Abstract
It is dogma that action potentials are initiated at the soma/axon hillock of neurons. However, dendrites often exhibit conductances necessary for spike generation and represent functionally independent processing compartments within neurons. GnRH neurons provide an interesting neuronal phenotype with simple, relatively unbranched, unipolar or bipolar dendrites of extensive lengths (>1000 μm) covered in spines. These neurons control fertility and must integrate a variety of internal homeostatic and external environmental cues. We used imaging, electrophysiological, and modeling studies to understand how they integrate and process information along dendrites. Simultaneous recordings from distal dendrites and somata of individual GnRH neurons indicate distal dendrites are the primary site of spike initiation in these cells. Compartmental modeling indicates that sites of spike initiation depend upon location of excitatory input and dendrite geometry. Together, these studies demonstrate a novel pattern of spike generation in mammalian neurons and indicate that afferent inputs within distal dendritic microdomains directly initiate action potentials.
THE GnRH NEURONS represent the final output neurons of the neuronal network controlling fertility in all mammals. These neurons are unique in that they are born in the nasal placode and migrate into the brain during embryogenesis (1,2). Once located in the hypothalamus, the GnRH neurons exhibit simple unipolar or bipolar morphologies and extend axons from their somata to the median eminence from where they secrete GnRH into the hypophyseal portal system to control pituitary gonadotropin secretion (3).
The regulation of fertility requires the precise integration of a wide variety of internal homeostatic and external environmental cues (4). The mechanisms underlying this integration are unclear. Controversy has surrounded the role that the GnRH neurons themselves may play in the processing of this information because early electron microscopic studies failed to identify more than a few synapses on individual GnRH neurons (3). However, very recent dye-filling experiments have challenged this view by revealing that the GnRH neurons in situ exhibit numerous spines on both their cell somata and dendrites (5,6,7). More remarkably, these studies have revealed that a GnRH neuron’s dendrite extends for considerable distances, often over 1000 μm, only occasionally branches, and remains spiny until its endpoint (5,6,7). Thus, GnRH neurons are simple yet extraordinarily long unipolar or bipolar processing units. This morphology raises important questions about the ways in which electrical information is processed by GnRH neurons. Using imaging, simultaneous soma-dendrite recording, and modeling approaches, we report here that GnRH neuron dendrites are active and, indeed, represent the primary site of action potential generation in these cells.
Materials and Methods
Confocal imaging
We examined GnRH dendrites from transgenic mice that express green fluorescent protein (GFP) in their GnRH neurons (GnRH-GFP transgenic mice) (8) for voltage-gated sodium channels using immunocytochemistry and confocal fluorescence microscopy employing a primary antibody whose specificity has been characterized previously (9). Sagittal slices from adult female homozygous GnRH-GFP mice, in which essentially all GnRH neurons express GFP, were processed for fluorescence immunocytochemistry as reported previously (6) and with the approval of the University of Otago Animal Welfare and Ethics Committee. In brief, four mice were perfused with 4% paraformaldehyde, and 30-μm-thick sagittal sections cut on a freezing microtome. Free-floating sagittal sections were incubated in a cocktail of chicken anti-GFP (1:1000; Chemicon International, Inc., Temecula, CA) and rabbit anti-pan voltage-gated sodium channel (Na+v) [made against the peptide sequence SP19 (amino acids 1501–1518) of the rat sodium channel peptide sequence, 1:750; Chemicon]. Sections incubated without the SP19 primary antibody served as a negative control. Subsequently, sections were incubated in Alexa Fluor 594 goat antirabbit and Alexa Fluor 488 goat antichicken Ig (both at 1:200; Molecular Probes, Eugene, OR). A series of optical images at 0.36-μm intervals were collected on a Zeiss 510 LSM from 10–12 randomly chosen GnRH neurons located in the rostral preoptic area in each animal.
Electrophysiological recordings
Sagittal hypothalamic slices (300 μm) were prepared from GnRH-GFP mice (8), and whole-cell recordings were obtained as described previously (10). Studies were approved by the Institutional Animal Care and Use Committee at Emory University. Each recording was obtained from a different mouse (n = 12). GnRH-GFP somata were identified using a brief exposure with epifluorescence. The diffuse distribution of GnRH neurons/dendrites combined with robust GFP fluorescence of dendrites allowed for high-fidelity tracking of living dendrites from individual somata. Extension of the dendrite was tracked to at least 250 μm before attempting to establish a dendritic recording. To limit exposure, the light source was shuttered such that the slice was illuminated for only 100 msec of each 1.5 sec during identification. Due to the very small dendrite diameters (∼0.1 μm) (6,7), dendrite recordings employed a tight-seal attached approach. Cell-attached recordings were obtained using the 2B-Axoclamp in series with the AM Systems 3000 amplifier. Electrodes (9–12 mΩ) for cell-attached recordings were filled with artificial cerebrospinal fluid and coated with Sylgard to reduce capacitance. Seal resistances were 300–500 mΩ.
