Abstract
Background
Biomass fuel is the primary source of domestic fuel in much of rural China. Previous studies have not characterized particle exposure through time–activity diaries or personal monitoring in mainland China.
Objectives
In this study we characterized indoor and personal particle exposure in six households in northeastern China (three urban, three rural) and explored differences by location, cooking status, activity, and fuel type. Rural homes used biomass. Urban homes used a combination of electricity and natural gas.
Methods
Stationary monitors measured hourly indoor particulate matter (PM) with an aerodynamic diameter ≤ 10 μm (PM10) for rural and urban kitchens, urban sitting rooms, and outdoors. Personal monitors for PM with an aerodynamic diameter ≤ 2.5 μm (PM2.5) were employed for 10 participants. Time–activity patterns in 30-min intervals were recorded by researchers for each participant.
Results
Stationary monitoring results indicate that rural kitchen PM10 levels are three times higher than those in urban kitchens during cooking. PM10 was 6.1 times higher during cooking periods than during noncooking periods for rural kitchens. Personal PM2.5 levels for rural cooks were 2.8–3.6 times higher than for all other participant categories. The highest PM2.5 exposures occurred during cooking periods for urban and rural cooks. However, rural cooks had 5.4 times higher PM2.5 levels during cooking than did urban cooks. Rural cooks spent 2.5 times more hours per day cooking than did their urban counterparts.
Conclusions
These findings indicate that biomass burning for cooking contributes substantially to indoor particulate levels and that this exposure is particularly elevated for cooks. Second-by-second personal PM2.5 exposures revealed differences in exposures by population group and strong temporal heterogeneity that would be obscured by aggregate metrics.
Keywords: biomass fuels, China, exposure assessment, household energy, indoor air pollution, particulate matter, PM10, PM2.5, rural health
About half the world’s population relies on biomass fuels as the primary domestic energy source (Smith et al. 2004). In rural China, biomass fuels account for about 80% of domestic energy (World Resources Institute 1998). Biomass combustion results in severe indoor air pollution, especially particulate matter (PM). Exposure to PM has been associated with increased risk for a suite of negative health outcomes, such as acute respiratory infection, chronic respiratory disease, and mortality (U.S. Environmental Protection Agency 2004).
The Chinese population suffers a high health burden from lung diseases, and respiratory disease is the primary cause of death in rural China (Schmidt 2002). Indoor PM from biomass fuels is one of the most serious yet least studied environmental health problems in China. In fact, little is known about human exposure to indoor PM in China and how different populations may be affected. Data are particularly lacking for rural China (Schmidt 2002). Several studies conducted in other parts of the world have investigated exposures to indoor air pollution from biomass fuels, finding that exposure patterns differed by sex, location in the home, and activity patterns (e.g., cooking vs. noncooking) (Balakrishnan et al. 2002; Ezzati et al. 2000).
Indoor air pollution in China has been explored in several previous studies. Respirable particles [RPM; PM with a median aerodynamic diameter ≤ 4 μm (PM4)], carbon monoxide, and sulfur dioxide were measured using stationary monitors at the household level in four Chinese provinces. The two provinces using biomass as the primary fuel had the highest PM4 concentrations (Jin et al. 2005). He et al. (2005) monitored multiple pollutants (PM4, CO, SO2, fluoride, and arsenic) at four points inside homes consuming coals and/or biomass fuels in the Guizhou and Shaanxi provinces. PM4 was higher in Guizhou households than in those in Shaanxi because of the fuel and stove combination (i.e., biomass fuel instead of coal, traditional stove instead of improved stove). Wang et al. (2006) investigated PM with an aerodynamic diameter ≤ 10 μm (PM10), PM with an aerodynamic diameter ≤ 2.5 μm (PM2.5), and 18 PM2.5 chemical components in four hospitals and adjacent outdoor environments in Guangzhou, China. Indoor PM2.5 levels in the hospitals were significantly higher than the U.S. Environmental Protection Agency ambient PM2.5 standard.
Additional summaries of research on exposure to indoor air pollution in China are provided elsewhere (Mestl et al. 2007a; Sinton et al. 1995). Despite these important studies, several unanswered questions remain. Specifically, estimates of indoor PM exposure typically were based on stationary monitors, often in combination with daily activity dairies, rather than continuous personal PM monitoring. Time–activity patterns were recorded by participants rather than researchers, introducing potential bias. Published studies do not include time–activity data for mainland China. In addition, monitoring generally applied exposure metrics of daily or hourly values and therefore has been unable to illuminate heterogeneity in exposure at smaller time scales. Finally, with the notable exception of Wang et al. (2006), most studies focused on PM10 or PM4 rather than PM2.5, although PM2.5 appears to be more closely linked to adverse health effects.
In this study we investigated indoor PM10 and PM2.5 levels in northeastern China using stationary and personal monitoring and time–activity diaries generated by direct observation. We compared exposures for cooks and noncooks, indoor and outdoor levels, urban and rural homes, and fuel type. Personal monitoring data include second-by-second measurements, allowing analysis of variation at small time scales. To the best of our knowledge, this is the first study to employ personal PM2.5 monitoring to assess individual exposures in China. In addition, we believe this study to be one of the first in mainland China to collect time–activity data.
Materials and Methods
Research location and sampling periods
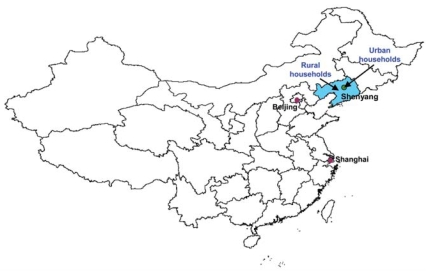
This study was conducted in six households of Shenyang, the capital city of Liaoning Province in northeastern China (Figure 1). Three households were located in Shenyang rural areas, and three in Shenyang metropolitan areas. Household selection was based on feasibility and guidance of local environmental and governmental agencies.
Figure 1.
Map of study area showing Shenyang City (green circle), the capital of Liaoning Province (blue), China. Arrows note the approximate locations of the urban households (in Shenyang city) and rural households (in Liaozhong County). Liaozhong County is approximately 69 km southwest of Shenyang City.
