Abstract
Pulsatile release of GnRH-1 stimulates the anterior pituitary and induces secretion of gonadotropin hormones. GnRH-1 release is modulated by many neurotransmitters that act via G protein-coupled membrane receptors. cAMP is the most ubiquitous effector for these receptors. GnRH-1 neurons express hyperpolarization-activated cyclic nucleotide-modulated (HCN) channel protein in vivo. HCN channels are involved in neuronal pacemaking and can integrate cAMP signals. cAMP-dependent protein kinase (PKA) is also activated by cAMP signals, and PKA-dependent phosphorylation modulates voltage-activated channels. In this report, these two pathways were examined in GnRH-1 neurons as integrators of forskolin (FSK)-induced stimulation. The HCN3 isoform was detected in GnRH-1 neurons obtained from mouse nasal explants. ZD7288, a HCN channel blocker, significantly reduced the efficiency of FSK to stimulate GnRH-1 neurons, whereas blockade of PKA with Rp-adenosine-3′,5′-cyclic monophosphorothioate triethylammonium did not attenuate the FSK-induced stimulation. To ensure that disruption of HCN channels on GnRH-1 neurons was responsible for reduction of FSK stimulation, experiments were performed removing γ-aminobutyric acid (GABA), the major excitatory input to GnRH-1 neurons in nasal explants. Under these conditions, Rp-adenosine-3′,5′-cyclic monophosphorothioate triethylammonium, but not ZD7288, altered the FSK-induced response of GnRH-1 neurons. These studies indicate that PKA-dependent phosphorylation is involved in the FSK-induced stimulation of GnRH-1 neurons rather than HCN channels, and HCN channels integrate the FSK-induced stimulation on GABAergic neurons. In addition, blockade of HCN channels did not modify basal GnRH-1 neuronal activity when GABAergic input was intact or removed, negating a role for these channels in basal GABAergic or GnRH-1 neuronal activity.
IN ALL MAMMALS, the pulsatile release of GnRH-1 controls fertility (1). It is widely held that in vivo GnRH-1 release is caused by a coordinated increase in activity of GnRH-1 neurons (1). Data from different models demonstrate that GnRH-1 neurons exhibit intrinsic oscillatory properties. In vitro, isolated hypothalamic explants show pulsatile GnRH-1 release (2,3,4) as well as GT1 cells, an immortalized GnRH-1 cell line (5) and GnRH-1 neurons maintained in nasal explants (6,7,8). Consistent with GnRH-1 intrinsic rhythmicity, electrophysiological recordings, and calcium imaging of GT1 cells (9,10), GnRH-1 neurons in nasal explants (11,12,13) or GnRH-1 neurons in hypothalamic slices (14,15,16) reveal spontaneous electrical activity as well as intracellular calcium oscillations. However, to date, the mechanisms underlying the pulsatile release of GnRH-1 as well as their intrinsic rhythmicity remain unclear.
In reproductively mature animals, the pulsatile release of GnRH-1 can be regulated by external and internal cues (17). In vivo GnRH-1 neurons express a variety of neurotransmitter receptors (1) and receive many neuronal inputs shown to modulate the pattern of pulsatile release (1). Recently, it has been documented that neurotransmitters and hormones can alter GnRH-1 neuronal excitability (1) and/or the synchronicity among GnRH-1 neurons (13,18). Many neurotransmitters, acting through G protein-coupled receptors (GPCR), alter cellular behavior by changing intracellular levels of cAMP (19). Gs protein-coupled receptors increase cAMP level by activating adenylyl cyclase (AC), and Gi protein-coupled receptors decrease cAMP level by inhibiting AC and activating phosphodiesterases (PDE) (20). In vitro, a positive correlation between cAMP level and GnRH-1 release has been shown in hypothalamic explants (21,22,23) and GT1 cells (24). An understanding of the modulatory mechanisms of GnRH-1 neurons, i.e. stimulation or inhibition, might highlight a crucial component(s) of their intrinsic rhythmic behavior.
Cyclic nucleotide-activated ion channels are one putative pathway for integrating cAMP signals (25). These channels belong to two families: cyclic nucleotide-gated channels and hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels (26). The diterpene forskolin (FSK) is known to directly activate adenylyl cyclase and to raise cAMP in a wide variety of cell types (27) and as such is often used as a tool to investigate cellular target(s) able to integrate cAMP signals. Previous studies have shown that FSK was able to increase the pacemaking activity of GT1 cells (28). A recent study by our lab (29) showed that cyclic nucleotide-gated channels are not necessary for FSK-induced stimulation of GnRH-1 neurons or synchronized oscillatory behavior. Although initially identified in sinoatrial node cells (30), the current generated by HCN channels has now been found in a variety of cell types with spontaneous activity such as thalamic relay neurons (31) and superior colliculus-projecting neurons (32). The function of HCN channels has also been expanded from pacemaking to include the control of membrane resting potential and membrane overshooting (33). A recent study has shown immunoreactivity for HCN channels in GnRH-1 neurons in vivo (34). In adult mice, two different studies have shown responsiveness of GnRH-1 neurons elicited by either hyperpolarizing current (35) or voltage pulse (36). Thus, these channels may be important for regulating GnRH-1 neuronal activity. Alternatively, cAMP-dependent protein kinase (PKA) is one of the most common pathways activated by cAMP signals, and PKA-dependent phosphorylation is well known as a modulator of the properties of voltage-activated channels.
The goal of the present study was to investigate the role of HCN channels and PKA phosphorylation in basal and FSK-stimulated GnRH-1 neuronal activity. Our data indicate that HCN channels are not involved in the endogenous activity of GnRH-1 neurons and that the FSK-induced stimulation is transduced in GnRH-1 neurons through cAMP-dependent activation of PKA rather than HCN channels.
Materials and Methods
In vitro nasal explants
Nasal regions were cultured as previously described (37). Embryos were obtained from timed pregnant animals in accordance with NIH guidelines. Briefly, nasal pits of embryonic d-11.5 staged NIH Swiss mice were isolated under aseptic conditions and adhered onto coverslips by a plasma (Cocalico Biologicals, Reamstown, PA)/thrombin (Sigma Chemical Co., St. Louis, MO) clot. Nasal explants were maintained at 37 C in a humidified atmosphere with 5% CO2 in a defined serum-free medium (SFM) (37). On culture d 3, fresh medium containing fluorodeoxyuridine (8 × 10−5 m; Sigma) was given for 3 d to inhibit proliferation of dividing olfactory neurons and nonneuronal explant tissue. On culture d 6, and every 2 d afterward, the medium was changed with fresh SFM. Explants were used between 6–10 d in vitro (div) (Fig. 1A).
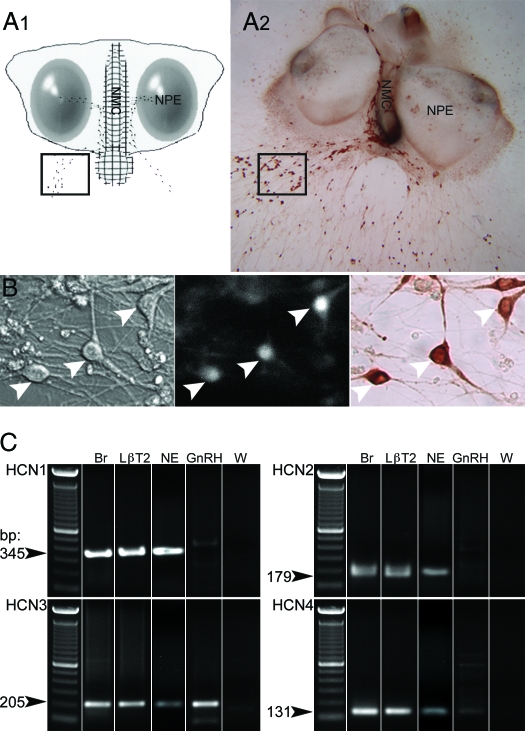
Figure 1.