Compartmental modeling
The multicompartmental, conductance-based models used in this study were constructed in the General Neural Simulation System (GENESIS) and based on those described previously (7). Morphologies (a simple unbranching bipolar morphology and one with a single branching dendrite) were based on reconstructions of biocytin-filled GnRH neurons, from which active and passive electrical activity was measured in whole-cell recordings. Genetic algorithm automated parameter searches were used to tune passive (membrane capacitance and resistance and axial resistance) and active (conductance densities and kinetics of voltage-gated currents) electrical parameters in the models to duplicate measured electrophysiological responses to somatic current injection. In these active dendrite models, the somata had fast Na+ delayed rectifier (11), inward rectifier K+ (11,12), and L-type Ca2+ conductances (11,13). The axons and dendrites had fast Na+ and delayed rectifier K+ conductances. The initial formulations for conductances were taken from mathematical models of GT1-7 cells in (14), a model cell line of GnRH neurons (15). To construct multicompartmental models and thus preserve the structure and potential function of extended dendrite (which are not represented in single compartment models), densities of the conductances in the various parts of the models were determined by genetic algorithm tuning by matching the shape and frequency of measured somatic action potentials generated by somatic current injection in living GnRH neurons.
Sensitivity analysis of conductances in the model was performed by systematically changing one of the conductance amplitudes at a time by ±10%. The model was then run with this perturbed parameter set, and a fitness value was calculated and compared with that for the best model from the genetic algorithm. For variations in the Na+ and K+ conductances of ±10% in all model compartments, the fitness value changed by less than 20%, and the basic behavior of the model was unchanged. The models were most sensitive to variations in the Ca2+ conductance amplitude, but nonetheless, an increase in the Ca2+ conductance of 5% resulted in only a 6% change in model fitness.
Results
Immunocytochemistry reveals voltage-gated sodium channels in dendrites of GnRH neurons
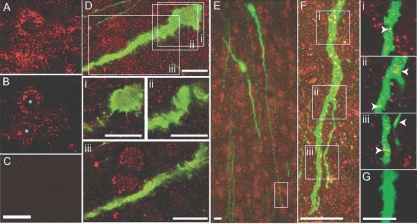
Na+v immunoreactivity (Na+v-ir) existed in a punctate but heterogeneous pattern throughout the forebrain (Fig. 1, A and B). In many brain regions, such as the hippocampus, Na+v-ir was found associated with the cell soma. All GnRH neurons (43 of 43) examined in all four mice were found to express Na+v-ir (Fig. 1, D–F). Staining for Na+v-ir was detected in the soma as well as throughout the entire length of the dendrites of each GnRH neuron (Fig. 1, D–F). No differential distribution of Na+v-ir was apparent in different subcellular portions. Figure 1E shows Na+v-ir in a distal dendritic element located 390 μm from the GnRH neuron soma. Immunoreactivity, detected in single optical images, was present along the length of the distal dendrite (Fig. 1, E and F, insets i–iii). Absence of the primary antibody resulted in a complete absence of signal for Na+v (Fig. 1, C and G). This observation indicates that GnRH neuron dendrites express conductances that could lead to active properties and facilitate the impact of synapses in distal dendritic regions of GnRH neurons.
Figure 1.
GnRH neuron dendrites express Na+v channels. A, Projected confocal image of Na+v-ir in the CA1 hippocampus of GnRH-GFP mice; B, single optical slice (0.36 μm) through two neurons (asterisks) showing with membrane-localized Na+v-ir; C, omitting Na+v primary antibody eliminates Na+v-ir; D, projected confocal stack of a GnRH soma and proximal dendrite (green) and Na+v-ir (red): i–iii, below, single optical slices (0.36 μm) corresponding to regions indicated by white boxes in D; E, low-power projected confocal image of GnRH neuron cell bodies and dendrites and Na+v-ir; F, projected confocal stack of a portion of dendrite 390 μm from the cell body of a GnRH neuron shown in E (white box); i–iii, far right, single optical slices (0.36 μm) corresponding to regions indicated by white boxes in F, with arrows highlighting yellow pixels indicative of Na+v-ir localized in the distal segment of a GnRH dendrite; G, omitting Na+v primary antibody eliminates Na+v-ir. Scale bars, 10 μm (A–E) and 5 μm (G).