Exposure analysis included a) stationary indoor and outdoor PM10 monitors, b) personal PM2.5 monitors, and c) time–activity dairies for study participants. Sampling was conducted within 25 May to 10 August 2006. During this time of year, biomass burning is used just for cooking, whereas in other time periods biomass is used for both cooking and heating. Thus, our results can be interpreted as isolating the impact of biomass burning for cooking. Stationary monitoring occurred over 5 consecutive days. In rural homes, stationary monitors were used to assess hourly PM10 levels in kitchens, and a single outdoor monitor at a rural home was used to measure ambient PM10. For urban homes, stationary monitors assessed hourly PM10 levels in kitchens and sitting rooms.
Adult household residents were surveyed to determine whether each person was a primary cook for the household and the time spent at home per day. All cooks and the noncooks spending most of their time at home were requested to participate in the personal monitoring and time–activity diary portion of the study. Ten of the 18 adult residents participated. Verbal consent to participate in the study was obtained from each participant. Personal monitors estimated continuous PM2.5 exposure with second-by-second resolution over 3 consecutive days for each participant. During the 3 days coinciding with personal monitoring, researchers kept time–activity dairies for each participant. Table 1 provides the sampling periods for stationary and personal monitoring for each household. The sampling period covers weekends and weekdays, although the work and activity patterns of this population are similar across days.
Table 1.
Sampling periods for exposure analysis.
| Type and location of monitoring | Sampling period |
|---|---|
| Stationary monitoring (PM10) | |
| Urban kitchen 1 and urban sitting room 1 | 2–6 Aug 2006 |
| Urban kitchen 2 and urban sitting room 2 | 5–9 Aug 2006 |
| Urban kitchen 3 and urban sitting room 3 | 7–11 Aug 2006 |
| Urban outdoorsa | 2–11 Aug 2006 |
| Rural kitchen 1 | 25–29 May 2006 |
| Rural kitchen 2 | 26–30 May 2006 |
| Rural kitchen 3 | 26–30 May 2006 |
| Rural outdoors | 25–30 May 2006 |
| Personal monitoring (PM2.5) and time–activity dairies | |
| Urban cook 1 and urban noncook 1 | 2–4 Aug 2006 |
| Urban cook 2 | 5–7 Aug 2006 |
| Urban cook 3 and urban noncook 3 | 8–10 Aug 2006 |
| Rural cook 1 and rural noncook 1 | 25–27 May 2006 |
| Rural cook 2 | 26–28 May 2006 |
| Rural cook 3 and rural noncook 3 | 28–30 May 2006 |
The three urban households are designated urban 1, urban 2, and urban 3, and likewise for rural households. Urban cook 1 corresponds to a participant in household urban 1, etc.
Urban outdoor PM10 levels were obtained from the Shenyang Environmental Bureau.
Stationary PM10 monitoring
Stationary PM10 monitors were placed in 10 locations: three urban kitchens, three urban sitting rooms, three rural kitchens, and an outdoor rural location. Hourly concentrations were measured using P-5L2C Digital Dust Indicators manufactured by Beijing Binta Green Technology Co., Ltd. (Beijing, China). These devices determine relative PM10 concentrations based on the intensity of light scattered by particles passing through an illumination chamber. This intensity is measured by a photo multiplier tube located at a 90° angle to the light source and converted to pulses, which are indicated in count per minute values that are then converted to mass PM10 concentrations (Beijing Binta Green Technology Co. 2007).
For rural households, PM10 levels in indoor kitchens were measured approximately 1 m from the stove. Outdoor PM10 levels were measured in the yard of rural household 2 approximately 0.8 m from the house. Rural household 2 was 80 m and 50 m from the other rural households. For urban homes, monitors were approximately 1 m from the gas stove in kitchens and in the center of sitting rooms. All stationary monitors were placed on a flat surface at a height of approximately 0.6 m. Other criteria for choosing the sampling positions were access to electricity to power the monitors, the safety and stabilization of equipment, and avoidance of interference with household activities. During sampling periods, PM10 monitors operated continuously each day for approximately 14 hr from 0530 to 1930 hours for rural households and for approximately 10 hr from 0830 to 1830 hours for urban households.
Urban PM10 levels were obtained from the Shenyang Environmental Bureau, which measures 24-hr PM10 at eight locations across the city (Er Mao, Tai Yuan Street, Xiao He Yan, Wen Yi Road, North Mausoleum, Cannon School, Zhang Shi, and Dong Ruan). Daily PM10 levels were calculated from publicly available air pollution index values (Shenyang Environmental Bureau 2007) based on the guidelines provided by the China National Environmental Monitoring Center (2007).
The Shenyang Environmental Bureau used automated continuous sampling methods for PM10, which is measured using tapered element oscillating microbalance technology and reported at averaging times of 24 hr (Zhou, Shenyang Environmental Bureau Monitoring Center, personal communication).
Personal PM2.5 monitoring
Personal PM2.5 exposures for 10 participants were measured using model AM510 SidePak personal aerosol monitors (aTSI Inc. 2006a). These monitors use light-scattering technology to determine mass concentration in real time at 1-sec intervals. An aerosol sample is drawn into the sensing chamber in a continuous stream. A laser illuminates one section of the aerosol stream. A lens at 90° to both the aerosol stream and laser beam collects light scattered by particles and focuses it onto a photo detector. The detection circuitry converts the light into voltage, which is proportional to the mass concentrations of aerosols. The voltage is read by the processor and multiplied by an internal calibration constant to provide mass concentration (bTSI Inc. 2006b).
These lightweight monitors were equipped with personal pumps and attached to the belts of participants. Tubing connected the inlet of each monitor to the individual’s collar to sample the breathing zone (Figure 2). Each individual was instructed to carry the monitor indoors and outdoors during waking hours (~ 15 hr/day) throughout the sampling period, except while sleeping, showering, and using the restroom. Participants were instructed to place the monitors at approximately 1–1.5 m above the ground surface (i.e., close to the breathing zone) when the monitor could not be carried.
Figure 2.
Study participant with the personal monitor in a rural setting: using biomass to fuel the stove (A) and cooking (B).
Time–activity diaries
Throughout the personal monitoring sampling periods, the principal researcher (R.J.) and a research assistant maintained written 24-hr time–activity diaries for each participant (Table 1). Whereas most previous research had subjects record their own activities, this study applied direct observation in real time to eliminate recall bias and ensure uniform treatment across participants. Time–activity diaries recorded participants’ location (outdoors vs. indoors) and activities in 30-min intervals. Activities were divided into the following categories: cooking (e.g., preparation for cooking, such as cleaning stove, lighting, and tending fire), sleeping at nighttime, eating, socializing (e.g., conversing), relaxing (e.g., watching television, playing with children, napping during daytime), cleaning, and other (e.g., outside, all other activities not listed above). Researchers also noted housing characteristics and fuel type for cooking in each home.
