CREB-binding protein or p300. The involvement of such mechanisms has been examined in other systems. For example, GR interferes with Oct-2A-dependent transcription in a DNA-binding-independent manner in HeLa cells but apparently not in lymphoid cells (64). In this case, the involvement of a putative rate-limiting coactivator was proposed.
Furthermore, because GnRH is a key hormone regulating αGSU transcription in gonadotropes, it was important to examine whether there was cross-talk between responses to these hormones. Our results revealed a synergistic relationship between GnRH and glucocorticoids (Fig. 8), uncovering a novel mechanism that remains to be further studied. This newly found synergism and the implied involvement of CREB bring more insight to the mechanism of GR-induced αGSU gene expression. Studies have previously shown that GnRH-stimulated CREB phosphorylation is necessary for transcriptional activation of the αGSU gene in the pituitary (65). Additionally, GR and CREB have been shown to synergistically activate the transcription of composite promoters, such as that of phosphoenolpyruvate carboxykinase (PEPCK) and somatostatin, which, like the αGSU promoter, contain both GRE and CRE sequences that are close in proximity (59,66). These studies revealed a protein-protein interaction in vitro between GR and CREB that might account for the role of the CRE in the glucocorticoid response of the PEPCK gene (59) and could contribute to the GnRH-glucocorticoid synergy on the αGSU gene. In addition to synergism between GR and CREB, interaction between GR and other transcription factors such as nuclear factor 1, specificity protein 1, and CACCC-binding proteins in genes encoding tyrosine amino transferase and rat tryptophan oxygenase (67,68) have also been reported. Therefore, the synergistic transcriptional activation of αGSU may involve the interactions between CREB and GR when they are both bound to their proximal response elements. Interestingly, our results with CREB overexpression (data not shown) suggest that overexpression of this molecule alone is insufficient for a synergistic induction with GR. This is probably due to the requirement not just for CREB molecules, but also for phosphorylated CREB, to induce transcriptional activation (69).
Activin, a member of the TGFβ family of growth factors, can regulate the transcription of genes by binding specific serine/threonine kinase receptors (70). These receptors then activate the intracellular signaling system that involves Smad proteins as intracellular signaling mediators. There are three classes of Smad proteins: receptor-regulated Smads (R-Smad), comediator Smads (Co-Smad), and inhibitory Smads (I-Smad) (70). Through these different classes of Smad, activin can cause activation or repression of gene transcription. In our results, activin treatment did not show induction of human αGSU expression but rather produced a small repression of about 27% (Fig. 8). More significant levels of repression of human αGSU by activin were previously shown by Attardi et al. (37) in αT3-1 cells that did not express GR. These cells express higher basal levels of αGSU expression compared with LβT2 cells, which may explain the higher repression levels of αGSU transcription by activin (71). Moreover, in our results in LβT2 cells, activin synergistically repressed Dex-induced human αGSU gene expression (Fig. 8). Interestingly, a recent study in our lab has shown that activin and glucocorticoids synergistically activate transcription of the FSHβ gene in LβT2 cells (41). These findings indicate that there are response elements in the αGSU promoter, not found in the FSHβ promoter, that allow for transcriptional repression by activin. Likewise, there may be response elements in the FSHβ promoter that are not found in the αGSU promoter that allow for transcriptional activation by activin. Attardi et al. (37) used a series of 5′ deletions (−507 to −133) of the mouse αGSU promoter to map regions that were essential for activin responsiveness. They found significant stepwise losses of activin responsiveness when sequences between −507 and −424, −424 and −288, and −288 and −205 bp were eliminated. However, these elements may be species specific and do not infer any interaction between activin responsiveness and glucocorticoid responsiveness for the human αGSU promoter. The interaction between activin and glucocorticoid regulation of the human αGSU promoter is thus highly specific to the cell type and target gene.
In summary, our findings demonstrate that glucocorticoids, upon binding and activating GR, directly induce the expression of human αGSU gene at the level of the gonadotrope and that this regulation occurs through binding of the ligand-bound receptor to the −111 GRE (GRE2) in the proximal promoter. Additionally, this activation is specific to GR because other steroid receptors such as PR, AR, and ER, did not induce human αGSU gene expression. These studies also indicate that the −124 CRE2 mediates this mechanism, most likely through the transcription factor CREB, which has been previously shown to interact with GR and to have a stimulatory synergistic effect on promoter transcription. In conjunction with the findings that glucocorticoids can up-regulate expression of FSHβ gene directly at the level of the gonadotrope, it makes physiological sense that the αGSU is also up-regulated by glucocorticoids to produce a functional FSH heterodimer.