Simultaneous recordings from somata and dendrites demonstrate active back-propagation of spikes in GnRH neurons
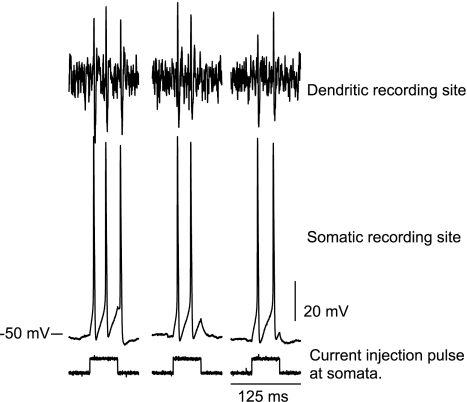
The above results indicated that the dendrites of GnRH neurons might exhibit active electrical properties. We tested this hypothesis directly using simultaneous somatic and dendritic recordings from individual GFP-identified GnRH neurons in brain slices. Dendrites were recorded at least 250 μm from the soma. Somatic current injection (Fig. 2; bottom traces) drove action potentials in GnRH somata (Fig. 2; middle traces) and resulted in the detection of action potentials at the dendritic recording site (Fig. 2; top traces). In all cases, dendritic action potentials followed somatic action potentials, a finding that is consistent with back-propagation from a somatic site of origination. The placement of the dendritic recording at least 250 μm from somata exceeds the distance over which voltage from action potentials of somatic origin could be conveyed passively, based on our initial multicompartmental modeling study (7).
Figure 2.
Back-propagation of action potentials from GnRH somata to dendrites. Current pulses applied to somata induced action potentials. These action potentials were subsequently detected at dendrite recording sites that were at least 250 μm from somata.
Thus, dendrites exhibit back-propagation of action potentials, indicative of active electrical properties in dendrites. Peak latencies between somatic action potentials (n = 16; elicited in response to current injection in the whole-cell recording configuration) and subsequent dendritic action potentials (in the cell-attached recording configuration) were 0.37 ± 0.12 msec (mean ± sd). The variation in peak latencies probably reflects differences in placement of the somatic and dendritic recording pipettes. We cannot define an exact rate of propagation because the course of the dendritic profile between the two recording pipettes was not a straight line.
Simultaneous recordings from somata and dendrites of GnRH neurons demonstrate predominant dendritic sites of action potential initiation
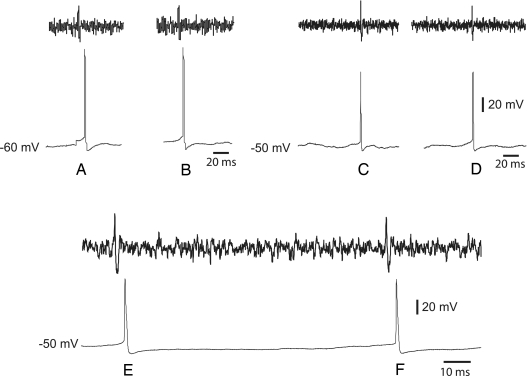
To evaluate the temporal relationship between somatic and dendritic spikes under conditions of spontaneous activity, we made dual soma-dendrite recordings from 12 spontaneously active GnRH neurons (Fig. 3). During these recordings, no current was applied to either the somata or dendrite. Strikingly, in eight GnRH neurons, all 57 endogenously generated action potentials (range, one to 11 action potentials per neuron in 4–7 min of recording) were detected first at the dendritic recording site over 250 μm distant from the soma (Fig. 3, action potentials A, B, E, and F). In the remaining four GnRH neurons (13 action potentials; range, two to five action potentials per neuron in 4–7 min of recording), a mixture of somatic- and dendritic-initiated action potentials were recorded with about 35% of the total number of action potentials first detected at the somatic recording site (Fig. 3, action potentials C and D) and about 65% recorded first at the dendrite. Peak latencies in propagation of the spontaneous action potentials from the dendrite to the soma (0.45 ± 0.26 msec) were not different to those of back-propagating action potentials (0.37 ± 0.12 msec).