Data analysis
We compared stationary PM10 measurements between rural and urban households, kitchens, and sitting rooms in urban households, and indoor and outdoor levels. We examined personal PM2.5 exposures by activity pattern (e.g., cooking vs. noncooking) and participant group (e.g., urban cook vs. rural cook). We applied descriptive statistics, Pearson’s correlation coefficients, linear regression analysis, and analysis of variance (ANOVA). Minitab statistical software (Minitab Inc., State College, PA, USA), TrakPro data analysis software, version 3.41 (TSI Inc., Shoreview, MN, USA) and the R statistical package, version 2.4.1 (http://www.r-project.org), were used.
We analyzed the relationship between hourly PM10 levels in urban kitchens and sitting rooms with linear regression as follows:
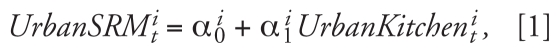 |
where UrbanSRMti is the PM10 concentration for hour t for the sitting room of urban house-hold i, UrbanKitchenti is the PM10 concentration for hour t for the kitchen of urban household i, and α0i α1i are the regression coefficients for the relationship between PM10 kitchen and sitting room levels of household i.
We examined the relationship between rural indoor and outdoor hourly PM10 levels as follows:
where RuralKitchenti is the PM10 concentration for hour t for the kitchen of rural household i, RuralOutdoorst is the PM10 concentration for hour t for the rural outdoor monitor located at rural household 2, and β0i β1i are the regression coefficients for the relationship between PM10 levels outdoors and in the kitchen of household i.
The above regression analysis was performed separately for each rural household for cooking times, noncooking times, and the entire study period. A cooking episode was designated for any hour for which cooking took place in a time–activity diary for that household. A single outdoor monitor, located outside rural household 2, was used to estimate representative ambient concentrations for the rural community.
Results
Housing and participant characteristics
All rural homes in the study were one-story houses with large yards for crop cultivating and animal husbandry. All the urban homes were apartments located in central Shenyang, two on the eleventh floor and one on the fourth floor. For all households, kitchens and living areas were separate. The rural homes used biomass fuels (corn) for cooking, whereas the urban homes used a combination of natural gas and electricity. Exhaust fans existed in all the urban kitchens; none were in the rural kitchens.
Ten household residents participated in the personal monitoring and time–activity diary portions of the study: three female cooks, a female noncook, and a male noncook in urban households and three female cooks, a female noncook, and a male noncook in rural households. All subjects were adults, with an average age of 61 years (range, 19–85 years).
Stationary PM10 monitoring results
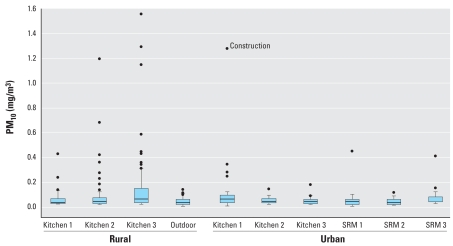
Table 2 summarizes PM10 levels based on hourly measurements from stationary monitors at various indoor locations on average across specific groups of households (rural or urban) and the outdoor locations. Urban outdoor levels, likely resulting from transportation networks and growing urbanization, exceeded rural outdoor levels and urban indoor levels. Figure 3 provides box plots of the hourly PM10 stationary monitors. An outlier value of 1287.0 μg/m3 occurred in urban kitchen 1 during a period of indoor construction. PM10 levels in rural kitchens were 64% higher on average than in urban kitchens and 2.5 times higher than outdoor levels. Urban kitchen and sitting rooms had similar concentrations, which were lower than the urban outdoors concentration. Rural kitchen PM10 levels had the highest recorded levels and exhibited the largest variability.
Table 2.
PM10 levels from stationary monitors (μg/m3).
| Location | Mean ± SD | Median | Minimum–maximum |
|---|---|---|---|
| Indoors | |||
| Rural kitchens | 100.6 ± 203.1 | 45.00 | 14.00–1571.0 |
| Urban kitchens | 61.34 ± 111.8
52.77 ± 44.08a |
43.00
43.00a |
2.00–1287.0
2.00–335.0a |
| Urban sitting rooms | 48.46 ± 51.97 | 36.00 | 0.00–448.0 |
| Outdoors | |||
| Rural | 40.23 ± 26.50 | 32.00 | 2.00–133.0 |
| Urbanb | 89.20 ± 17.84 | 80.00 | 74.00–126.0b |
Three households each were used to estimate concentrations for rural kitchens, urban kitchens, and urban sitting rooms. Median, minimum, and maximum refer to hourly levels. The minimum and maximum represent the lowest and highest hourly levels recorded in any household. Urban indoor values are based on 10-hr sampling periods, and rural values, on 14-hr sampling periods.
Excludes outlier value from indoor construction for urban kitchen 1.
Mean urban outdoor levels were based on 24-hr averages; minimum and maximum outdoor levels reflect daily values.
Figure 3.
Box plots of hourly measurements from PM10 stationary monitors (mg/m3). SRM, sitting room. An outlier value for urban kitchen 1 took place during indoor construction.
Comparison of PM10 levels in urban and rural kitchens
Kitchen measurements were divided into cooking and noncooking times to explore how different fuel types and kitchen designs affect PM10 levels. A cooking time was defined as a period with “cooking” in the activity diary for at least one participant in the household. Table 3 shows PM10 concentrations in kitchens based on stationary hourly measurements, divided by cooking and noncooking times, averaged by home type (urban or rural). During cooking, kitchen PM10 levels in rural households were on average 3.0 times higher than in urban households (one-way ANOVA, p< 0.05), whereas the PM10 levels for urban and rural households during noncooking times are not statistically different (p > 0.05).
Table 3.
PM10 levels for urban and rural kitchens, stratified by cooking and noncooking periods (μg/m3).
| Total study period
|
Cooking times
|
Noncooking times
|
||||
|---|---|---|---|---|---|---|
| Household type | No. of hours | PM10 (mean ± SD) | No. of hours | PM10 (mean ± SD) | No. of hours | PM10 (mean ± SD) |
| Rural | 190 | 100.6 ± 203.1 | 76 | 202.1 ± 293.6 | 114 | 33.01 ± 15.31 |
| Urban | 144
143a |
61.34 ± 111.8
52.77 ± 44.08a |
29 | 67.00 ± 32.58 | 115
114a |
59.40 ± 123.8
48.62 ± 44.83a |
Excludes outlier value from indoor construction for urban kitchen 1.
