Abstract
Wheat gliadin induces severe intestinal symptoms and small-bowel mucosal damage in coeliac disease patients. At present, the only effective treatment for the disease is a strict life-long gluten-free diet. In this study we investigated whether probiotics Lactobacillus fermentum or Bifidobacterium lactis can inhibit the toxic effects of gliadin in intestinal cell culture conditions. The ability of live probiotics to inhibit peptic-tryptic digested gliadin-induced damage to human colon cells Caco-2 was evaluated by measuring epithelial permeability by transepithelial resistance, actin cytoskeleton arrangements by the extent of membrane ruffling and expression of tight junctional protein ZO-1. B. lactis inhibited the gliadin-induced increase dose-dependently in epithelial permeability, higher concentrations completely abolishing the gliadin-induced decrease in transepithelial resistance. The same bacterial strain also inhibited the formation of membrane ruffles in Caco-2 cells induced by gliadin administration. Furthermore, it also protected the tight junctions of Caco-2 cells against the effects of gliadin, as evinced by the pattern of ZO-1 expression. We conclude thus that live B. lactis bacteria can counteract directly the harmful effects exerted by coeliac-toxic gliadin and would clearly warrant further studies of its potential as a novel dietary supplement in the treatment of coeliac disease.
Keywords: Bifidibacterium lactis, coeliac disease, gliadin, Lactobacillus fermentum
Introduction
Coeliac disease is an autoimmune-mediated intestinal disorder induced by prolamins present in wheat (gliadin), rye (secalin) and barley (hordein), and belongs to the most common food-related disorders in western countries. The disease has a strong genetic component (human leucocyte antigen DQ2 or DQ8) and is characterized by small-intestinal villous atrophy, crypt hyperplasia and a profound immune response in the mucosa.
Gluten, the coeliac toxic component in wheat, is a glutamine- and proline-rich cereal grain storage protein. It can be divided classically into aqueous alcohol-insoluble glutenin polymers and soluble gliadin monomers. Only the gliadin fraction of wheat has been traditionally considered toxic to coeliac disease patients, but according to recent results it seems obvious that glutenins are equally harmful [1,2]. Because of its high proline and glutamine content gliadin is conferred resistant to the enzymes of the human digestive tract and is only partially cleaved. Experiments have shown that treating gliadin with a physiological repertoire of digestive enzymes leads to the appearance of several peptides, including the 33-mer and its split product peptide fragments known to be harmful for coeliac patients [3,4]. According to the prevailing hypothesis, these immunodominant peptides enter the small-intestinal lamina propria and are deamidated by transglutaminase 2, thus resulting in their better binding to DQ2 and DQ8 molecules on antigen-presenting cells [5]. These latter cells then activate CD4+ T helper lymphocytes, which leads eventually to inflammation and intestinal tissue damage [6].
The incomplete digestion of gliadin by digestive tract enzymes also leads to the generation of peptides other than the immunodominant ones. For example, peptide p31–43 of α-gliadin is a classical toxic peptide able to induce small-intestinal epithelial cell apoptosis without stimulating CD4-positive cells [7]. The peptide is believed to cause damage by inducing a stress response by an innate immunity mechanism [7–10]. Regardless of the precise pathogenetic mechanism underlying coeliac disease, the common denominator is a permanent intolerance to gluten.