Experimental conditions. Calcium imaging recordings were performed on cells maintained in mouse nasal explants for 6–10 div. A1, Schematic showing the structure of a nasal explant obtained from embryonic d-11.5 mouse and maintained 7 div, with nasal pit epithelium (NPE; ovals) and the nasal midline cartilage (NMC; meshed area), surrounded by mesenchyme. GnRH-1 neurons (dots) migrate from NPE and follow olfactory axons to the NMC and off the explant into the periphery. The boxed area delimits a typical field where cells were recorded. A2, A 9-div nasal explant immunostained for GnRH-1 (brown). B, Identified by their bipolar morphology (left panel) and loaded with a calcium-sensitive dye (middle panel), GnRH-1-like cells were recorded. The phenotype of the recorded cells was confirmed a posteriori by immunocytochemistry (right panel, brown). Arrows indicate same cells in all fields. C, Representative gel of PCR products from total nasal explant RT-PCR (NE), single-cell RT-PCR performed on GnRH-1 cells (GnRH) extracted from nasal explant using specific primers for HCN1, HCN2, HCN3, and HCN4 subunits. No bands were detected in GnRH-1 cells with HCN1- or HCN2-specific primers (n = 5). Thirty-five percent of the tested cells (n = 20) exhibited a band with HCN3-specific primers, and a weak band was observed in one of five cells with HCN4-specific primers. Adult brain (Br) and LβT2 cell line (LβT2) showed the expected band, whereas water (W) was negative.
PCR on nasal explants and single GnRH-1 cells
GnRH-1-like neurons, identified in vitro by their bipolar morphology, association with outgrowing axons, and location within the explant, were removed with pulled glass capillaries. cDNA were produced and PCR amplification was performed as previously described (38,39). cDNA were also produced using similar techniques from whole nasal explants.
Based on the technique used to generate the cDNA pools, -3′-untranslated region biased primers are necessary. Primers were designed with the 5′-primer being less than 300 bases from the polyA site and the -3′ primer close to, but not into, the polyadenylation region. All designed primers were screened using BLAST to ensure specificity of binding. For each reaction, 30.5 μl H2O, 5 μl 10× PCR buffer (Applied Biosystems, Foster City, CA), 4 μl 25 mm MgCl2 (Applied Biosystems), 5 μl dNTP mix (25 μl of each 100 mm dNTP, 900 μl H2O), 2 μl 6.25 μm forward primer, 2 μl 6.25 μm reverse primer, and 0.5 μl AmpliTaq Gold (Applied Biosystems) were added to 1 μl template cDNA. PCR was performed at 94 C for 10 min, 94 C for 30 sec, 55 or 65 C, depending on primers, for 30 sec, and 72 C for 2 min for 40 cycles, with a terminal elongation at 72 C for 10 min. Amplified products were run on a 1.5% agarose gel. Specific bands of the predicted size were observed in the control total brain lane, whereas no bands were seen in water. All cDNA were initially screened by PCR for GnRH-1 (assuring the GnRH-1 cell phenotype), III-tubulin, and L19 (two housekeeping genes, respectively microtubule and ribosomal; for primers sequences see Ref. 40). Only cells and explants positive for all three transcripts were used (39,40).
PCRs were performed on total nasal explant cDNA (n = 4) and single GnRH-1 cell cDNA (6–8 div, n = 20) for the predominant HCN isoform in adult GnRH-1 cells (34), HCN3 [accession no. NM_008227; forward primer 5′-AGT GAG AAT CCA CGT CTG-3′, reverse primer 5′-ACA TGA GGA TTC AGC CCC TG-3′; melting temperature (Tm), 52 C; size of product, 205 bp]. Screening for the other isoforms HCN1 (accession no. NM_010408; forward primer 5′-AGA AGC TGT GGC CTA CAT GC-3′, reverse primer 5′-AAC AAC CTA GAC GCA GAG G-3′; Tm, 58 C; size of product, 354 bp), HCN2 (accession no. NM_008226; forward primer 5′-TCA AGT GCA ATA CTC GGC CC-3′, reverse primer 5′-CGT CAG TAC ATT CAT GGC GA-3′; Tm, 58 C; size of product, 179 bp), and HCN4 (accession no. NM_001081192; forward primer 5′-TCC TCA GGT TCT TTG CCA CC-3′, reverse primer 5′-AGT TTG GAGCGT ACT GGC T-3′; Tm, 58 C; size of product, 131 bp) were performed on total nasal explant cDNA (n = 4) and single GnRH-1 cell cDNA (6–7 div, n = 5). Two immortalized cell lines were also evaluated, the GnRH-1 cell line GT-1 (41) and the pituitary cell line, LβT2 (42). Pituitary cDNA was run in parallel as a native tissue control as well as brain cDNA. A negative control (water lane) validated the experimental run. In a similar manner, whole nasal explants were screen for all four HCN isoforms.
In vitro calcium imaging
Calcium imaging has been previously described as a valid method for investigating in vitro GnRH-1 neuronal activity (29). Calcium Green-1 calcium imaging was performed as reported previously (13,29). Briefly, Calcium Green-1 AM (Molecular Probes, Eugene, OR) was diluted to 2.7 mm in 20% pluronic F-127/dimethylsulfoxide solution (Molecular Probes). This solution was diluted 1:200 with SFM to a final concentration of 13.5 μm. Nasal explants were incubated with this solution (20 min) and then washed twice with fresh SFM (10 min each). Explants were mounted into a perfusion chamber and were continuously perfused with medium (∼280 μl/min), using a gravity system (Warner Instruments, Hamden, CT) as inflow and a peristaltic pump as outflow. Calcium Green-1 was visualized using an inverted microscope (Nikon) through a ×20 fluorescence objective and a digital CCD camera (Retiga; Qimaging, Burnaby, Canada) connected to a Macintosh computer. Experiments were piloted by imaging software (IPLab Spectrum; Scanalytics Inc., Rockville, MD), controlling the shutter (Uniblitz; Vincent Associates, Rochester, NY) and the acquisition. Excitation wavelengths were provided through a medium-width excitation bandpass filter at 465–495 nm, and emission was monitored through a 40-nm bandpass centered on 535 nm. Pictures were acquired each second for short recordings (∼16 min) and every 5 sec for long recordings (∼83 min).
FSK, (−)-bicuculline chloride (BIC), H89, and enantiomers to p-adenosine-3′,5′-cyclic monophosphorothioate triethylammonium that exhibit inhibitory and stimulatory behavior, Rp-cAMPS and Sp-cAMPS, respectively, were obtained from Sigma. ZD7288 was purchased from Tocris (Bristol, UK). All stock solutions were stored at −20 C, and solutions were prepared before each experiment by diluting stock solutions (1/500 to 1/2000) into SFM (used for growing nasal explants) (37). Subdivision into three to four periods was done for experiments as indicated and subsequent analysis [i.e. for three periods: control period in SFM (5 min), treatment period (3 min), and washout period in SFM (5 min)]. All recordings were terminated by a 40 mm KCl stimulation to ensure the viability of the recorded cells.
Electrophysiological recording of HCN current
HCN current was evaluated in GnRH-1 neurons (6–7 div) in voltage-clamp mode, whole-cell configuration. Pipettes were back-filled with a 0.22 μm filtered solution containing (in mM) 140 KCl, 1.8 MgCl2, 0.3 GTP, 4 MgATP, 10 HEPES, and 10 EGTA (pH 7.3 with KOH). Extracellular solution was SFM (Na ∼124 mm) supplemented with KCl (20 mm final), barium (1 mm), 4-aminopyridine (1 mm), cadmium (1 mm), tetraethyl ammonium (6 mm), and tetrodotoxin (1 μm). Whole-cell data were 10 kHz filtered and digitized at 20 kHz. Electrodes were pulled with a resistance of about 3 mΩ, and seal resistances ranged from 1–3 GΩ. From a resting potential clamped at −40 mV, a test pulse was performed at −140 mV for 1.5 sec. LβT2 cells were used as a positive control to validate our experimental conditions.
Statistical analysis
Calcium imaging recordings were performed and intracellular calcium fluctuations on individually identified cells were analyzed a posteriori with IPLab software. Calcium Green-1 fluorescence intensity was plotted with Excel. MATLAB (MathWorks, Natick, MA) was used to specify calcium peaks. A calcium fluctuation was first identified when a value was greater than the five previous and five following points. Then, that calcium fluctuation had to be greater than the mean of the five previous and five next points plus a minimal value (which represented small fluctuations in baseline) to be considered as a calcium oscillation (peak). Calcium oscillation frequency was calculated as the ratio of the number of detected calcium peaks per time unit (minute).