Comparison of kitchen and sitting room PM10 levels for urban households
PM10 levels in urban sitting rooms were similar to but lower than concentrations in urban kitchens (Table 2). The relationship between PM10 levels in these two types of areas was analyzed with correlation coefficients and with linear regression for each urban household (Table 4). Regression analysis results are presented as the percent change in the urban sitting room PM10 level per 10 μg/m3 increase in the urban kitchen PM10 levels and 95% confidence interval (CI), evaluated at the mean sitting room level for that household. Findings indicate that in each household, PM10 levels in sitting rooms and kitchens are strongly related.
Table 4.
Relationship between hourly urban sitting room PM10 levels and urban kitchen PM10 levels.
| Home designation | Correlation coefficient (p-value) | Percent change in urban sitting room PM10 per 10 μg/m3 increase in kitchen PM10 (95% CI) |
|---|---|---|
| Urban home 1 | 0.77 (0.051)
0.77 (< 0.001)a |
1.79 (0.04–3.53)
14.40 (10.97–17.82)a |
| Urban home 2 | 0.86 (< 0.001) | 24.27 (20.51–28.03) |
| Urban home 3 | 0.93 (< 0.001) | 29.60 (25.32–33.88) |
Excludes outlier value from indoor construction for urban kitchen 1.
Comparison of indoor and outdoor PM10 levels for rural households
Table 5 shows correlation coefficients comparing the hourly PM10 measurements from the rural kitchens to the rural outdoor monitor, stratified by cooking and noncooking periods. Table 5 also presents results from the regression analysis for each household, stratified by cooking and non-cooking periods. Although the outdoor monitor is located near rural home 2, this home does not exhibit the strongest relationship between indoor and outdoor PM10 levels. Rural home 1 had higher indoor (kitchen) PM10 levels during periods of higher outdoor levels (p < 0.05), yet no relationship was observed between outdoor and kitchen levels for the other homes, based on data for the entire study period. No relationship was observed between outdoor and kitchen PM10 levels during cooking periods. However, during noncooking periods, rural kitchen and outdoor PM10 levels were positively associated (statistically significant for rural homes 1 and 3).
Table 5.
Relationship between rural kitchen and rural outdoor PM10 levels.
| Correlation coefficients between rural kitchen and rural outdoor PM10 levels (p-value)
|
Percent increase in rural kitchen PM10 per 10 μg/m3 increase in rural outdoor PM10 levels, evaluated at the mean (95% CI)
|
|||||
|---|---|---|---|---|---|---|
| Home designation | Entire study period | Cooking times | Noncooking times | Entire study period | Cooking times | Noncooking times |
| Rural home 1 | 0.397 (0.004) | 0.121 (0.633) | 0.900 (< 0.001) | 8.80 (3.10 to 14.50) | 1.22 (−3.98 to 6.12) | 23.58 (19.57 to 27.59) |
| Rural home 2 | −0.043 (0.760) | −0.018 (0.936) | 0.157 (0.407) | −4.16 (−30.76 to 22.43) | −1.29 (−32.10 to 29.52) | 14.26 (−18.91 to 47.43) |
| Rural home 3 | −0.057 (0.658) | −0.270 (0.183) | 0.845 (< 0.001) | −4.40 (−23.74 to 14.96) | −11.98 (−29.09 to 5.13) | 23.95 (18.86 to 29.04) |
Time–activity patterns
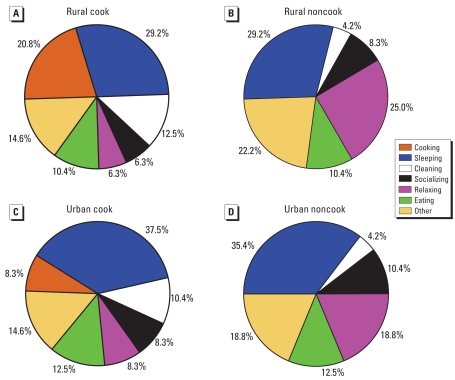
Figure 4 describes the participants’ time–activity budgets based on 24-hr assessments for various participant categories: rural cooks (n = 3), rural noncooks (n = 2), urban cooks (n = 3), and urban non-cooks (n = 2). Generally, cooks spent 8.3–20.8% of the total time cooking. Rural cooks averaged 5 hr/day cooking, versus 2 hr/day for urban cooks. Cooking took place three times per day for each rural household, twice per day for urban homes 1 and 2, and once per day for urban home 3. The average time for each cooking event was 1.7 hr for rural homes and 1.2 hr for urban homes.
Figure 4.
Time–activity budgets of three rural cooks (A), two rural noncooks (B), three urban cooks (C), and two urban noncooks (D).
Comparison of PM2.5 levels from personal monitoring by participant and activity
Table 6 shows average PM2.5 exposure by participant category (cook or noncook) and urban or rural designation, and by activity, as measured during waking hours (approximately a 16-hr period). Cooking periods had higher personal PM2.5 exposures than all other activity categories for cooks, but especially for rural cooks. The various noncooking activities had similar PM2.5 levels. Personal PM2.5 exposure for rural cooks was 3.3 times higher than for urban cooks (one-way ANOVA, p < 0.05) and 3.6 times higher than for rural noncooks (p < 0.05).
Table 6.
Personal exposure PM2.5 levels (mean ± SD) by urban or rural designation, participant type, and activity based on second-by-second measurements (μg/m3).