Acknowledgments
We thank Djurdjica Coss and other members of the Mellon lab for helpful discussions and comments. We thank Susan Mayo for technical assistance. We thank Jorma Palvimo for the pSG5-rAR plasmid, Benita Katzenellenbogen for the pCMV5-rPRB plasmid, and Keith Yamamoto for the pSG5-rGR plasmid and the N499 GR rabbit polyclonal antibody. We also thank Margaret Shupnik for providing the pcDNA3.1-ERβ plasmid and Mark Lawson for the human 1.8αGSU-luc plasmid. We thank Maria Morasso for the pCI-Dlx3 plasmid. We also thank the University of Colorado Cancer Center Tissue Culture Core Facility for baculovirus production and the University of California San Diego Cancer Center DNA Sequencing Shared Resource for dideoxynucleotide sequencing.
Footnotes
This work was supported by the National Institute of Child Health and Human Development, National Institutes of Health (NIH), through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (P.L.M.). This work was also supported by NIH Grant R01 HD020377 (to P.L.M.). S.H.L. was supported by an Endocrine Society Student Summer Research Fellowship. V.G.T. was supported by NIH F32 DK065437 and NIH T32 HD007203. P.L.M. is a member of the Biomedical Sciences Graduate Program.
Present address for R.S.: Betastim Corp., 2 Hatohen Street, POB 3143, Caesarea, Israel, 38900.
Present address for S.H.L.: Department of Epidemiology, UCLA School of Public Health Box 951772, Los Angeles, California 90095-1772.
Disclosure summary: The authors have nothing to disclose.
First Published Online April 10, 2008
Abbreviations: AP-1, Activating protein-1; AR, androgen receptor; CAT, chloramphenicol acetyltransferase; ChIP, chromatin immunoprecipitation; CRE, cAMP response element; CREB, CRE-binding protein; Dlx3, Distal-less 3; DBD, DNA-binding domain; Dex, dexamethasone; DN, dominant-negative; ER, estrogen receptor; GR, glucocorticoid receptor; GRE, glucocorticoid response element; αGSU, glycoprotein hormone α-subunit; hCG, human chorionic gonadotropin; JRE, junctional regulatory element; luc, luciferase; PR, progesterone receptor.
References
- Gharib SD, Wierman ME, Shupnik MA, Chin WW 1990 Molecular biology of the pituitary gonadotropins. Endocr Rev 11:177–199 [DOI] [PubMed] [Google Scholar]
- Fiddes JC, Goodman HM 1981 The gene encoding the common α-subunit of the four human glycoprotein hormones. J Mol Appl Genet 1:3–18 [PubMed] [Google Scholar]
- Shupnik MA 1996 Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod 54:279–286 [DOI] [PubMed] [Google Scholar]
- Keri RA, Andersen B, Kennedy GC, Hamernik DL, Clay CM, Brace AD, Nett TM, Notides AC, Nilson JH 1991 Estradiol inhibits transcription of the human glycoprotein hormone α-subunit gene despite the absence of a high affinity binding site for estrogen receptor. Mol Endocrinol 5:725–733 [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Suhr A, Marshall JC 1990 Gonadal regulation of gonadotropin subunit gene expression: evidence for regulation of follicle-stimulating hormone-β messenger ribonucleic acid by nonsteroidal hormones in female rats. Endocrinology 127:798–806 [DOI] [PubMed] [Google Scholar]
- Kerrigan JR, Dalkin AC, Haisenleder DJ, Yasin M, Marshall JC 1993 Failure of gonadotropin-releasing hormone (GnRH) pulses to increase luteinizing hormone β-messenger ribonucleic acid in GnRH-deficient female rats. Endocrinology 133:2071–2079 [DOI] [PubMed] [Google Scholar]
- Paul SJ, Ortolano GA, Haisenleder DJ, Stewart JM, Shupnik MA, Marshall JC 1990 Gonadotropin subunit messenger RNA concentrations after blockade of gonadotropin-releasing hormone action: testosterone selectively increases follicle-stimulating hormone β-subunit messenger RNA by posttranscriptional mechanisms. Mol Endocrinol 4:1943–1955 [DOI] [PubMed] [Google Scholar]
- Clay CM, Keri RA, Finicle AB, Heckert LL, Hamernik DL, Marschke KM, Wilson EM, French FS, Nilson JH 1993 Transcriptional repression of the glycoprotein hormone α-subunit gene by androgen may involve direct binding of androgen receptor to the proximal promoter. J Biol Chem 268:13556–13564 [PubMed] [Google Scholar]