Figure 3.
Dendrites exhibit spontaneous action potential initiation in GnRH neurons. Simultaneous recordings from GnRH somata (thin traces) and dendrites (thick traces) in two neurons reveal action potentials originating in dendrites in one neuron (action potentials A and B), whereas others originate in somata of a different GnRH neuron (action potentials C and D). In a third GnRH neuron, action potentials have dendritic sites of origin (action potentials E and F).
Location of excitatory synaptic input determines whether spike initiation is somatic or dendritic in model GnRH neurons
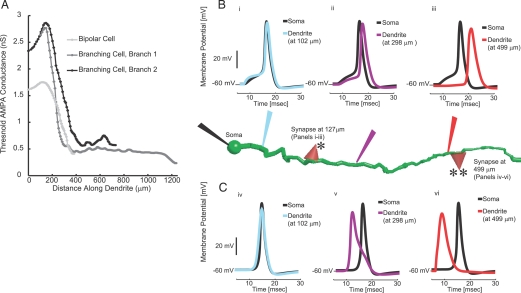
To understand the significance of the different sites of action potential generation (i.e. somata or dendrites) we used our multicompartmental, conductance-based models of GnRH neurons with active dendrites and determined the minimal conductance amplitude of a synaptic event to generate a spike. For this synaptic threshold survey, we used models of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-type glutamatergic synapses (10). However, the same behavior was observed with short-pulse current injections. The threshold conductance value was found to vary markedly depending upon the location of the simulated synapse on the dendrite. At dendritic distances beyond about 130 μm, the threshold of activation decreased precipitously to a level well below that for the initiation of somatic action potentials (Fig. 4A). The conductance threshold for somatic action potential generation was 1.6 nS for the bipolar cell model, and 2.3 nS for the larger branching cell. Thresholds increase to maxima of 1.8 nS at 118 μm of dendrite length in the bipolar cell and 2.9 nS at 150 μm of dendrite length in the branching cell. From the maxima, the synaptic amplitudes necessary to cause an action potential decreased to 0.41 and 0.23 nS at the distal tips of the dendrites. To understand this dependence of threshold conductance on the distance of the synapse from the soma, we examined the time courses of the voltages of the individual compartments in the simulations. The activation of synapses less than 130 μm from the soma (Fig. 4B, i–iii) resulted in action potentials that were initiated in the soma (black traces) and were followed by action potentials at later times along the increasing length of dendrite (colored traces). In contrast, activation of dendritic synapses greater than 200 μm from the soma (Fig. 4C, iv–vi) resulted in spikes of dendritic origin (colored traces) that preceded somatic action potentials (in black traces). These findings support a model of dual soma-dendritic control of action potential generation in GnRH neurons.
Figure 4.
Differences in action potential thresholds for simulated synapses applied along the length of model GnRH dendrites. A, The minimum synaptic strength to initiate a spike varies with the location on the dendrite. Each curve indicates a dendrite branch, either the single dendrite in the bipolar cell model or each of the two branches of model with a branching dendrite. Simulated synapses were placed on dendrites at increasing distances from the soma. Action potential thresholds reach a maximum of 1.8 nS at 118 μm of dendrite length in the bipolar cell and 2.9 nS at 150 μm of dendrite length in the branching cell and then decreased to 0.41 and 0.23 nS, respectively, at the distal tips of the dendrites. B, Shift from somatic to dendritic spike initiation along the length of the model GnRH dendrite. In each panel, the black trace corresponds to somatic membrane potential changes (black electrode). The colored traces indicate voltages in dendrite compartments whose recording electrode and simulated voltage trace has the corresponding color. Voltage responses in the top panels correspond to activation of a simulated synapse at 127 μm of dendrite length (indicated by the red triangle with a single asterisk). Voltage responses in the lower panels correspond to activation of a simulated synapse at 499 μm of dendrite length (indicated by the red triangle with a double asterisk). In i, the postsynaptic potential is seen first in the dendrite (blue), but the action potential is initiated at the soma (black). The purple and red traces show active back-propagation of the somatic action potential into dendrite compartments at 298 μm of dendrite length (ii; purple trace) and 499 μm of dendrite length (iii; red trace). In the upper traces, the black somatic spike precedes spikes in all dendrite compartments, and thus, these action potentials have somatic sites of initiation. In the lower panels, activation of the simulated synapse at 499 μm of dendrite length led to a locally initiated action potential in the dendrite (vi) where the dendrite action potential (red trace) precedes the somatic action potential (black trace). The action potential was actively propagated from its initiation site in the distal dendrite (at 499 μm) toward the soma to more proximal dendrite compartments at 298 μm (purple trace in v) and 102 μm (blue trace in iv) and then invades the soma (black trace in iv–vi). Accordingly, regions on either side of the maximal threshold in A differ in spike initiation zone. The axonal compartments have been removed from the model neuron in the figure but were included during simulations.