| Rural cook (n = 3) | Rural noncook (n = 2) | Urban cook (n = 3) | Urban noncook (n = 2) | |
|---|---|---|---|---|
| Cooking | 487.9 ± 874.9 | — | 90.1 ± 120.9 | — |
| Noncooking | ||||
| Cleaning | 76.9 ± 58.2 | 73.8 ± 58.5 | 62.4 ± 29.5 | 83.5 ± 145.1 |
| Socializing | 51.3 ± 27.8 | 42.2 ± 31.1 | 52.5 ± 31.3 | 62.6 ± 30.3 |
| Relaxing | 39.5 ± 26.3 | 50.7 ± 37.8 | 48.7 ± 104.9 | 61.9 ± 36.2 |
| Eating | 86.9 ± 65.5 | 70.2 ± 64.8 | 60.7 ± 30.5 | 73.4 ± 44.4 |
| Other | 59.9 ± 64.1 | 65.8 ± 31.1 | 54.3 ± 32.4 | 72.5 ± 49.6 |
| Total | 201.5 ± 539.8 | 56.4 ± 51.1 | 61.7 ± 48.3 | 71.5 ± 72.9 |
Variability in PM2.5 exposures was investigated using the second-by-second measurements from personal monitors. Cooking times exhibited more heterogeneity in PM2.5 levels than did any noncooking activity for urban or rural cooks, particularly for rural cooks (Table 6). Second-by-second PM2.5 personal exposures for a rural cook over a 1-day period are shown in Figure 5, demontrating the higher PM2.5 levels and variability during cooking periods. During the three cooking periods, mean PM2.5 concentrations for this participant were 349.8, 256.8, and 387.7 μg/m3, compared with 37.5 and 22.9 μg/m3 during the noncooking periods. The standard deviations of PM2.5 during cooking periods were 661.5, 463.2, and 464.3 μg/m3, compared with 7.2 and 17.0 μg/m3 during noncooking times.
Figure 5.
Variations of personal PM2.5 in a rural cook, based on second-by-second concentrations. Horizontal lines reflect the average for each cooking period.
Discussion
Our results indicate higher PM levels for households using biomass compared with those using cleaner fuels, cooks compared with noncooks, and cooking times compared with noncooking periods for households using biomass in northeastern China. Although the generalizability of our results is limited by the small sample size, these findings confirm similar results identified by studies in other regions (Ezzati and Kammen 2002) and add to the growing body of evidence that biomass fuels can result in highly elevated indoor air pollution levels, which in turn can contribute to adverse health effects.
Unique aspects of our study, in addition to the location, include the use of personal PM2.5 monitoring at second-by-second resolution, allowing analysis of heterogeneity at small time scales. The personal exposure monitoring data exhibit variation that would be obscured by the use of more aggregate measures, especially during cooking periods. Another unique aspect is the use of researchers rather than participants to record time–activity dairies in real time, which avoids recall bias and encourages consistency. To the best of our knowledge, this study is the first to employ personal PM2.5 monitoring to assess PM exposures in China, and the first study in mainland China to collect time–activity data.
Measured concentrations for rural kitchens were lower than PM kitchen levels of rural households burning biomass as measured in other studies (Table 7). The lower levels observed in this study may be related to the good conditions of the stoves, because all stoves in the participating rural households were refurbished within the 5 years preceding the study. Also, this study was conducted at the end of May, which is not a major season of rural biomass consumption. Thus, PM levels are likely to be even higher during the winter season, when biomass is used for heating as well as cooking in rural households. Our findings show that high PM levels are experienced even under conditions of refurbished stoves in the nonheating season.
Table 7.
Comparison of the rural indoor kitchen or cooking room particulate levels based on stationary monitoring in this study and previous studies.
| Location, study period (reference) | PM size | Type of fuel | Mean (μg/m3) | Notes |
|---|---|---|---|---|
| China | ||||
| Shenyang, China, May 2006 (this study) | PM10 | Crop residue | Total: 100.6
Cooking times: 202.1 |
Based on ~14 hr/day (0530–1930) for 5 consecutive days
3 households |
| Jilan, China, Nov–Dec 2001, Feb–Mar 2003, 2004 (Fischer and Koshland 2007) | PM4 | Multiple fuels: coal, biomass, gas, electricity | Daily average: 312
1-hr peak: 1,880 |
Based on 24-hr periods and 1-hr peak values
70 household-days 37 households |
| Gansu, China, Mar–Apr 2003, Dec 2003–Jan 2004 (Jin et al. 2005) | PM4 | Wood and crop residue | Mar–Apr 2003: 518
Dec 2003–Jan 2004: 661 |
Based on 24-hr/day periods
Mar–Apr 2003: 72 households with 1 day of measurement, 6 households with 4 days of measurement Dec 2003–Jan 2004: 17 households with 1 day of measurement, 6 households with 2–3 days of measurement |
| Guizhou, China, Mar–Apr 2003, Dec 2003–Jan 2004 (Jin et al. 2005) | PM4 | Coal, wood, and crop residue | Mar–Apr 2003: 352
Dec 2003–Jan 2004: 301 |
Based on 24-hr/day periods
Mar–Apr 2003: 76 households with 1 day of measurement, 7 households with 2–4 days of measurement Dec 2003–Jan 2004: 16 households with 1 day of measurement, 6 households with 2–3 days of measurement |
| Inner Mongolia, China, Dec 2003–Jan 2004 (Jin et al. 2005) | PM4 | Wood and crop residue | 718 | Based on 24-hr/day periods
49 households with 1 day of measurement, 4 households with 3 days of measurement |
| Shaanxi, China, Mar–Apr 2003, Dec 2003–Jan 2004 (Jin et al. 2005) | PM4 | 50% coal, 50% wood and crop residue | Mar–Apr 2003: 187
Dec 2003–Jan 2004: 223 |
Based on 24-hr/day periods
Mar–Apr 2003: 75 households with 1 day of measurement, 6 households with 4 days of measurement Dec 2003–Jan 2004: 18 households with 1 day of measurement, 6 households with 3 days of measurement |
| Guizhou, China, Jan 2003 (He et al. 2005) | PM4 | Coal (3 households), coal and biomass (1 household) | 1,944 | Based on 24-hr/day periods for 4 consecutive days
4 households |
| Shaanxi, China, Feb 2003 (He et al. 2005) | PM4 | Coal and biomass for cooking, coal for heating | 205 | Based on 24-hr/day periods for 4 consecutive days
4 households |
| Zhejiang, Hubei, and Shaanxi, China, Jun–Aug 2002, Dec 2002–Jan 2003 (Edwards et al. 2007) | PM4 | Crop residues | Summer: 282.9
Winter: 456.4 |
Based on 24-hr periods
48 households for summer, 25 for winter |
| Bolivia | ||||
| Cantuyo, Jan 1994–Oct 1995 (Albalak et al. 1999) | PM10 | Dung | 1,830 | Based on 6-hr periods (0500–1100)/day every 6 days for 3 consecutive weeks for each month of study period
12 households |
| India | ||||
| Pauri District, Garhwal Himalaya, northern India, Aug 1989–Jul 1990 (Saksena et al. 1992) | TSP | Wood | Cooking times: 5,600
Noncooking times: 820 |
Based on 15-hr periods
12 households |
| Tamil Nadu, southern India, Jul–Dec 1999 (Balakrishnan et al. 2002) | PM4 | Wood and crop residue | Indoor kitchen without partitions: 1,442
Kitchen with partitions: 970 |
Based on 10- to 12-hr/day periods for 1–3 days
Without partitions: 105 households With partitions: 68 households |
| Andhra Pradesh, southern India, Jan–May 2001 (Balakrishnan et al. 2004) | PM4 | Wood
Dung |
Wood: 500
Dung: 732 |
Based on 22- to 24-hr/day sampling periods for 3–4 days
Wood: 270 households Dung: 97 households |
| Guatemala | ||||
| Quetzaltenango, May–Nov 1993 (Naeher et al. 2000) | PM2.5 PM10 TSP |
Wood | PM2.5: 527.9,a 96.5b PM10: 717.1,a 186.3b TSP: 835.8,a 275.5b |
Based on three 22-hr sampling periods
3 households |
| La Victoria, Jan 1999 (Bruce et al. 2004) | PM3.5 | Wood and crop residue | 1,019a 351b |
Based on one 24-hr measurement/ household
11 households with open fires, 5 with planchas |
| La Victoria, Dec 1998–Jul 1999 (Albalak et al. 2001) | PM3.5 | Wood | 1,560a 280b |
Based on 24-hr measurements taken 6 times at 1-month intervals
10 households of each stove type |
TSP, total suspended particles.
