Abstract
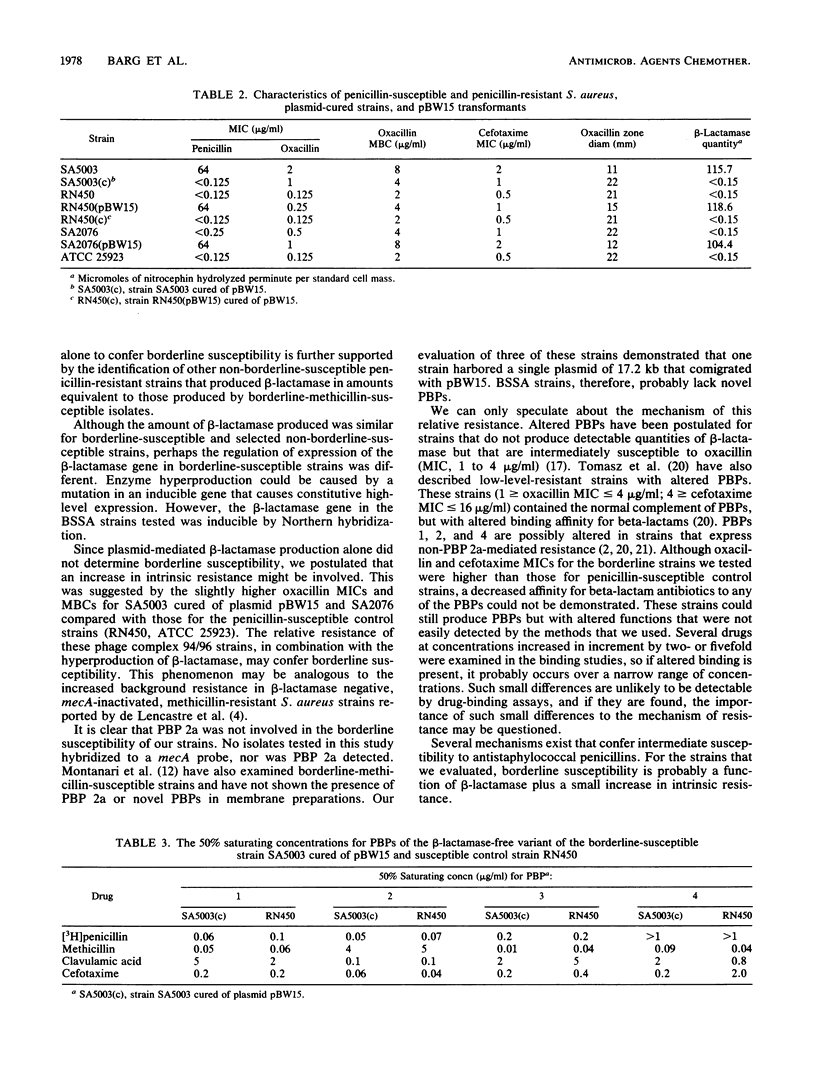
Staphylococcus aureus strains bearing the 17.2-kb beta-lactamase plasmid pBW15 and belonging to phage group 94/96 exhibit borderline susceptibility to the antistaphylococcal penicillins. Borderline susceptibility within phage group 94/96 is thought to be mediated by the hyperproduction of type A staphylococcal beta-lactamase. Evaluation of 84 non-94/96 phage type S. aureus strains that also produced the type A enzyme identified 7 additional hyperproducing strains. However, none of these isolates contained pBW15, and only one met the criteria for borderline susceptibility. To determine the role of pBW15 and the 94/96 phage type in the expression of borderline susceptibility, pBW15 was transformed in two plasmid-free, penicillin-susceptible strains, one of which belonged to phage group 94/96. Penicillin MICs for both transformants and quantitative beta-lactamase activity were comparable to those for the parent pBW15-containing strain. A fourfold difference in the oxacillin MICs for the 94/96 and non-94/96 phage type transformants (1.0 and 0.25 microgram/ml, respectively) was identified, and only the 94/96 phage type transformant met the criteria for borderline susceptibility. Chromosomal DNA from borderline-susceptible phage group 94/96 strains did not hybridize with a probe for mecA, and the beta-lactam binding affinity of PBPs 1, 2, 3, and 4 from a penicillin-susceptible 94/96 phage type strain and a non-94/96 phage type strain were comparable. Although hyperproduction of the type A beta-lactamase appears to be necessary for the expression of borderline susceptibility within certain phage group 94/96 strains, beta-lactamase production of a comparable magnitude by a group of S. aureus strains belonging to other phage types does not confer borderline susceptibility. These data suggest that borderline susceptibility is not solely due to the hyperproduction of beta-lactamase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Hartman B. J., Tomasz A. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest. 1985 Jul;76(1):325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernodle D. S., McGraw P. A., Stratton C. W., Kaiser A. B. Use of extracts versus whole-cell bacterial suspensions in the identification of Staphylococcus aureus beta-lactamase variants. Antimicrob Agents Chemother. 1990 Mar;34(3):420–425. doi: 10.1128/aac.34.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum J. S., Projan S. J., Moghazeh S. L., Novick R. P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63(1):75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray L. W., Kernodle D. S., Barg N. L. Characterization of a widespread strain of methicillin-susceptible Staphylococcus aureus associated with nosocomial infections. J Infect Dis. 1990 Sep;162(3):759–762. doi: 10.1093/infdis/162.3.759. [DOI] [PubMed] [Google Scholar]
- Montanari M. P., Tonin E., Biavasco F., Varaldo P. E. Further characterization of borderline methicillin-resistant Staphylococcus aureus and analysis of penicillin-binding proteins. Antimicrob Agents Chemother. 1990 May;34(5):911–913. doi: 10.1128/aac.34.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Tomasz A. Involvement of multiple genetic determinants in high-level methicillin resistance in Staphylococcus aureus. J Bacteriol. 1989 Feb;171(2):874–879. doi: 10.1128/jb.171.2.874-879.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Sierra-Madero J. G., Knapp C., Karaffa C., Washington J. A. Role of beta-lactamase and different testing conditions in oxacillin-borderline-susceptible staphylococci. Antimicrob Agents Chemother. 1988 Dec;32(12):1754–1757. doi: 10.1128/aac.32.12.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Drugeon H. B., de Lencastre H. M., Jabes D., McDougall L., Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989 Nov;33(11):1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin E., Tomasz A. Beta-lactam-specific resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Oct;30(4):577–583. doi: 10.1128/aac.30.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost S. C., Jones J. M., Pattee P. A. Sequential transposition of Tn916 among Staphylococcus aureus protoplasts. Plasmid. 1988 Jan;19(1):13–20. doi: 10.1016/0147-619x(88)90058-3. [DOI] [PubMed] [Google Scholar]
- de Lencastre H., Sá Figueiredo A. M., Urban C., Rahal J., Tomasz A. Multiple mechanisms of methicillin resistance and improved methods for detection in clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Apr;35(4):632–639. doi: 10.1128/aac.35.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]