For long-term recordings, calcium oscillations were detected in real time in all individual GnRH-1 neurons and binned into 30-sec bins. The GnRH-1 neuronal activity in the whole explant was evaluated by summing the number of calcium oscillations per 30-sec bin. A synchronized event was defined as a 30-sec period exhibiting calcium oscillations greater than the mean + 2 sd. The inter-event interval was calculated by subtracting (time of event i + 1) from (time of event i).
Statistical analysis was performed using paired t tests to identify a drug effect on the peak frequency among a pool of cells; inter-event intervals obtained during long experimental conditions were compared using an ANOVA test. A P value of 0.05 was chosen for significance. In Results, n and N represent the number of cells and explants recorded, respectively.
In vitro GnRH-1 immunocytochemistry
To confirm the phenotype of recorded cells, nasal explants were immunocytochemically stained for GnRH-1 as previously described (Fig. 1B) (37). Briefly, after calcium imaging recordings, explants were fixed (4% formaldehyde, 1 h), rinsed in PBS, and placed in cryoprotectant until staining. Explants were washed in PBS, blocked in 10% normal goat serum/0.3% Triton X-100 for 1 h, washed several times in PBS, and then incubated in anti-GnRH-1 antibody (rabbit, 1:3000, SW-1) (43) overnight at 4 C. The next day, explants were washed in PBS, incubated for 1 h in biotinylated secondary antibody (goat antirabbit, 1:500 in PBS/0.3% Triton X-100; Vector Laboratories, Inc., Burlingame, CA), washed in PBS, and processed for avidin-biotin-horseradish peroxidase/3′,3-diaminobenzidine cytochemistry.
Results
Expression of HCN3 subunit in GnRH-1 neurons
PCR examination of cDNAs from nasal explants and single GnRH-1 cells were performed for the four HCN isoforms. All four isoforms were present in the nasal explants (Fig. 1C, NE, n = 4). HCN3 transcript was detectable in 35% of the individual GnRH-1 neurons screened (6–10 div, n = 20 cells), whereas none of the cells tested were positive for HCN1 or HCN2, and a weak band for HCN4 was detected in only one GnRH-1 cell (6–7 div, n = 5 cells). As previously described (34), the immortalized GnRH-1 cell line, GT1, was found to be positive for all HCN isoforms. The immortalized pituitary cell line, LβT2, was also positive for all HCN isoforms, whereas pituitary tissue was positive for HCN2, HCN3, and HCN4, as found in the GH3 cell line (44). Thus, as in adult GnRH-1 neurons (34), HCN3 is the most abundant HCN channel present in embryonic GnRH-1 cells.
HCN channels and GnRH-1 neuronal activity
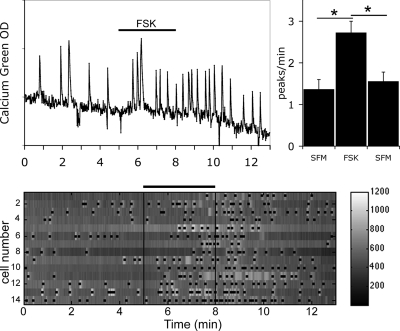
As reported previously (29), FSK was able to stimulate GnRH-1 neurons by increasing their excitability, i.e. the frequency of calcium oscillations, without significantly altering their amplitude (Fig. 2; 1 μm FSK; 2.73 ± 0.27 peaks/min in FSK vs. 1.37 ± 0.23 peaks/min in SFM; n = 32, N = 3) (29). This effect was reversible after a 3-min washout (Fig. 2) and prevented by the application of an inhibitor of adenylyl cyclase before FSK (29). To ensure that the stimulation induced by FSK at 1 μm was submaximal, a second 3-min stimulation was applied at 2 μm, after a washout period. The cells responded to, and were able to recover after, exposure to 2 μm FSK, and the response to 2 μm FSK was significantly greater than that observed after 1 μm FSK (1.65 ± 0.19 peaks/min in SFM, 2.13 ± 0.23 peaks/min in 1 μm FSK, 2.72 ± 0.23 peaks/min in 2 μm FSK; n = 25, N = 1; t test P < 0.05). These results verified the ability of cAMP to modulate GnRH-1 neuronal activity. A similar effect was induced by the inhibition of PDE by 3-isobutyl-1-methyl-xanthine (IBMX, 100 μm), confirming the ability of endogenous adenylyl cyclase to modulate GnRH-1 neuronal activity (29).
Figure 2.
FSK-stimulated GnRH-1 neuronal activity. FSK (1 μm), activator of adenylyl cyclase, induced an increase in calcium oscillations (left panel). Histogram (right panel) shows average peaks per minute (± sem) during the three experimental periods. A significant and reversible increase in calcium peaks in the presence of FSK occurred (*, P < 0.05, Student’s paired t test; N = 3, n = 32). The lower panel shows gray level changes in intracellular calcium in 14 cells simultaneously recorded during the experimental paradigm (SFM-FSK-SFM). Each row represents changes in intracellular calcium in a single cell, and dots mark significant calcium peaks. Lines indicate the time for drug applications as shown on the single-cell trace.
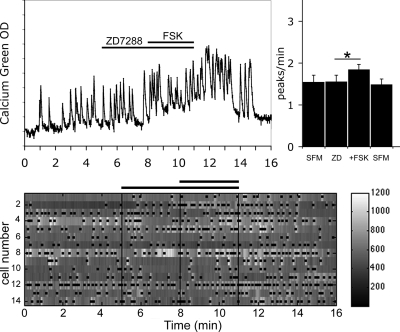
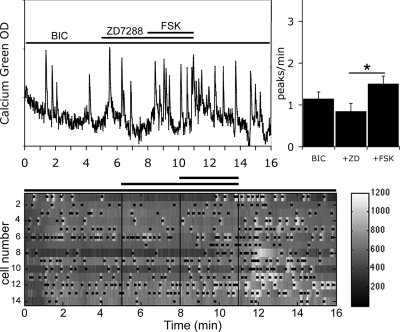
HCN channels are known as a cellular pathway integrating cyclic nucleotide signals, both cAMP and cGMP (cAMP > cGMP) (45). To determine whether HCN channels are involved in the FSK-induced stimulation of GnRH-1 neuronal activity, treatment was performed with ZD7288 (5 μm), a specific blocker of HCN channels (46), before the application of FSK. In the presence of ZD7288, an increase in the frequency of calcium oscillations in response to the application of FSK was still observed in GnRH-1 neurons (Fig. 3; 1.85 ± 0.12 peaks/min in ZD7288+FSK vs. 1.56 ± 0.15 peaks/min in ZD7288; n = 53, N = 2; paired t test P < 0.05) but was significantly attenuated in comparison with the response induced by FSK alone (150 ± 51% increase in the frequency of peaks in FSK; n = 26, N = 3; vs. 56 ± 16% increase in the frequency of peaks in ZD7288+FSK; n = 52, N = 2; t test P < 0.05). Because all four HCN isoforms were detected in nasal explants, it was unclear whether the blockade of HCN channels, responsible for the decreased response, occurred directly on GnRH-1 neurons or indirectly on another cell type able to transduce the FSK stimulation to GnRH-1 neurons.
Figure 3.
ZD7288 partially abolished FSK-induced stimulation. Recording is from a single cell exposed to ZD7288 (5 μm), inhibitor of HCN channels, followed by FSK (left panel). Treatment with ZD7299 partially prevented the stimulation of GnRH-1 neuronal activity by FSK without altering the endogenous activity (right panel; N = 2, n = 53). The number of peaks per minute is not modified by ZD7288 alone, and in the presence of ZD7288, the number of peaks per minute was still increased by FSK (*, P < 0.05, Student’s paired t test), however the extent of the FSK response was attenuated (compare Figs. 2 and 3). The lower panel shows changes in intracellular calcium in 14 cells simultaneously recorded during the experimental paradigm (SFM-ZD7288-FSK-SFM). Each row represents changes in intracellular calcium in a single cell, and dots mark significant calcium peaks. Lines indicate the time for drug applications as shown on the single-cell trace.