Traditional open fire stove.
Improved plancha stove–equipped.
PM10 levels in rural kitchens were 64% above those in urban kitchens and 2.5 times higher than outdoor concentrations, consistent with earlier work finding higher PM levels in rural kitchens compared with the ambient environment (Table 8). Higher pollution levels in kitchens compared with other rooms for households using biomass fuel have been documented in other regions. In a study of rural homes using biomass in Mpala Ranch, central Kenya, PM10 concentrations were 3.5–7.5 times higher in areas close to the stove compared with other areas (Ezzati et al. 2000). In Andra Pradesh, India, PM4 levels were 1.5–2 times higher in kitchens than in living rooms in rural households burning biomass (Balakrishnan et al. 2004).
Table 8.
Comparison of PM levels in rural kitchens and outdoor environments based on stationary monitoring in this study and previous studies.
| Location (reference) | PM size | Type of fuel | Kitchen mean PM (μg/m3) | Outdoor mean PM (μg/m3) |
|---|---|---|---|---|
| Shenyang, China (this study) | PM10 | Crop residue | 100.6 | 40.23 |
| Andhra Pradesh, southern India (Balakrishnan et al. 2004) | PM4 | Wood
Dung |
500
732 |
87
99 |
| Guizhou, China (He et al. 2005) | PM4 | Coal and biomass | 1,944–2,334 | 206 |
| Shaanxi, China (He et al. 2005) | PM4 | Coal and biomass | 456 | 122 |
| Cantuyo, Bolivia (Albalak et al. 1999) | PM10 | Dung | 3,690 (mean)
1,830 (geometric mean) |
60 (mean)
50 (geometric mean) |
| Tanzania (Kilabuko et al. 2007) | PM10 | Wood | 656.2 (cooking times)
96.1 (noncooking times) |
40.1 |
We found that urban households, which used cleaner fuels (natural gas), had significantly lower indoor and personal PM levels than did rural households, which used biomass. Rural kitchens were equipped with low-efficiency chimneys compared with the highly efficient exhaust fans in urban kitchens. This conclusion is supported by earlier findings, such as those of Röllin et al. (2004), who reported elevated indoor RPM levels in non-electrified dwellings relying on biomass as domestic energy, compared with homes using electricity or a mix of electricity and biomass fuels for cooking, in rural South Africa. In Tamil Nadu, India, cooks using biomass fuels experienced higher indoor PM4 levels than did cooks using clean fuels such as kerosene or gas (Balakrishnan et al. 2002).
During cooking, rural cooks had personal PM2.5 exposure 5.4 times higher than urban cooks. Whereas urban cooks and noncooks had similar personal exposures, rural cooks’ PM2.5 exposure was 3.6 times higher than that of rural noncooks. Other studies revealing different personal PM exposure based on cooking fuels include research of rural households in southern India, which found concentrations of respiratory particles for cooks using wood and crop residue to be 2.6 times higher than for noncooks and 2.8 times higher than for cooks using clean fuel (Balakrishnan et al. 2002). In rural Kenya, adult women had the highest PM10 levels (4,898 μg/m3), and both young and adult women had higher exposures than did their male counterparts. For cooks, the high-intensity emission episodes accounted for 31–61% of total exposure (Ezzati et al. 2000). In Maputo, Mozambique, biomass users were exposed to significantly higher PM levels during cooking (540–1,200 μg/m3) than were users of liquefied petroleum gas and electricity (200–380 μg/m3) (Ellegård 1996). A study of three households in Highland Guatemala showed that personal exposures of mothers and children using biomass are higher than exposures of those using natural gas (Naeher et al. 2000).
Time–activity diaries recorded by researchers in 30-min intervals showed that rural cooks spent 2.5 times more hours per day cooking than did urban cooks, with a higher frequency and length of cooking events. One reason for this difference is that rural cooks need to clear the stove, fetch biomass, and light biomass before their cooking activities, whereas the urban cooks in this study used the more efficient and less time-consuming fuels natural gas and/or electricity. Jin et al. (2006) reported that women in four Chinese provinces spend approximately 2–3 hr/day cooking, whereas in our study, rural cooks spent 5 hr/day cooking and urban cooks 2 hr/day.
Other studies evaluated time–activity diary data according to time spent in various microenvironments (e.g., kitchen, living room). In Tami Nadu, India, women cooks spent 6.76 hr/day in the kitchen, compared with 0.76 hr for women not involved in cooking (Balakrishnan et al. 2002). In central Kenya, some household members, primarily cooks, spent more time in the kitchen close to the fire when pollution concentrations were high, while other household members were outside the kitchen (Ezzati and Kammen 2001). Similar results were found in Kenya (Ezzati et al. 2000), rural Bolivia (Albalak et al. 1999), Andhra Pradesh, India (Balakrishnan et al. 2004), and the Shanxi Province of China (Mestl et al. 2006).