Discussion
We report here that the simple, long dendrites of GnRH neurons are not only active but also the probable primary site of action potential generation in these cells. The identification of voltage-gated Na+ channels throughout their dendritic length and the back-propagation of somatic action potentials into dendrites at lengths greater than 250 μm all demonstrate that GnRH neuron dendrites exhibit active properties like some other neurons. This had not been shown before for GnRH neurons. The striking finding of this study, however, has been that dendrites of GnRH neurons appear to be the principle site of action potential generation in this neuronal phenotype. All 12 spontaneously active GnRH neurons recorded simultaneously at their somata and dendrites showed evidence of dendrite-initiated spikes. Somatic action potentials occurred only after dendritic spike initiation in eight of these neurons, whereas the other four exhibited mixed dendritic and somatic spike initiation.
Although it is now well established that dendrites can exist as independent functional modules within neurons able to generate dendritic spikes (16,17), examples of neurons in which dendrites reliably drive action potential firing are rare. Dopaminergic neurons in the substantia nigra exhibit robust dendrite spikes that reflect largely back-propagation because the axon generally originates from the dendrite (18). The specialized tufts of olfactory mitral cells located on their distal dendrites have been shown to activate somatic impulses (19), and the dendrites of hippocampal interneurons activate action potentials in response to high-intensity stimulation (20). The findings here with GnRH neurons are remarkable in that the great majority of action potentials appear to be spontaneously initiated in the dendrites.
Based on our modeling studies, it seems probable that the site of spike initiation in GnRH neurons depends upon the location of excitatory synaptic input and the geometry of the dendrite. Compared with the soma and very proximal dendrite, the high axial resistance and low capacitance of distal dendrites will tend to isolate the effects of inputs and enhance postsynaptic potentials (16). These properties would result in highly localized synaptic integration and action potential initiation in the distal dendrites. As shown by the modeling work, even small synaptic currents at the distal dendrites would be effective in initiating action potentials, and indeed, most endogenous, single synaptic currents in GnRH neurons are known to be relatively small (∼0.4 nS) (10,21). In contrast, as suggested by dynamic clamping experiments (10), the larger-diameter proximal dendrite would enable a more global mode of synaptic integration and action potential initiation near the soma.
Although the majority of action potentials in GnRH neurons are initiated from distal dendritic sites, the dual soma-dendrite recordings clearly show that some cells are initiating action potentials from both somatic and dendritic sites. This probably reflects the shifting balance of excitatory and inhibitory synapse activation along the soma and dendrite (17). The density of spines within the first 50 μm of proximal GnRH neuron dendrites is twice that of distal dendritic elements in GnRH neurons, but this is equaled by a similar density gradient of GABAergic synapses (6). Reasons for the dominance of dendritic sites in activating action potentials in GnRH neurons are not known. The morphology of these neurons is unique in that they are simple, relatively unbranched unipolar or bipolar neurons of substantial length. As a result, there is an extensive, cable-like, distal dendrite endowed with active conductances and excitatory synaptic inputs. Thus, from a conceptual standpoint, this would be predicted to act as a highly efficient dendritic spike-initiating unit (17). From a physiological standpoint, GnRH neurons must integrate multiple internal and external cues that regulate reproduction (4). The ability of dendritic domains to initiate action potentials suggests that the integration of inputs may occur in a spatially distinct manner along the GnRH neuron dendrite. This greatly expands the processing options for these cells because the impact of an individual input on cell firing no longer depends on its proximity to the axon hillock but, instead, their spatial relationship to other inputs. The clustering of modality-specific inputs at a particular point on the dendrite would then enable focused mode-specific integration that would impact upon the output of GnRH neurons.
In summary, these observations represent a critical new insight into the way in which electrical information processing is occurring within the GnRH neuronal phenotype. More generally, these observations provide the initial demonstration that dendrites can in fact be a principal site of action potential initiation and establish further the critical role of dendrites as functionally active units within the neuron.