HCN channels and γ-aminobutyric acid (GABA)A inputs deprived GnRH-1 neuronal activity
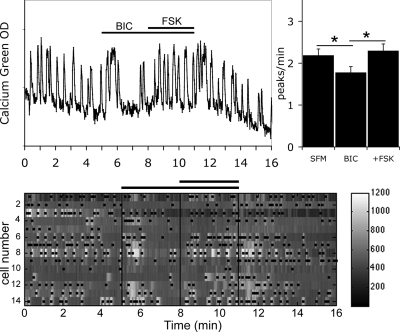
A subpopulation of GABAergic neurons is known to be present in nasal explants and regulates GnRH-1 neuronal activity via an excitatory input (11,13,47). Thus, the neuronal activity recorded from GnRH-1 neurons is an integration of at least two neuronal activities, i.e. GABA-dependent activation of GnRH-1 neurons in addition to their own activation (GABA independent). Although both GABAA and GABAB receptors have been shown to be important in GnRH-1 neuronal activity in older nasal explants, transduction via GABAA receptors predominates in GnRH-1 neurons at 1 wk (13). Therefore, explants were treated with BIC (20 μm; GABAA receptor antagonist) to evaluate GABA-independent GnRH-1 neuronal activity (1.78 ± 0.14 peaks/min in BIC vs. 2.19 ± 0.15 peaks/min in SFM; n = 59, N = 2, t test P < 0.05). Previously, we showed that the increase in calcium oscillations induced in GnRH-1 neurons by FSK persisted (Fig. 4; 2.30 ± 0.16 peaks/min in BIC+FSK vs. 1.78 ± 0.14 peaks/min in BIC; n = 59, N = 2) (29) but was reduced after removal of endogenous GABAergic activity (150 ± 51% increase in the frequency of peaks in FSK; n = 26, N = 3; vs. 64 ± 16% increase in the frequency of peaks in BIC+FSK; n = 59, N = 2; t test P < 0.05). These results suggest, respectively, 1) the ability of GnRH-1 neurons to directly integrate cAMP signals and 2) a potentiation of FSK-induced stimulation of GnRH-1 neurons by GABAergic neurons.
Figure 4.
BIC did not prevent FSK-induced stimulation. Recording from a single cell shows that FSK-induced stimulation occurred after removal of endogenous GABAergic inputs (GABAA antagonist BIC; 20 μm, left panel). The number of peaks per minute is significantly decreased by BIC (N = 2, n = 59) and then increased by FSK (*, P < 0.05, Student’s paired t test), showing that GnRH-1 neurons were able to directly integrate FSK-induced signals. The lower panel shows changes in intracellular calcium in 14 cells simultaneously recorded during the experimental paradigm (SFM-BIC-FSK-SFM). Each row represents changes in intracellular calcium in a single cell, and dots mark significant calcium peaks. Lines indicate the time for drug applications as shown on the single-cell trace.
To determine whether the reduction in the FSK-stimulation observed in the presence of ZD7288 occurred at the level of GABA neurons and/or GnRH-1 neurons, the involvement of HCN channels in FSK-stimulated GnRH-1 neuronal activity was tested after removal of GABAergic inputs. FSK-induced stimulation of GnRH-1 neuronal activity was still observed in the presence of BIC+ZD7288 (Fig. 5; 1.51 ± 0.18 peaks/min in BIC+ZD7288+FSK vs. 0.85 ± 0.19 peaks/min in BIC+ZD7288; n = 28, N = 2; paired t test P < 0.05), and the magnitude of the FSK-induced stimulation was not significantly reduced in comparison with the response induced by BIC+FSK alone (69 ± 19% increase in the frequency of peaks in BIC+ZD7288+FSK; n = 28, N = 2; vs. 64 ± 16% increase in the frequency of peaks in BIC+FSK; n = 59, N = 2). Thus, an effect of ZD7288 was observed only when the GABAergic pathway was intact. These results indicate that HCN channels are involved in the GABAergic driven response to FSK rather than directly in the response of GnRH-1 neurons to FSK.
Figure 5.
ZD72888 did not block FSK-induced stimulation after blockade of endogenous GABAergic signals. Recording from a single cell shows that in presence of BIC, ZD7288 failed to prevent the FSK-induced stimulation (left panel). The number of peaks per minute was not modified by BIC+ZD in comparison with BIC alone (N = 2, n = 28). In the presence of BIC+ZD, the number of peaks per minute increased by FSK (*, P < 0.05, Student’s paired t test) showing that ZD7288 exerted its blockade on GABAergic neurons rather than GnRH-1 neurons. The lower panel shows changes in intracellular calcium in 14 cells simultaneously recorded during the experimental paradigm (BIC-ZD7288-FSK-BIC). Each row represents changes in intracellular calcium in a single cell, and dots mark significant calcium peaks. Lines indicate the time for drug applications as shown on the single-cell trace.
To determine whether the lack of a ZD728 effect was due to the concentration (5 μm), the experiment was performed with a 10-fold higher concentration of ZD7288 (50 μm). In the presence of BIC+ZD7288 (50 μm), FSK was still able to stimulate GnRH-1 neuronal activity [1.87 ± 0.14 peaks/min in BIC+ZD7288 (50 μm) +FSK vs. 1.60 ± 0.15 peaks/min in BIC+ZD7288 (50 μm); n = 43, N = 2; paired t test P < 0.05] with the extent of the FSK-induced stimulation similar to that seen with the lower concentration of ZD7288 (approximately a 51 ± 20% increase in the frequency of the peaks).
HCN channels and basal GnRH-1 neuronal activity
Calcium imaging experiments in nasal explants derived from a variety of species have shown that a population of the recorded GnRH-1 neurons exhibit synchronized calcium oscillations (12,13). Initially, HCN channels were identified as critical to pacemaking activity (30) and were proposed as a possible mechanism of synchronized GnRH-1 neuronal activity (48). Therefore, we examined whether HCN channels were involved in basal and/or synchronized GnRH-1 neuronal activity.
Treatment with ZD7288 alone did not alter GABA-dependent GnRH-1 activity (Fig. 3; 1.56 ± 0.15 peaks/min in ZD7288 vs. 1.55 ± 0.16 peaks/min in SFM; n = 53, N = 2) or GABA-independent GnRH-1 neuronal activity (Fig. 5; 0.85 ± 0.19 peaks/min in BIC+ZD7288 vs. 1.15 ± 0.16 peaks/min in BIC; n = 28, N = 2). Taken together, these results show that HCN channels are not involved in the neuronal activity of GnRH-1 neurons driven by GABAergic inputs or endogenous GnRH-1 neuronal activity and suggest a regulatory role of HCN channels in the neuronal activity rather than a crucial component of the rhythmicity in developing GnRH-1 neurons.
Previous studies on nasal explants have shown that GnRH-1 neurons exhibit synchronized calcium oscillations that occur with the same periodicity as GnRH-1 release (12,13). This correlation led us to further evaluate the role of HCN channels in the oscillatory activity of GnRH-1 neurons using long-term recordings performed in the presence of ZD7288. To ensure the stability of the experimental conditions over approximately 83 min, the recording period was divided into four equal periods (A–D). GnRH-1 neuronal activity was similar over the entire recording period when explants were maintained in SFM (period A, 0.62 ± 0.05 peaks/min; period B, 0.68 ± 0.05 peaks/min; period C, 0.67 ± 0.05 peaks/min; period D, 0.63 ± 0.04 peaks/min; n = 89, N = 5; paired t test for pairs A-B, B-C, and C-D) or during a long-term exposure to ZD7288 (period A, 0.63 ± 0.05 peaks/min; period B, 0.63 ± 0.04 peaks/min; period C, 0.65 ± 0.04 peaks/min; period D, 0.65 ± 0.04 peaks/min; n = 109, N = 5; paired t test for pairs A-B, B-C, and C-D). Synchronicity of calcium oscillations was then evaluated. In the presence of ZD7288, the oscillatory electrical activity exhibited a similar periodicity as that observed for GnRH-1 neurons maintained in SFM (16.00 ± 3.46 min, N = 5, in ZD7288 vs. 16.23 ± 2.48 min, N = 5, in SFM). These data demonstrate that HCN channels are not necessary for synchronized GnRH-1 neuronal activity.
HCN channels current
To investigate whether HCN channels could generate a hyperpolarization-gated current, whole-cell patch clamp was performed on GnRH-1 neurons (n = 6, N = 2). None of the recorded cells exhibited any inward current in recording conditions in which a current, sensitive to ZD7288 50 μm, was recorded in LβT2 cells (data not shown, similar to GH3) (44).