- Jorgensen JS, Nilson JH 2001 AR suppresses transcription of the α-glycoprotein hormone subunit gene through protein-protein interactions with cJun and activation transcription factor 2. Mol Endocrinol 15:1496–1504 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Wilson EM, Nilson JH 1997 Transcriptional repression of the α-subunit gene by androgen receptor occurs independently of DNA binding but requires the DNA-binding and ligand-binding domains of the receptor. Mol Endocrinol 11:1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstrom SJ, McAndrews JM, Rahal JO, Schwartz NB 1991 Cortisol in vivo increases FSHβ mRNA selectively in pituitaries of male rats. Endocrinology 129:2793–2795 [DOI] [PubMed] [Google Scholar]
- Kilen SM, Szabo M, Strasser GA, McAndrews JM, Ringstrom SJ, Schwartz NB 1996 Corticosterone selectively increases follicle-stimulating hormone β-subunit messenger ribonucleic acid in primary anterior pituitary cell culture without affecting its half-life. Endocrinology 137:3802–3807 [DOI] [PubMed] [Google Scholar]
- McAndrews JM, Ringstrom SJ, Dahl KD, Schwartz NB 1994 Corticosterone in vivo increases pituitary follicle-stimulating hormone (FSH)-β messenger ribonucleic acid content and serum FSH bioactivity selectively in female rats. Endocrinology 134:158–163 [DOI] [PubMed] [Google Scholar]
- Gurr JA, Kourides IA 1989 Regulation of the transfected human glycoprotein hormone α-subunit gene by dexamethasone and thyroid hormone. DNA 8:473–480 [DOI] [PubMed] [Google Scholar]
- Akerblom IE, Slater EP, Beato M, Baxter JD, Mellon PL 1988 Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science 241:350–353 [DOI] [PubMed] [Google Scholar]
- Chatterjee VK, Madison LD, Mayo S, Jameson JL 1991 Repression of the human glycoprotein hormone α-subunit gene by glucocorticoids: evidence for receptor interactions with limiting transcriptional activators. Mol Endocrinol 5:100–110 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL 2001 Cell-specific transcriptional regulation of FSHβ by activin and GnRH in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL 2006 Androgens, progestins and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn F, Windle JJ, Barnhart KM, Mellon PL 1992 Tissue-specific gene expression in the pituitary: the glycoprotein hormone α-subunit gene is regulated by a gonadotrope-specific protein. Mol Cell Biol 12:2143–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen T, Palvimo JJ, Janne OA 1998 Heterodimerization is mainly responsible for the dominant negative activity of amino-terminally truncated rat androgen receptor forms. FEBS Lett 430:393–396 [DOI] [PubMed] [Google Scholar]
- Resnick EM, Schreihofer DA, Periasamy A, Shupnik MA 2000 Truncated estrogen receptor product-1 suppresses estrogen receptor transactivation by dimerization with estrogen receptors α and β. J Biol Chem 275:7158–7166 [DOI] [PubMed] [Google Scholar]
- Stauber C, Altschmied J, Akerblom AE, Marron JL, Mellon PL 1992 Mutual cross-interference between glucocorticoid receptor and CREB inhibits transactivation in placental cells. New Biol 4:527–540 [PubMed] [Google Scholar]
- Slinker BK 1998 The statistics of synergism. J Mol Cell Cardiol 30:723–731 [DOI] [PubMed] [Google Scholar]
- Griffin JE, Ojeda SR 2000 Textbook of endocrine physiology. 4th ed. New York: Oxford University Press [Google Scholar]
- Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf HJ, Herrlich P, Cato AC 1994 A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J 13:4087–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G 1998 DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531–541 [DOI] [PubMed] [Google Scholar]
- Rogers SL, Nabozny G, McFarland M, Pantages-Torok L, Archer J, Kalkbrenner F, Zuvela-Jelaska L, Haynes N, Jiang H, Four mutations in the GR dimerization domain result in perinatal lethal mice. Keystone Symposia, Nuclear Receptors: Steroid Sisters, Keystone, CO 2004, p 194 (Abstract 316) [Google Scholar]
- Beato M, Arnemann J, Chalepakis G, Slater E, Willmann T 1987 Gene regulation by steroid hormones. J Steroid Biochem 27:9–14 [DOI] [PubMed] [Google Scholar]
- Diamond M, Miner J, Yoshinaga S, Yamamoto K 1990 Transcription factor interactions: Selectors of positive or negative regulation from a single DNA element. Science 249:1266–1272 [DOI] [PubMed] [Google Scholar]
- Hoeppner MA, Mordacq JC, Linzer DI 1995 Role of the composite glucocorticoid response element in proliferin gene expression. Gene Expr 5:133–141 [PMC free article] [PubMed] [Google Scholar]
- Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS 2005 Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol 19:2624–2638 [DOI] [PubMed] [Google Scholar]
- Schoderbek WE, Kim KE, Ridgway EC, Mellon PL, Maurer RA 1992 Analysis of DNA sequences required for pituitary-specific expression of the glycoprotein hormone α-subunit gene. Mol Endocrinol 6:893–903 [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR 1989 Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59:675–680 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Meermann S, Morasso MI, Mulvaney-Musa JM, Zhang T 2001 A role for the homeobox protein Distal-less 3 in the activation of the glycoprotein hormone α-subunit gene in choriocarcinoma cells. J Biol Chem 276:10016–10024 [DOI] [PubMed] [Google Scholar]
- Bailey JS, Rave-Harel N, Coss D, McGillivray SM, Mellon PL 2004 Activin regulation of the follicle-stimulating hormone β-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol 18:1158–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Thackray VG, Deng CX, Mellon PL 2005 Activin regulates luteinizing hormone β-subunit gene expression through smad-binding and homeobox elements. Mol Endocrinol 19:2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi B, Klatt B, Little G 1995 Repression of glycoprotein hormone α-subunit gene expression and secretion by activin in αT3-1 cells. Mol Endocrinol 9:1737–1749 [DOI] [PubMed] [Google Scholar]
- Ben-Menahem D, Shraga-Levine Z, Mellon PL, Naor Z 1995 Mechanism of action of gonadotropin-releasing hormone upon gonadotropin α-subunit mRNA levels in the αT3-1 cell line: Role of Ca++ and protein kinase C. Biochem J 309:325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL 2004 A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon JL, Kimura Y, Waring DW, Mellon PL 1996 Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol 10:439–450 [DOI] [PubMed] [Google Scholar]
- McGillivray SM, Thackray VG, Coss D, Mellon PL 2007 Activin and glucocorticoids synergistically activate follicle-stimulating hormone β-subunit gene expression in the immortalized LβT2 gonadotrope cell line. Endocrinology 148:762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AM, Blount AL, Donaldson CJ, Bilezikjian LM, Vale WW 2003 Regulation of follicle-stimulating hormone secretion by the interactions of activin-A, dexamethasone and testosterone in anterior pituitary cell cultures of male rats. Neuroendocrinology 77:298–304 [DOI] [PubMed] [Google Scholar]
- Sakakura M, Takebe K, Nakagawa S 1975 Inhibition of luteinizing hormone secretion induced by synthetic LRH by long-term treatment with glucocorticoids in human subjects. J Clin Endocrinol Metab 40:774–779 [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ 2004 Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology 145:692–698 [DOI] [PubMed] [Google Scholar]
- Wilbur JF, Utiger RD 1969 The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 48:2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faglia G, Ferrari C, Beck-Peccoz P, Spada A, Travaglini P, Ambrosi B 1973 Reduced plasma thyrotropin response to thyrotropin releasing hormone after dexamethasone administration in normal subjects. Horm Metab Res 5:289–292 [DOI] [PubMed] [Google Scholar]
- Re RN, Kourides IA, Ridgway EC, Weintraub BD, Maloof F 1976 The effect of glucocorticoid administration on human pituitary secretion of thyrotropin and prolactin. J Clin Endocrinol Metab 43:338–346 [DOI] [PubMed] [Google Scholar]
- Nicoloff JT, Fisher DA, Appleman Jr MD 1970 The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 49:1922–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau H, Delgado A, MacIntosh-Hardt B, Fortier C 1972 Metabolic clearance and secretion rates of TSH following adrenalectomy and corticosterone administration in the rat. Endocrinology 90:973–980 [DOI] [PubMed] [Google Scholar]
- Fang VS, Shian LR 1981 Adrenal influence on pituitary secretion of thyrotropin and prolactin in rats. Endocrinology 108:1545–1551 [DOI] [PubMed] [Google Scholar]