Currently, the only effective treatment for coeliac disease is life-long withdrawal of gluten from the diet. The consensus is that gluten-free dieting should be as strict as possible, but a diet completely devoid of gluten is almost impossible to maintain, as many gluten-free products contain trace amounts of gluten. Coeliac disease patients are thus exposed to gluten contaminations in food every day. As adherence to a strict gluten-free diet is burdensome, new treatment options are warranted. Currently, several research lines are concentrated in developing novel forms of therapy for coeliac disease. These include detoxification of the disease-driving gluten and gliadin peptides as well as blockage of the gluten-induced inflammatory response [11]. Gluten can basically be detoxified after ingestion in the gastrointestinal tract or be hydrolyzed prior to ingestion during food processing. The first approach is adopted in the development of new drugs for coeliac disease patients given as oral enzyme supplementation designed to accelerate the gastrointestinal degradation of gluten [12,13]. The second mode, gluten detoxification during food processing, has been established by sourdough fermentation. Several papers have reported that the use of probiotic bacteria in sourdough fermentation increases the degradation of gluten during the process [14–18]. Although several studies have addressed the ability of probiotic bacteria to detoxify gliadin after an extensive incubation period, to our knowledge none has investigated whether different live probiotic bacteria can inhibit gliadin-induced toxic effects directly on epithelial cells.
The toxicity of gliadin and gliadin peptides as well as the mechanism by which they function can be studied in cell culture. It is known that intestinal epithelial cell lines Caco-2 and T84 respond to gluten/gliadin treatment in a specific manner. When Caco-2 cells are exposed to a peptic-tryptic digest of gliadin (PT-gliadin), there is a significant increase in the permeability of the epithelial layer, measured as increased transepithelial resistance (TER), presumably because of decreased expression of several tight junctional proteins [19]. Furthermore, both cells types react to gliadin treatment by reorganizing their actin cytoskeleton [19,20]. This rearrangement in Caco-2 cells can be detected by large membrane ruffles at the edges of cell islets when grown in medium containing gliadin [21]. These gliadin-induced in vitro effects on epithelial cell behaviour offer a cheap and easy means of verifying gliadin detoxification after specific treatment, and thus methods which measure gliadin toxicity/non-toxicity provide a suitable approach in assessing the potency of the novel therapeutics.
The present study aimed to establish whether live probiotic bacteria could inhibit directly the toxic effects of wheat-derived gliadin in epithelial cell culture.
Materials and methods
Intestinal epithelial cell culture
The human colon epithelial cell line Caco-2 (American Cell Type Collection, HTB-37, Rockville, MD, USA; passage 23–60) was cultured in minimum essential medium (MEM; Gibco invitrogen, Paisley, UK) supplemented with 20% fetal bovine serum (FBS; Gibco invitrogen), penicillin–streptomycin (Gibco invitrogen), sodium pyruvate (Sigma-Aldrich, Seelze, Germany), sodium bicarbonate (Gibco invitrogen) and non-essential amino acids (Gibco invitrogen). Cells were cultured in 5% CO2 atmosphere at 37°C and passaged 105/cm2 when they reached 80% confluence.
Preparation of PT-gliadin and bovine serum albumin
Gliadin was extracted from wheat flour (Raisio Oyj, Raisio, Finland) obtained from a local grocery store. Salt-soluble proteins were first removed by extracting 10 g of wheat flour at room temperature with 30 ml of 1 M NaCl for 1 h in a shaker. The sample was centrifuged at 4000 g for 20 min, after which the pellet was washed with 40 ml of water and centrifuged as above. The resulting pellet was suspended with 25 ml of 70% ethanol and the mixture was incubated at 60°C for 60 min in a shaker. After centrifugation, the supernatant containing the gliadin fraction was collected, frozen and lyophilized.
Gliadin (60 mg) was dissolved in 10 ml of 50 mM Na-acetate buffer, pH 4·0. Pepsin (3 mg, P-6887; Sigma-Aldrich) was added and the mixture incubated for 2 h at 37°C under agitation; 71 mg of Na2HPO4 was then added to the solution and the pH was adjusted to 7·0 by NaOH. Trypsin (3 mg, T-7418; Sigma-Aldrich) was added and the reaction mixture incubated for another 2 h at 37°C under agitation. The reaction was stopped by heating (> 95°C, 10 min) and the resulting PT-gliadin mixture was frozen and lyophilized. Lyophilized fractions were stored at −20°C. Fractions were added to Caco-2 cell monolayers at a concentration of 1 mg/ml.