The higher PM exposures from biomass burning in this study are likely associated with adverse health effects. A review of health studies researching the burning of coal and biomass fuels in Chinese households found evidence of a severe health burden, including respiratory disease and impaired lung function (Zhang and Smith 2007). Several studies have linked fuel and stove type to health effects, such as increased risk of cataracts for women cooks in Nepal and India (Pokhrel et al. 2005), asthma symptoms in children in homes with open fires compared with children in homes with improved stoves with chimneys (Schei et al. 2004), more eye discomfort for women using open fires compared with those using improved stoves (Díaz et al. 2007), and higher frequency of cytogenetic alterations in blood lymphocytes for users of biomass fuels compared with women using liquefied petroleum gas (Musthapa et al. 2004). In Tanzania, acute respiratory illness was more prevalent for cooks and children < 5 years of age compared with men and noncooking women, likely because of differences in biomass burning exposure (Kilabuko et al. 2007). Risk of lung cancer was lower for residents of houses with separate kitchens or improved air circulation in Guangzhou, China (Liu et al. 1993). Mestl et al. (2006) estimated that lower household solid fuel use in Shanxi Province would reduce childhood asthma and adult respiratory disease.
Indoor air pollution is responsible for an estimated 4–5% of deaths in developing countries (Smith and Mehta 2003) and 4–6% of the burden of disease (Smith 2000). Approximately 3.5 million deaths per year in China are attributable to indoor air pollution (Mestl et al. 2007b). Programs to reduce these health responses include installation of improved stove types that increase energy efficiency, substitution of cleaner fuels, and new technologies such as biodigesters (Edwards et al. 2004; Zhang and Smith 2007). Still, the dominance of biomass fuels for energy use in rural Chinese households is anticipated to continue (Li et al. 2007).
Our findings indicate that the use of biomass for cooking by rural households greatly elevates PM exposures, particularly for those performing the cooking, and emphasizes the need for additional research on alternative fuels and kitchen and stove designs, as well as on the health burden from this pollution. Further, research is needed to investigate the intersection between policies aimed at improving indoor air quality and those intended to lower emissions of greenhouse gases (Smith 2002).
Footnotes
We thank study participants, X. Sun and J. Liu from the Northeastern Electricity Corporation Research Institute in China, and K. Ebisu of Yale University.
Funding for R.J. was provided by the Yale University Tropical Research Institute, East Asian Studies Summer Research and Travel Grants, and Cameron Speth Fellowship. Funding for M.L.B. was provided by the Health Effects Institute through the Walter A. Rosenblith New Investigator Award (4720-RFA04-2/04-16) and the National Institute of Environmental Health Sciences Outstanding New Environmental Scientist (ONES) Award (R01 ES015028).
This article represents the views of the authors and not necessarily those of the sponsoring agencies. Mention of commercial products does not constitute endorsement or recommendation.
References
- Albalak R, Bruce N, McCraken JP, Smith KR, de Gallardo T. Indoor respirable particulate matter concentrations from an open fire, improved cook stove, and LPG/open fire combination in a rural Guatemalan community. Environ Sci Technol. 2001;35:2650–2655. doi: 10.1021/es001940m. [DOI] [PubMed] [Google Scholar]
- Albalak R, Keeler GJ, Frisancho AR, Haber M. Assessment of PM10 concentrations from domestic biomass fuel combustion in two rural Bolivian highland villages. Environ Sci Technol. 1999;33:2505–2509. [Google Scholar]
- Balakrishnan K, Sambandam S, Ramaswamy P, Mehta S, Smith KR. Exposure assessment for respirable particulates associated with household fuel use in rural districts of Andhra Pradesh, India. J Exp Anal Environ Epidemiol. 2004;14:S14–S25. doi: 10.1038/sj.jea.7500354. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K, Sankar S, Parikh J, Padmavathi R, Srividya K, Venugopal V, et al. Daily average exposures to respirable particulate matter from combustion of biomass fuels in rural households of southern India. Environ Health Perspect. 2002;110:1069–1075. doi: 10.1289/ehp.021101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijing Binta Green Technology Co. P-5L2C Digital Dust Indicator User’s Manual. 2007. [[accessed 9 May 2007]]. Available: http://www.sibata.co.jp/%7Eoverseas/index.html.
- Bruce N, McCracken J, Albalak R, Schei MA, Smith KR, Lopez V, et al. Impact of improved stoves, house construction and child location on levels of indoor air pollution exposure in young Guatemalan children. J Exp Anal Environ Epidemiol. 2004;14:S26–S33. doi: 10.1038/sj.jea.7500355. [DOI] [PubMed] [Google Scholar]
- China National Environmental Monitoring Center. Technological Rules Concerned “Ambient Air Quality Daily Report.”. 2007. [[accessed 1 March 2007]]. Available: http://www.zhb.gov.cn/english/airqualityinfo.htm.
- Díaz E, Smith-Sivertsen T, Pope D, Lie RT, Díaz A, McCracken J, et al. Eye discomfort, headache and back pain among Mayan Guatemalan women taking part in a randomized stove intervention trial. J Epidemiol Community Health. 2007;61:74–79. doi: 10.1136/jech.2006.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RD, Liu Y, He G, Yin Z, Sinton J, Peabody J, et al. Household CO and PM measured as part of a review of China’s National Improved Stove Program. Indoor Air. 2007;17:189–203. doi: 10.1111/j.1600-0668.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- Edwards RD, Smith KR, Zhang J, Ma Y. Implications of changes in household stoves and fuel use in China. Energy Policy. 2004;32:395–411. [Google Scholar]
- Ellegård A. Cooking fuel smoke and respiratory symptoms among women in low income areas of Maputo. Environ Health Perspect. 1996;104:980–985. doi: 10.1289/ehp.104-1469451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Kammen DM. Quantifying the effects of exposure to indoor air pollution from biomass combustion on acute respiratory infections in developing countries. Environ Health Perspect. 2001;109:481–488. doi: 10.1289/ehp.01109481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Kammen DM. The health impacts of exposure to indoor air pollution from solid fuels in developing countries: knowledge, gaps, and data needs. Environ Health Perspect. 2002;110:1057–1068. doi: 10.1289/ehp.021101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Saleh H, Kammen DM. The contributions of emissions and spatial microenvironments to exposure to indoor air pollution from biomass combustion in Kenya. Environ Health Perspect. 2000;108:833–839. doi: 10.1289/ehp.00108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SL, Koshland CP. Daily and peak 1 h indoor air pollution and driving factors in a rural Chinese village. Environ Sci Technol. 2007;41:3121–3126. doi: 10.1021/es060564o. [DOI] [PubMed] [Google Scholar]