PKA and FSK-induced stimulation of GnRH-1 neurons
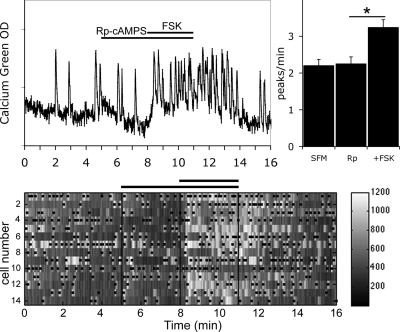
In addition to cyclic nucleotide-gated channels and HCN channels, PKA is another pathway able to integrate cAMP signals via PKA-dependent phosphorylation. To determine whether PKA activation is involved in FSK-induced stimulation of GnRH-1 neuronal activity, a pretreatment was performed before the application of FSK with Rp-cAMPS, a specific membrane-permeable inhibitor of activation of PKA by cAMP, at the concentration (10 μm) used previously (49). In the presence of Rp-cAMPS, the response of GnRH-1 neurons to FSK persisted (Fig. 6; 3.25 ± 0.20 peaks/min in Rp-cAMPS+FSK vs. 2.26 ± 0.18 peaks/min in Rp-cAMPS; n = 62, N = 3) and although the increase in calcium oscillations was attenuated, it was not significantly modified in comparison with the response induced by FSK alone (150 ± 51% increase in the frequency of peaks in FSK; n = 26, N = 3; vs. 88 ± 21% increase in the frequency of peaks in Rp-cAMPS+FSK; n = 61, N = 3). To ensure that the blockade of PKA was efficient, experiments were performed with another blocker of PKA, H89, at a concentration (1 μm) used previously (50). In the presence of H89, the response of GnRH-1 neurons to FSK persisted (2.25 ± 0.17 peaks/min in H89+FSK vs. 1.98 ± 0.16 peaks/min in H89; n = 54, N = 4), consistent with the Rp-cAMPS results. These data show that the PKA-dependent pathway is not the main pathway involved in the FSK-induced stimulation of GnRH-1 neurons when they are driven by GABAergic input.
Figure 6.
Rp-cAMPS did not prevent FSK-induced stimulation. Recording from a single cell shows that Rp-cAMPS (10 μm), inhibitor of PKA, did not alter endogenous activity or prevent stimulation of neuronal activity by FSK (left panel). The number of peaks per minute was not modified by Rp alone (N = 3, n = 62), and in the presence of Rp, the number of peaks per minute were still increased by FSK (*, P < 0.05, Student’s paired t test). The lower panel shows changes in intracellular calcium in 14 cells recorded simultaneously during the experimental paradigm (SFM-RpcAMPS-FSK-SFM). Each row represents changes in intracellular calcium in a single cell, and dots mark significant calcium peaks. Lines indicate the time for drug applications as shown on the single-cell trace.
PKA and FSK-induced stimulation of GABAA-deprived GnRH-1 neurons
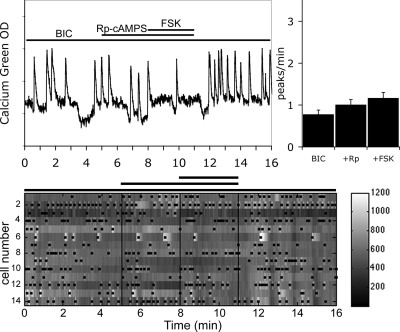
Next, the GABA-independent FSK-induced stimulation of GnRH-1 neurons, insensitive to the HCN channels blocker, was investigated after the application of Rp-cAMPS. In the presence of BIC, the FSK-induced response of GnRH-1 neuronal activity was inhibited by pretreatment with Rp-cAMPS (Fig. 7; 1.17 ± 0.13 peaks/min in BIC+Rp-cAMPS+FSK vs. 1.01 ± 0.12 peaks/min in BIC+Rp-cAMPS; n = 57, N = 3) as well as the magnitude of the change (30 ± 18% increase in the frequency of the peaks in BIC+Rp-cAMPS+FSK, n = 48, N = 3; vs. 88 ± 21% increase in the frequency of the peaks in Rp-cAMPS+FSK, n = 60, N = 3; t test P < 0.05). These results suggest that a PKA-dependent pathway is involved in the GABA-independent FSK-induced stimulation and that GnRH-1 neurons are able to integrate cAMP signals via PKA-dependent phosphorylation rather than cyclic nucleotide-activated ion channels.
Figure 7.
Rp-cAMPS abolished FSK-induced stimulation after blockade of endogenous GABAergic signals. Recording from a single cell shows that in presence of BIC, Rp-cAMPS prevented the FSK-induced stimulation (left panel). The number of peaks per minute was not modified by BIC+Rp in comparison with BIC alone (N = 3, n = 57). However, in the presence of BIC+Rp, the number of peaks per minute did not increase by FSK, revealing the involvement of a PKA-dependent pathway in the integration of FSK-induced signals in GnRH-1 neurons. The lower panel shows changes in intracellular calcium in 14 cells simultaneously recorded during the experimental paradigm (BIC-RpcAMPS-FSK-BIC). Each row represents changes in intracellular calcium in a single cell, and dots mark significant calcium peaks. Lines indicate the time for drug applications as shown on the single-cell trace.
Stimulation of PKA in GABAA-deprived GnRH-1 neurons
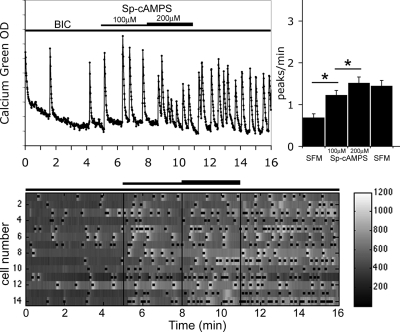
To ensure that GnRH-1 neurons can be directly modulated by the activation of cAMP-dependent protein kinases, experiments were performed with Sp-cAMPS (100 and 200 μm), a potent and specific activator of PKA. A significant increase in GnRH-1 neuronal activity was observed (Fig. 8; 0.69 ± 0.09 peaks/min in BIC vs. 1.23 ± 0.11 peaks/min in 100 μm Sp-cAMPS, 1.52 ± 0.14 peaks/min in 200 μm Sp-cAMPS; n = 54, N = 3; t test P < 0.05). Note in Fig. 8 that the Sp-cAMPS-induced stimulation persisted after the 3-min washout due to its resistance to cyclic nucleotide PDE (51).
Figure 8.
Activation of PKA increased GnRH-1 neuronal activity. Recording from a single cell shows that Sp-cAMPS stimulates calcium oscillations (left panel). The number of peaks per minute was modified by Sp-cAMPS in comparison with SFM (N = 2, n = 19; *, P < 0.05, Student’s paired t test). The lower panel shows changes in intracellular calcium in 14 cells simultaneously recorded during the experimental paradigm (BIC-SpcAMPs-SFM). Each row represents changes in intracellular calcium in a single cell, and dots mark significant calcium peaks. Lines indicate the time for drug application as shown on the single-cell trace.
PKA and basal GnRH-1 neuronal activity
Next, PKA-dependent phosphorylation was examined in basal and synchronized GnRH-1 neuronal activity. Preventing PKA activation by endogenously synthesized cAMP did not alter GnRH-1 neuronal activity when GABAergic inputs were intact (Fig. 6; 2.26 ± 0.18 peaks/min in Rp-cAMPS vs. 2.21 ± 0.16 peaks/min in SFM; n = 62, N = 3; paired t test P > 0.05; 1.98 ± 0.16 peaks/min in H89 vs. 1.82 ± 0.15 peaks/min in SFM; n = 54, N = 4; paired t test P > 0.05) or suppressed by BIC (Fig. 7; 1.01 ± 0.12 peaks/min in BIC+Rp-cAMPS vs. 0.78 ± 0.10 peaks/min in BIC; n = 57, N = 3; paired t test P > 0.05). These data are consistent with previous data showing no changes in GnRH-1 neuronal activity after blockade of adenylyl cyclase (29) and suggested that PKA-dependent phosphorylation is a modulatory mechanism rather than a component of an intrinsic oscillatory mechanism.