To distinguish the effects exerted by gliadin itself and possible minor amounts of pepsin and trypsin in the gliadin digests, peptic-tryptic digest of bovine serum albumin (PT-BSA) was used as a control. BSA was purchased from Sigma-Aldrich (A8806) and the pepsin-trypsin digestion was performed similarly to the gliadin digestion.
Bacterial cultures
Lactobacillus fermentum and Bifidobacterium lactis probiotic bacterial cell lines were cultured in de Man Rogosa Sharpe broth (LabM™; International Diagnostics Group plc, Bury, UK) at 37°C in anaerobic conditions [AnaeroGen™ 2·5 litre, AnaeroGen Anaerobic Indicator (reazurin); Oxoid Ltd, Basingstoke, Hampshire, UK]. B. lactis broth was supplemented with 0·05% Cystein-HCl (Merck KGaA, Darmstadt, Germany). Bacterial cultures were subdivided after 20 h growth and stored in anaerobic conditions at 4°C between culturing. The bacteria were exposed to epithelial cells at passages 105, 106 and 107 colony forming units (cfu)/ml.
Transepithelial resistance measurements
Caco-2 cells were plated onto Millicell Culture inserts (Millipore Corporate, Billerica, MA, USA) and grown until confluence. The resistance of the cell monolayer was measured using a Millicell-ERS volt-ohm meter (Millipore Corporate). Caco-2 cells were regarded as confluent when TER exceeded 600 ohms/cm2. Confluent monolayers were washed twice with Hank's balanced salt solution (HBSS; Gibco invitrogen) and incubated overnight in MEM supplemented with non-essential amino acids, sodium puryvate, sodium bicarbonate and 1% FBS, but without antibiotics prior to gliadin and bacteria exposure. After addition of PT-BSA, PT-gliadin and/or bacteria, TER was measured immediately after changing the media as well as after 1, 3, 5 and 24 h. The experiments were performed in duplicate three times independently.
Immunofluorescence microscopy on intestinal cell lines
For immunofluorescence Caco-2 cells were plated onto eight chamber glass slides (BD Biosciences, Erembodegem, Belgium). After 4 days of culture the monolayers were washed twice with HBSS (Gibco invitrogen) and incubated overnight in MEM supplemented with non-essential amino acids, sodium puryvate, sodium bicarbonate, 1% FBS and without antibiotics before 24-h exposure to PT-BSA, PT-gliadin and/or bacteria. Cells were then washed twice with phosphate-buffered saline, fixed in 4% paraformaldehyde (Merck) and permeabilized with 0,1% Triton X-100 (Sigma-Aldrich). To visualize membrane ruffle formation the cells were stained for intracellular F-actin with phalloidin–fluorescein isothiocyanate (Sigma-Aldrich). The extent of actin cytoskeleton arrangement, evinced as membrane ruffling, was quantified by measuring the cellular edge covered by membrane ruffles as a percentage of the total length of the cell cluster. The measurements were made by analySIS software (Olympus Soft Imaging System GmbH, Munster, Germany) from five pictures taken from each experiment performed in duplicate three times independently.
To study the tight junctions, unspecific binding was blocked (1:20 normal goat serum: Vector Laboratories Inc., Peterborough, UK; 5% milk powder: Valio Oy, Lapinlahti, Finland; 5% albumin bovin serum: Sigma-Aldrich; 15 min) prior to incubation in primary antibody (1:100 mouse anti-ZO-1 0.5 mg/ml; Zymed, San Francisco, CA, USA) for 60 min and in secondary antibody (1:1000 Alexa Fluor 568 goat anti-mouse immunoglobulin G; Invitrogen, Carlsbad, CA, USA) for 30 min. After washes, the cover slips (Mentzel-Gläser, Braunschweig, Germany) were mounted with Vectashield Mounting Medium for fluorescence with 4,6-diamino-2-phenylindole (Vector Laboratories). The results were analysed using a BX60 fluorescence microscope (Olympus, Hamburg, Germany). ZO-1 stainings were observed blindly by two individual observers in duplicate samplings performed three times.