- He G, Ying B, Liu J, Gao S, Shen S, Balakrishnan K, et al. Patterns of household concentrations of multiple indoor air pollutants in China. Environ Sci Technol. 2005;39:991–998. doi: 10.1021/es049731f. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ma X, Chen X, Cheng Y, Baris E, Ezzati M, et al. Exposure to indoor air pollution from household energy use in rural China: the interactions of technology, behavior, and knowledge in health risk management. Soc Sci Med. 2006;62:3161–3176. doi: 10.1016/j.socscimed.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Jin Y, Zhou Z, He G, Wei H, Liu J, Liu F, et al. Geographical, spatial, and temporal distributions of multiple indoor air pollutants in four Chinese provinces. Environ Sci Technol. 2005;39:9431–9439. doi: 10.1021/es0507517. [DOI] [PubMed] [Google Scholar]
- Kilabuko JH, Matsuki H, Nakai S. Air quality and acute respiratory illness in biomass fuel using homes in Bagamoyo, Tanzania. Int J Environ Res Public Health. 2007;4:39–44. doi: 10.3390/ijerph2007010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Duan L, Wang S, Duan J, Guo X, Yi H, et al. Emission characteristics of particulate matter from rural household biofuel combustion in China. Energy Fuels. 2007;21:845–851. [Google Scholar]
- Liu Q, Sasco AJ, Riboli E, Hu MX. Indoor air pollution and lung cancer in Guangzhou, People’s Republic of China. Am J Epidemiol. 1993;137:145–154. doi: 10.1093/oxfordjournals.aje.a116654. [DOI] [PubMed] [Google Scholar]
- Mestl HES, Aunan K, Seip HM. Potential health benefit of reducing household solid fuel use in Shanxi province, China. Sci Total Environ. 2006;372:120–132. doi: 10.1016/j.scitotenv.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Mestl HE, Aunan K, Seip HM. Health benefits from reducing indoor air pollution from household solid fuel use in China: three abatement scenarios. Environ Int. 2007a;33:831–840. doi: 10.1016/j.envint.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Mestl HE, Aunan K, Seip HM, Wang S, Zhao Y, Zhang D. Urban and rural exposure to indoor air pollution from domestic biomass and coal burning across China. Sci Total Environ. 2007b;377:12–26. doi: 10.1016/j.scitotenv.2007.01.087. [DOI] [PubMed] [Google Scholar]
- Musthapa MS, Lohani M, Tiwari S, Mathur N, Prasad R, Rahman Q. Cytogenetic biomonitoring of Indian women cooking with biofuels: micronucleus and chromosomal aberration tests in peripheral blood lymphocytes. Environ Mol Mutagen. 2004;43:243–249. doi: 10.1002/em.20018. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Leaderer BP, Smith KR. Particulate matter and carbon monoxide in highland Guatemala: indoor and outdoor levels from traditional and improved wood stoves and gas stoves. Indoor Air. 2000;10:200–205. doi: 10.1034/j.1600-0668.2000.010003200.x. [DOI] [PubMed] [Google Scholar]
- Pokhrel AK, Smith KR, Khalakdina A, Deuja A, Bates MN. Case-control study of indoor cooking smoke exposure and cataract in Nepal and India. Int J Epidemiol. 2005;34:702–708. doi: 10.1093/ije/dyi015. [DOI] [PubMed] [Google Scholar]
- Röllin HB, Mathee A, Bruce N, Levin J, von Schirnding YER. Comparison of indoor air quality in electrified and un-electrified dwellings in rural South African villages. Indoor Air. 2004;14:208–216. doi: 10.1111/j.1600-0668.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- Saksena S, Prasad R, Pal RC, Joshi V. Patterns of daily exposure to TSP and CO in the Garhwal Himalaya. Atmos Environ. 1992;26A:2125–2134. [Google Scholar]
- Schei MA, Hessen JO, Smith KR, Bruce N, McCracken J, Lopez V. Childhood asthma and indoor woodsmoke from cooking in Guatemala. J Expo Anal Environ Epidemiol. 2004;14:S110–S117. doi: 10.1038/sj.jea.7500365. [DOI] [PubMed] [Google Scholar]
- Schmidt CW. Economy and environment: China seeks a balance. Environ Health Perspect. 2002;110:A516–A522. doi: 10.1289/ehp.110-a516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenyang Environmental Bureau. Air Quality Report [in Chinese] 2007. [[accessed 1 Oct 2006]]. Available: http://www.syepb.gov.cn/zhengwu/panel.asp?code=0302.
- Sinton JE, Smith KR, Hansheng H, Junzhuo L. Indoor Air Pollution Database for China. WHO/EHG/95.8. Geneva: World Health Organization; 1995. [Google Scholar]
- Smith KR. Inaugural article: national burden of disease in India from indoor air pollution. Proc Natl Acad Sci USA. 2000;97:13286–13293. doi: 10.1073/pnas.97.24.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR. Indoor air pollution in developing countries: recommendations for research. Indoor Air. 2002;12:198–207. doi: 10.1034/j.1600-0668.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- Smith KR, Mehta S. The burden of disease from indoor air pollution in developing countries: comparison of estimates. Int J Hyg Environ Health. 2003;206:279–289. doi: 10.1078/1438-4639-00224. [DOI] [PubMed] [Google Scholar]
- Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor air pollution from household solid fuel use. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization; 2004. pp. 1435–1493. [Google Scholar]
- TSI Inc. SidePak™ AM510 Personal Aerosol Monitor Theory of Operation. Application Note ITI-085. 2006a. [[accessed 1 May 2007]]. Available: http://www.tsi.com/documents/ITI-085.pdf.
- TSI Inc. Model AM510 SidePak™ Personal Aerosol Monitor User Guide. 1980456, Revision E. Shoreview, MN: TSI Inc; 2006b. [Google Scholar]
- U.S. Environmental Protection Agency. Air Quality Criteria for Particulate Matter. EPA/600/P-99/002aF, EPA/600/P-99/ 002bF. Research Triangle Park, NC: U.S. Environmental Protection Agency; 2004. [Google Scholar]
- Wang X, Bi X, Sheng G, Fu J. Hospital indoor PM10/PM2.5 and associated trace elements in Guangzhou, China. Sci Total Environ. 2006;366:124–135. doi: 10.1016/j.scitotenv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- World Resources Institute. World Resources 1998–99: Environmental Change and Human Health. Washington, DC: World Resources Institute, United Nations Environment Programme, United Nations Development Programme, World Bank; 1998. [Google Scholar]
- Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ Health Perspect. 2007;115:848–855. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]