Long-term recordings were then performed in the presence of Rp-cAMPS to determine the role of PKA in the oscillatory activity within the GnRH-1 neuronal population. Interestingly, during a long exposure to Rp-cAMPS, the GnRH-1 neuronal activity started to slow during the fourth period of the recording (after ∼60 min; period A, 0.73 ± 0.04 peaks/min; period B, 0.76 ± 0.04 peaks/min; period C, 0.78 ± 0.04 peaks/min; period D, 0.68 ± 0.04 peaks/min; n = 145, N = 5; paired t test for pairs A-B and B-C and P < 0.05 for pair C-D), suggesting a long-term regulation of the rhythm by PKA. However, in the presence of Rp-cAMPS, the oscillatory electrical activity was still detected and exhibited a similar periodicity to cells recorded in SFM (18.31 ± 2.91 min, N = 5, in Rp-cAMPS vs. 16.23 ± 2.48 min, N = 5, in SFM). These data demonstrate that PKA is not necessary for synchronized calcium oscillations detected in developing GnRH-1 neurons.
Discussion
The present study investigated HCN channels and PKA as potential integrators of cAMP signals in GnRH-1 neurons. Application of the HCN channel blocker ZD7288 before FSK significantly decreased the FSK-induced stimulation of GnRH-1 neuronal activity. However, after removal of GABAA signals to GnRH-1 neurons, ZD7288 treatment no longer altered the FSK-induced stimulation of GnRH-1 neuronal activity. Together, these data suggest that the role of HCN channels in the integration of cAMP signals is at the level of GABAergic neurons rather than GnRH-1 neurons. In addition, under basal conditions, with intact GABAergic input, blockade of HCN channels did not alter GnRH-1 neuronal activity, removing HCN channel-dependent mechanisms from spontaneous activity in GABAergic neurons. Moreover, when deprived of GABAergic input, HCN channel blockade did not alter the residual endogenous GnRH-1 neuronal activity, removing HCN channel-dependent mechanisms from spontaneous activity in GnRH-1 neurons. Analysis of PKA pathways showed that when GABAergic inputs were removed, blockade of PKA significantly reduced extent of the FSK-induced stimulation of GnRH-1 neuronal activity (Fig. 9). These results indicate that in contrast to GABAergic neurons, PKA is involved in the cAMP-activated pathway leading to the direct stimulation of GnRH-1 neurons.
Figure 9.
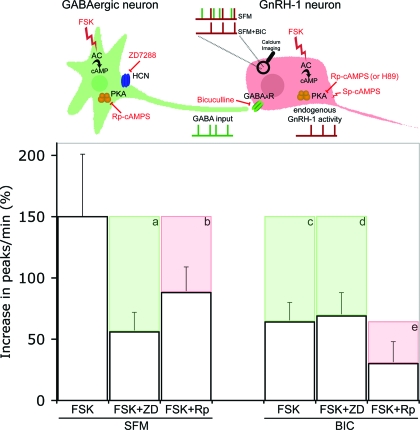
Summarized data. The upper panel represents the cellular partners evaluated in this study: GnRH-1 neurons (red) with their GABAergic input (green). Independent endogenous electrical activity of GnRH-1 and GABAergic neurons are represented by, respectively, red and green ticked lines under cells. The resulting intracellular calcium oscillations are recorded using calcium imaging, symbolized by a magnifying glass on the GnRH-1 cell body, and are represented by ticked lines above the GnRH-1 cell. Two experimental conditions are represented: SFM, which allows one to record the summation of the two cells’ endogenous activity (green/red), and SFM+BIC, which allows one to record the endogenous GnRH-1 neuronal activity (red). Pharmacological actions are symbolized in red. FSK stimulates AC, ZD7288 (ZD) blocks HCN channels, BIC antagonizes GABAA receptors, Rp-cAMPS (Rp) and H89 block PKA, and Sp-cAMPS stimulates PKA. Histograms show the percent increase in the number of peaks per minute in response to FSK under different experimental conditions. Pharmacological inhibitions are represented by shaded boxes showing loss of response (a–e). When GnRH-1 neurons are driven by GABAergic input (SFM), ZD inhibits the FSK-induced stimulation (a), whereas Rp is not as effective (b). However, in the presence of BIC, ZD fails to block the FSK-induced stimulation (d) in contrast with Rp (e). The inhibition induced by BIC alone (c) reflects the GABAergic contribution to the FSK-induced stimulation (c, green). Note that the inhibition of the FSK response induced by ZD in SFM (a) is similar to that detected after removal of GABAergic input alone (c) and in the presence of BIC+ZD (d). This suggests that in SFM, the inhibition exerted by ZD occurs on HCN channels localized to GABAergic neurons (a, green). The inhibition induced by Rp in BIC (e) can be directly attributed to a PKA-dependent pathway in GnRH-1 neurons (e, red) and likely corresponds to the inhibition observed in SFM (b, red), even though the PKA pathway cannot be totally excluded in GABAergic neurons.
In reproductively mature animals, the pulsatile release of GnRH-1 can be modulated by multiple factors such as breeding season, stress, or metabolic balance (52). Consistent with the ability of GnRH-1 neurons to integrate different signals that will lead to successful reproduction, GnRH-1 neurons express a variety of neurotransmitter receptors (53). Membrane receptors can be subdivided into two families: ionotropic receptors and metabotropic receptors, so-called GPCR. Ionotropic receptors represent an important family with members such as GABAA receptors and nicotinic acetylcholine receptors; nevertheless GPCR are the largest group of membrane receptors transducing about 80% of hormone or neurotransmitter signals (20). Intracellular cAMP level can be modulated by nearly 70% of GPCR (19). In vitro, a positive correlation between cAMP level and GnRH-1 release has been shown in hypothalamic explants (21,22,23) and GT1 cells (54). Increases in intracellular cAMP level can be transduced into changes in electrical activity through HCN channels (26). HCN channels have been immunocytochemically localized in vivo on GnRH-1 neurons in rat (34), and they have been proposed as a possible mechanism leading to GnRH-1 rhythmicity (48). In addition, Sim et al. (35) and Zhang et al. (36) have shown responses to hyperpolarizing protocols in GnRH-1 neurons from the preoptic area of adult mice.
Primary GnRH-1 neurons maintained in nasal explants have proven to be a useful model to investigate both intrinsic and extrinsic factors affecting neuronal activity within single GnRH-1 cells as well as population dynamics, using calcium imaging to evaluate GnRH-1 electrical activity (29). As shown here and in a previous study (29), stimulation of adenylyl cyclase by FSK (27) induced an increase in calcium oscillations in GnRH-1 neurons. Similar findings were obtained after inhibition of PDE by IBMX (55). Although in pituitary cells, FSK and IBMX can lead to increases in both intracellular cAMP and cGMP levels (50), it is clear that cAMP is the effector of FSK-induced stimulation in GnRH-1 neurons (29). Activation of GABAA receptors is a major developmental excitatory signal (13,47), but removal of the GABA input to GnRH-1 neurons only partially inhibited the FSK-induced (or IBMX-induced) response (29), indicating that GnRH-1 neurons can integrate cAMP signals directly. The pathways underlying this response were investigated, and a summary of the results and schematic of emerging characteristics of GnRH-1 neurons is shown in Fig. 9.
When GnRH-1 neurons are driven by endogenous GABAergic input, blockade of HCN channels significantly decreased the FSK-induced stimulation of GnRH-1 neuronal activity. However, after removal of GABAA signals, blockade of HCN channels no longer altered the FSK-induced stimulation of GnRH-1 neuronal activity. In addition, when deprived of GABAergic input, HCN channel blockade did not change the residual endogenous GnRH-1 neuronal activity. These data remove HCN channel-dependent mechanisms from both cAMP-induced and spontaneous activity in developing GnRH-1 neurons. These observations are consistent with a study showing that small afterdepolarizing potentials recorded in adult GnRH-1 neurons were insensitive to HCN channel blockers (56). In fact, we found only the transcript for the HCN3 subunit in GnRH-1 neurons, and it has been reported that HCN3 subunit alone is unable to form functional homomultimers (57). Furthermore, in successful conditions of overexpression, the current generated by HCN3 subunit was inhibited rather than activated by cyclic nucleotides (58). In adult rats, 43% of GnRH-1 neurons were immunopositive for HCN2, whereas 90% were positive for HCN3 (34). Although subunit expression was not examined, Sim et al. (35) and Zhang et al. (36) described a response to hyperpolarization in about 50% of the recorded cells, which is consistent with HCN2 detection in rats. Therefore, HCN2 may be developmentally regulated postnatally and responsible for the observed current in adults.