Acknowledgments
Electrophysiology was performed in Dieter Jaeger’s laboratory at Emory University. We also acknowledge his insight in the modeling components.
Footnotes
This work was supported by National Institutes of Health Grant HD-45436 (to K.J.S.) and by the Wellcome Trust (UK) and New Zealand Foundation for Research Science and Technology to R.E.C. and A.E.H. Analysis and modeling facilities were provided by the Computational Biology Initiative (http://www.cbi.utsa.edu) at the University of Texas San Antonio and the University of Texas Health Sciences Center at San Antonio, which is funded by a partnership between National Institutes of Health (RR013646) and the Vice Chancellor for Medical Affairs of the University of Texas.
Preliminary reports of this work were presented at the 2006 Society for Neuroscience Annual Meeting (Abstract 776.1), the Sixth International Congress for Neuroendocrinology (Abstract 208), and Symposium Advances in Neurobiology, at the 2008 Annual Meeting for Society for Integrative and Comparative Biology (Abstract S6-1.2).
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 10, 2008
Abbreviations: GFP, Green fluorescent protein; ir, immunoreactivity; Na+v, voltage-gated sodium channel.
References
- Wray S, Grant P, Gainer PH 1989 Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW 1989 Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164 [DOI] [PubMed] [Google Scholar]
- Silverman A-J, Livne I, Witkin JW 1994 The gonadotropin-releasing hormone (GnRH) neuronal systems: immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD, eds. Physiology of reproduction. New York: Raven Press; 1683–1710 [Google Scholar]
- Herbison AE 2006 Physiology of the GnRH neuronal network. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. San Diego: Academic Press; 1415–1482 [Google Scholar]
- Campbell RE, Han SK, Herbison AE 2005 Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology 146:1163–1169 [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Campell RE, Han SK, Herbison AE 2006 Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147:3652–3661 [DOI] [PubMed] [Google Scholar]
- Roberts CB, Best JA, Suter KJ 2006 Dendritic processing of excitatory synaptic input in hypothalamic gonadotropin releasing-hormone neurons. Endocrinology 147:1545–1555 [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Shimshek DR, Sprengel R, Seeburg PH 1999 GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, AS Sanders, EJ Duszyk, E Prasad, S Torkkeli, PH Haskins, J Murphy, RA 1993 Immunocytochemical localization of sodium channels in an insect central nervous system using a site-directed antibody. J Neurobiol 24:939–948 [DOI] [PubMed] [Google Scholar]
- Suter KJ 2004 Control of firing by small (S)-α-amino-3-hydroxy-5-methyl-isoxazolepropionic acid-like inputs in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Neuroscience 128:443–450 [DOI] [PubMed] [Google Scholar]
- Kusano K, Fueshko S, Gainer H, Wray S 1995 Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci USA 92:3918–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Grandy DK, Kelly MJ 1998 The peptide orphanin FQ inhibits β-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology 67:73–82 [DOI] [PubMed] [Google Scholar]
- Spergel DJ 2007 Calcium and small-conductance calcium-activated potassium channels in gonadotropin-releasing hormone neurons before, during, and after puberty. Endocrinology 148:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau AP, Van Goor F, Stojilkovic SS, Sherman A 2000 Modeling of membrane excitability in gonadotropin-releasing hormone-secreting hypothalamic neurons regulated by Ca2+-mobilizing and adenylyl cyclase-coupled receptors. J Neurosci 20:9290–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RL 1990 Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10 [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ 2003 Role of dendritic synapse location in the control of action potential output. Trends Neurosci 26:147–154 [DOI] [PubMed] [Google Scholar]
- Holthoff K, Kovalchuk Y, Konnerth A 2006 Dendritic spikes and activity-dependent synaptic plasticity. Cell Tissue Res 326:369–377 [DOI] [PubMed] [Google Scholar]
- Hausser M, Stuart G, Racca C, Sakmann B 1995 Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron 15:637–647 [DOI] [PubMed] [Google Scholar]
- Chen WR, Midtgaard J, Shepherd GM 1997 Forward and backward propagation of dendritic impulses and their synaptic control in mitral cells. Science 278:463–467 [DOI] [PubMed] [Google Scholar]
- Martina M, Vida I, Jonas P 2000 Distal initiation and active propagation of action potentials in interneuron dendrites. Science 287:295–300 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, DeFazio RA, Moenter SN 2003 Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J Neurosci 23:8578–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]