- Ross DS, Ellis MF, Milbury P, Ridgway EC 1987 A comparison of changes in plasma thyrotropin β- and α-subunits, and mouse thyrotropic tumor thyrotropin β- and α-subunit mRNA concentrations after in vivo dexamethasone or T3 administration. Metabolism 36:799–803 [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Dohler KD, Wilson CA 1978 Activity of the pituitary-adrenocortical system and thyroid gland during the oestrous cycle of the rat. J Endocrinol 78:359–366 [DOI] [PubMed] [Google Scholar]
- Nichols DJ, Chevins PF 1981 Plasma corticosterone fluctuations during the oestrous cycle of the house mouse. Experientia 37:319–320 [DOI] [PubMed] [Google Scholar]
- Tebar M, Uilenbroek JT, Kramer P, van Schaik RH, Wierikx CD, Ruiz A, de Jong FH, Sanchez-Criado JE 1997 Effects of progesterone on the secondary surge of follicle-stimulating hormone in the rat. Biol Reprod 57:77–84 [DOI] [PubMed] [Google Scholar]
- Szabo M, Kilen SM, Saberi S, Ringstrom SJ, Schwartz NB 1998 Antiprogestins suppress basal and activin-stimulated follicle-stimulating hormone secretion in an estrogen-dependent manner. Endocrinology 139:2223–2228 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Schultz K, Nilson JH 1995 Different composite regulatory elements direct expression of the human α-subunit gene to pituitary and placenta. J Biol Chem 270:26497–26504 [DOI] [PubMed] [Google Scholar]
- Barnhart KM, Mellon PL 1994 The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone α-subunit gene in pituitary gonadotropes. Mol Endocrinol 8:878–885 [DOI] [PubMed] [Google Scholar]
- Steger DJ, Hecht JH, Mellon PL 1994 GATA-binding proteins regulate the human gonadotropin α-subunit gene in placenta and pituitary. Mol Cell Biol 14:5592–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai E, Miner JN, Mitchell JA, Yamamoto KR, Granner DK 1993 Glucocorticoid receptor-cAMP response element-binding protein interaction and the response of the phosphoenolpyruvate carboxykinase gene to glucocorticoids. J Biol Chem 268:5353–5356 [PubMed] [Google Scholar]
- Johannessen M, Moller S, Hansen T, Moens U, Van Ghelue M 2006 The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell Mol Life Sci 63:268–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JS, Quirk CC, Nilson JH 2004 Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev 25:521–542 [DOI] [PubMed] [Google Scholar]
- Budworth PR, Quinn PG, Nilson JH 1997 Multiple characteristics of a pentameric regulatory array endow the human α-subunit glycoprotein hormone promoter with trophoblast specificity and maximal activity. Mol Endocrinol 11:1669–1680 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Schultz K, Nilson JH 1996 The cAMP response elements of the α-subunit gene bind similar proteins in trophoblasts and gonadotropes but have distinct functional sequence requirements. J Biol Chem 271:31650–31656 [DOI] [PubMed] [Google Scholar]
- Wieland S, Döbbeling U, Rusconi S 1991 Interference and synergism of glucocorticoid receptor and octamer factors. EMBO J 10:2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan WR, Shin JL, Jameson JL 1999 Estradiol suppresses phosphorylation of cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) in the pituitary: evidence for indirect action via gonadotropin-releasing hormone. Mol Endocrinol 13:1338–1352 [DOI] [PubMed] [Google Scholar]
- Liu JL, Papachristou DN, Patel YC 1994 Glucocorticoids activate somatostatin gene transcription through co-operative interaction with the cyclic AMP signalling pathway. Biochem J 301:863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R, Muller M, Kaltschmidt C, Renkawitz R 1988 Many transcription factors interact synergistically with steroid receptors. Science 242:1418 [DOI] [PubMed] [Google Scholar]
- Strähle U, Schmid W, Schütz G 1988 Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J 7:3389–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M 1992 Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell 70:105–113 [DOI] [PubMed] [Google Scholar]
- Ethier JF, Findlay JK 2001 Roles of activin and its signal transduction mechanisms in reproductive tissues. Reproduction 121:667–675 [DOI] [PubMed] [Google Scholar]
- Fowkes RC, King P, Burrin JM 2002 Regulation of human glycoprotein hormone α-subunit gene transcription in LβT2 gonadotropes by protein kinase C and extracellular signal-regulated kinase 1/2. Biol Reprod 67:725–734 [DOI] [PubMed] [Google Scholar]