Altogether, these observations suggest that the role of HCN channels in the integration of cAMP signals is at the level of GABAergic neurons rather than GnRH-1 neurons. This observation is consistent with recordings of HCN channel-generated current in interneurons that provide a tonic input to neurons (59,60) and the involvement of HCN channels in GABA release (61,62). However, under basal conditions, with intact GABAergic input, blockade of HCN channels did not alter GnRH-1 neuronal activity, removing HCN channel-dependent mechanisms from spontaneous activity in GABAergic neurons.
The studies reported in this paper, together with an earlier study (29), indicate that neither cyclic nucleotide-gated channels nor HCN channels are necessary for the FSK-induced stimulation of GnRH-1 neurons or synchronized oscillatory behavior. Thus, PKA remained as a pathway to integrate cAMP signals in GnRH-1 neurons. Our experiments showed that when GABAergic input was intact, blockade of PKA (with Rp-cAMPS) did not significantly reduce the FSK-induced stimulation of GnRH-1 neuronal activity (Fig. 9). However, after blockade with BIC, treatment with Rp-cAMPS significantly altered the FSK-induced stimulation of GnRH-1 neurons. These results indicate that in contrast to GABAergic neurons, PKA is involved in the cAMP-activated pathway leading to the direct stimulation of GnRH-1 neurons. These data fit with the delay in the washout of the FSK-induced stimulation (Fig. 2). A recent study showed that the long-lasting effect of FSK was directly linked to the activity of phosphatases and their ability to revert PKA phosphorylation (63). These results are in contrast with a previous study in GT1 cells (49), which favors a membrane integration of cAMP signals and shows that Rp-cAMPS did not prevent stimulation induced by Sp-cAMPS (a membrane-permeable activator of PKA). In addition, a negative feedback of PKA on basal GnRH-1 release was reported in GT1 cells (24,64). The present work did not observe such a feedback on GnRH-1 neuronal activity, either when driven by GABAergic inputs or when GABA independent. The immortalized cell line GT1 is derived from a tumor at the rostral boundary of the optic chiasm (41) and might retain some properties different from native cells (65). Moreover, endogenous basal stimulation of GPCR, known to alter FSK response, cannot be completely excluded in nasal explants because other transmitter phenotypes besides GABA may be present, i.e. activation of purinergic receptors (66), catecholaminergic receptors (67), and cholecystokinin receptors (68).
The experiments in the current report indicate that GnRH-1 neurons respond to FSK by modulating their neuronal activity through PKA-dependent phosphorylation. It is well known that phosphorylation can modify the properties of voltage-gated ion channels such as sodium channels (69), potassium channels (70), and calcium channels (71), all found in GnRH-1 neurons (11,35,72) and all able to contribute to membrane excitability. The PKA pathway is the most versatile pathway for integrating extracellular signals. The ability of this pathway to target proteins specifically has been widely studied. Changes in cAMP concentration are restricted to microdomains regulated in duration, amplitude, and extension by AC and PDE (25). In addition, a family of A-kinase anchoring proteins (AKAP) has been identified that bind PKA in the vicinity of its effector (in specific subcellular compartments), providing spatial and temporal specificity to cAMP signals (25). AKAP have been shown to be associated with channels on plasma membrane (potassium, calcium, and sodium channels) (25). Thus, PKA through association with AKAP may modulate the channels capable of integrating cAMP signals into GnRH-1 neuronal activity. In addition to fast membrane pathways, our data from long-term exposure to PKA blockers reveal a significant alteration in the spontaneous activity of GnRH-1 neurons that might result from PKA-dependent pathways mediating gene regulation. At least two pathways have been shown in PKA-dependent gene modulation: one involving cAMP-responsive element-binding protein (CREB) (73) and another including fractions of MAPK (p44/p42-MAPK) (73).
Although, the mechanisms underlying GnRH-1 spontaneous activity and rhythmicity remain to be determined, the present work excludes HCN channels from both basal and stimulated GABA-independent GnRH-1 neuronal activity and underlies the importance of the activation of PKA in the short- and a long-term modulation of GnRH-1 neurons.
Acknowledgments
We appreciate the help of Drs. Brett Shoelson (Mathworks) with MATLAB and Pascal Froment (Institut National de la Santé et de la Recherche Médicale Unité 418/Institut National de la Recherche Agronomique Unité Agronomique 953) for his comments. We thank the Newcomb Fellowship Program for providing a student intern, Cina Karodeh, who helped in this work.
Footnotes
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Disclosure Statement: The authors, S.C. and S.W., have nothing to declare.
First Published Online March 27, 2008
Abbreviations: AC, Adenylyl cyclase; AKAP, A-kinase anchoring protein; BIC, (−)-bicuculline chloride; div, days in vitro; FSK, forskolin; GABA, γ-aminobutyric acid; GPCR, G protein-coupled receptor; HCN, hyperpolarization-activated cyclic nucleotide-modulated; IBMX, 3-isobutyl-1-methyl-xanthine; PDE, phosphodiesterase; PKA, cAMP-dependent protein kinase; Rp-cAMPS, Rp-adenosine-3′,5′-cyclic monophosphorothioate triethylammonium; SFM, serum-free medium; Tm, melting temperature.
References
- Herbison AE 2006 Physiology of the gonadotropin-releasing hormone neuronal network. In: Knobil E, Neill J, eds. The physiology of reproduction. 3rd ed. New York: Academic Press; 1415–1482 [Google Scholar]
- Ramirez VD, Gallardo E, Hartter D 1980 Factors altering the secretion of LHRH from superfused fragments of rat hypothalamus. J Endocrinol Invest 3:29–37 [DOI] [PubMed] [Google Scholar]
- Bourguignon JP, Franchimont P 1984 Puberty-related increase in episodic LHRH release from rat hypothalamus in vitro. Endocrinology 114:1941–1943 [DOI] [PubMed] [Google Scholar]
- Rasmussen DD 1993 Episodic gonadotropin-releasing hormone release from the rat isolated median eminence in vitro. Neuroendocrinology 58:511–518 [DOI] [PubMed] [Google Scholar]
- Martinez de la Escalera G, Choi AL, Weiner RI 1992 Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci USA 89:1852–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Daikoku S, Shinohara K, Kimura F 2000 Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71:138–144 [DOI] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M 2000 Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J Reprod Fertil 120:391–396 [PubMed] [Google Scholar]
- Bosma MM 1993 Ion channel properties and episodic activity in isolated immortalized gonadotropin-releasing hormone (GnRH) neurons. J Membr Biol 136:85–96 [DOI] [PubMed] [Google Scholar]
- Hales TG, Sanderson MJ, Charles AC 1994 GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Neuroendocrinology 59:297–308 [DOI] [PubMed] [Google Scholar]
- Kusano K, Fueshko S, Gainer H, Wray S 1995 Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci USA 92:3918–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L 1999 Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Jr JP, Shang E, Wray S 2002 In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci 22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH 1999 GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM 2000 Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology 141:3731–3736 [DOI] [PubMed] [Google Scholar]
- Jasoni CL, Todman MG, Strumia MM, Herbison AE 2007 Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci 27:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL 2004 The neural basis of puberty and adolescence. Nat Neurosci 7:1040–1047 [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S 2004 Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 24:6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Li X, He J, Lu J, Diwu Z 2006 Real-time and high throughput monitoring of cAMP in live cells using a fluorescent membrane potential-sensitive dye. Assay Drug Dev Technol 4:461–471 [DOI] [PubMed] [Google Scholar]
- Kristiansen K 2004 Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther 103:21–80 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Katz KH, Costa ME 1985 Stimulation of cyclic adenosine-3′,5′-monophosphate production enhances hypothalamic luteinizing hormone-releasing hormone release without increasing prostaglandin E2 synthesis: studies in prepubertal female rats. Endocrinology 117:1175–1178 [DOI] [PubMed] [Google Scholar]
- Hartter DE, Ramirez VD 1985 Responsiveness of immature versus adult male rat hypothalami to dibutyryl cyclic AMP- and forskolin-induced LHRH release in vitro. Neuroendocrinology 40:476–482 [DOI] [PubMed] [Google Scholar]
- Lee BJ, Kim K, Cho WK 1990 Activation of intracellular pathways with forskolin and phorbol ester increases LHRH mRNA level in the rat hypothalamus superfused in vitro. Brain Res Mol Brain Res 8:185–191 [DOI] [PubMed] [Google Scholar]
- Weiner RI, Charles A 2001 Regulation of gonadotropin-releasing hormone release by cyclic AMP signalling pathways. Growth Horm IGF Res 11(Suppl A):S9–S15 [DOI] [PubMed] [Google Scholar]
- Tasken K, Aandahl EM 2004 Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84:137–167 [DOI] [PubMed] [Google Scholar]
- Craven KB, Zagotta WN 2006 CNG and HCN channels: two peas, one pod. Annu Rev Physiol 68:375–401 [DOI] [PubMed] [Google Scholar]
- Insel PA, Ostrom RS 2003 Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau AP, Van Goor F, Stojilkovic SS, Sherman A 2000 Modeling of membrane excitability in gonadotropin-releasing hormone-secreting hypothalamic neurons regulated by Ca2+-mobilizing and adenylyl cyclase-coupled receptors. J Neurosci 20:9290–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Wray S 2008 Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels. Endocrinology 149:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D 1986 Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature 324:470–473 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC 1990 Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol 431:291–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JS, Nerbonne JM 1993 Hyperpolarization-activated currents in isolated superior colliculus-projecting neurons from rat visual cortex. J Physiol 462:393–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC 1996 Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58:299–327 [DOI] [PubMed] [Google Scholar]
- Arroyo A, Kim B, Rasmusson RL, Bett G, Yeh J 2006 Hyperpolarization-activated cation channels are expressed in rat hypothalamic gonadotropin-releasing hormone (GnRH) neurons and immortalized GnRH neurons. J Soc Gynecol Investig 13:442–450 [DOI] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Herbison AE 2001 Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci 21:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ 2007 Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueshko S, Wray S 1994 LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol 166:331–348 [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R 1995 A novel family of genes encoding putative pheromone receptors in mammals. Cell 83:195–206 [DOI] [PubMed] [Google Scholar]
- Kramer PR, Krishnamurthy R, Mitchell PJ, Wray S 2000 Transcription factor activator protein-2 is required for continued luteinizing hormone-releasing hormone expression in the forebrain of developing mice. Endocrinology 141:1823–1838 [DOI] [PubMed] [Google Scholar]
- Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S 2004 Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci 24:4737–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI 1990 Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10 [DOI] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Weiner RI 1991 Immortalization of neuroendocrine cells by targeted oncogenesis. Recent Prog Horm Res 47:69–96 [DOI] [PubMed] [Google Scholar]
- Wray S, Gahwiler BH, Gainer H 1988 Slice cultures of LHRH neurons in the presence and absence of brainstem and pituitary. Peptides 9:1151–1175 [DOI] [PubMed] [Google Scholar]
- Kretschmannova K, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS 2006 Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing GH3 cells. J Neuroendocrinol 18:484–493 [DOI] [PubMed] [Google Scholar]
- Hofmann F, Biel M, Kaupp UB 2005 International Union of Pharmacology. LI. Nomenclature and structure-function relationships of cyclic nucleotide-regulated channels. Pharmacol Rev 57:455–462 [DOI] [PubMed] [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC 1993 Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol 110:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Fueshko SM, Kusano K, Gainer H 1996 GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol 180:631–645 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ 2002 GnRH neurons and episodic bursting activity. Trends Endocrinol Metab 13:409–410 [DOI] [PubMed] [Google Scholar]
- Charles A, Weiner R, Costantin J 2001 cAMP modulates the excitability of immortalized hypothalamic (GT1) neurons via a cyclic nucleotide gated channel. Mol Endocrinol 15:997–1009 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Jiang Y, Tomic M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS 2006 Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol Endocrinol 20:2231–2246 [DOI] [PubMed] [Google Scholar]
- Braumann T, Erneux C, Petridis G, Stohrer WD, Jastorff B 1986 Hydrolysis of cyclic nucleotides by a purified cGMP-stimulated phosphodiesterase: structural requirements for hydrolysis. Biochim Biophys Acta 871:199–206 [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Shimshek DR, Sprengel R, Seeburg PH 2001 Using reporter genes to label selected neuronal populations in transgenic mice for gene promoter, anatomical, and physiological studies. Prog Neurobiol 63:673–686 [DOI] [PubMed] [Google Scholar]
- Todman MG, Han SK, Herbison AE 2005 Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 132:703–712 [DOI] [PubMed] [Google Scholar]
- Martinez de la Escalera G, Choi AL, Weiner RI 1995 Signaling pathways involved in GnRH secretion in GT1 cells. Neuroendocrinology 61:310–317 [DOI] [PubMed] [Google Scholar]
- Levitan IB, Norman J 1980 Different effects of cAMP and cGMP derivatives on the activity of an identified neuron: biochemical and electrophysiological analysis. Brain Res 187:415–429 [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2006 Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci 26:11961–11973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajorat R, Brauer AU, Wasner U, Rolfs A, Strauss U 2005 Functional significance of HCN2/3-mediated I(h) in striatal cells at early developmental stages. J Neurosci Res 82:206–213 [DOI] [PubMed] [Google Scholar]
- Mistrik P, Mader R, Michalakis S, Weidinger M, Pfeifer A, Biel M 2005 The murine HCN3 gene encodes a hyperpolarization-activated cation channel with slow kinetics and unique response to cyclic nucleotides. J Biol Chem 280:27056–27061 [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ 1996 The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol 497(Pt 1):119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Samulack DD, Beaulieu C, LaCaille JC 1994 Membrane properties and synaptic responses of interneurons located near the stratum lacunosum-moleculare/radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices. J Neurophysiol 71:2217–2235 [DOI] [PubMed] [Google Scholar]
- Lupica CR, Bell JA, Hoffman AF, Watson PL 2001 Contribution of the hyperpolarization-activated current (Ih) to membrane potential and GABA release in hippocampal interneurons. J Neurophysiol 86:261–268 [DOI] [PubMed] [Google Scholar]
- Boyes J, Bolam JP, Shigemoto R, Stanford IM 2007 Functional presynaptic HCN channels in the rat globus pallidus. Eur J Neurosci 25:2081–2092 [DOI] [PubMed] [Google Scholar]
- Gervasi N, Hepp R, Tricoire L, Zhang J, Lambolez B, Paupardin-Tritsch D, Vincent P 2007 Dynamics of protein kinase A signaling at the membrane, in the cytosol, and in the nucleus of neurons in mouse brain slices. J Neurosci 27:2744–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis EA, Costantin JL, Tsai PS, Sakakibara H, Paruthiyil S, Iiri T, Martini JF, Taga M, Choi AL, Charles AC, Weiner RI 2000 Role of the cAMP signaling pathway in the regulation of gonadotropin-releasing hormone secretion in GT1 cells. Proc Natl Acad Sci USA 97:1861–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmanoff M 1997 Commentary on the use of immortalized neuroendocrine cell lines for physiological research. Endocrine 6:1–3 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Grendell RL, Golos TG 2005 Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol 19:2736–2747 [DOI] [PubMed] [Google Scholar]
- Izvolskaia M, Duittoz AH, Ugrumov MV, Tillet Y 2006 Tyrosine hydroxylase expression in the olfactory/respiratory epithelium in early sheep fetuses (Ovis aries). Brain Res 1083:29–38 [DOI] [PubMed] [Google Scholar]
- Giacobini P, Wray S 2007 Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology 148:63–71 [DOI] [PubMed] [Google Scholar]
- Schreibmayer W 1999 Isoform diversity and modulation of sodium channels by protein kinases. Cell Physiol Biochem 9:187–200 [DOI] [PubMed] [Google Scholar]
- Cole WC, Clement-Chomienne O, Aiello EA 1996 Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol 74:439–447 [DOI] [PubMed] [Google Scholar]
- Kamp TJ, Hell JW 2000 Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87:1095–1102 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR, Simonian SX, Skynner MJ, Sim JA 2001 Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Mol Cell Endocrinol 185:185–194 [DOI] [PubMed] [Google Scholar]
- Waltereit R, Weller M 2003 Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol 27:99–106 [DOI] [PubMed] [Google Scholar]