Statistical analysis
Statistical analysis was performed using the non-parametric two-tailed Mann–Whitney U-test. The data are presented as mean ± standard error of the mean. A P-value < 0·05 was considered significant.
Results
Bifidobacterium lactis counteracts the gliadin-induced increase in epithelial cell permeability
The efficacy of different numbers of both L. fermentum and B. lactis in inhibiting the gliadin-induced increase in Caco-2 cell permeability was assessed by TER measurement (Fig. 1). Changing the cell culture medium reduced TER immediately in all samples, even in the controls without gliadin addition, as described by Li and co-workers [22]. In all samples, TER started to recover after 1-h incubation, and in control samples without any supplementation TER returned to baseline level after 24 h incubation. The addition of PT-BSA to the cultures had only a minor effect on the recovery of TER. The reversion of TER to baseline levels was inhibited completely in the PT-gliadin-treated cultures. The addition of L. fermentum alone without gliadin did not affect the recovery of TER and reached the baseline level after 24 h (data not shown). Supplementation of L. fermentum at any of the tested concentrations was not able to inhibit the gliadin-induced block in the recovery of TER. The TER values with all the different bacterial concentrations remained at the same level as those with gliadin alone (Fig. 1a).
Fig. 1.
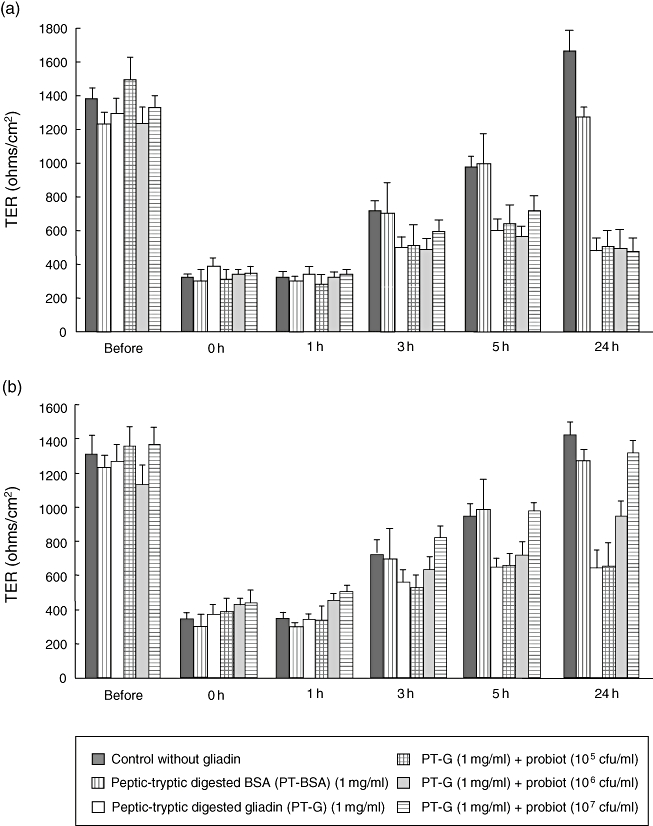
Effects of Lactobacillus fermentum and Bifidobacterium lactis supplementation on gliadin-induced decrease of transepithelial resistance (TER). (a) L. fermentum is not able to protect the permeability of Caco-2 cells from TER decrease caused by gliadin at any of the tested concentrations. (b) B. lactis inhibits the effects of gliadin on TER dose-dependently. The bars represent the mean value calculated from duplicate experiments repeated three times. The error bars represent standard error of the mean. Different experimental settings are indicated in the box below. PT-BSA, peptic-tryptic-digested bovine serum albumin; PT-G, peptic-tryptic-digested gliadin.
Similarly to L. fermentum, the addition of B. lactis alone to Caco-2 cell monolayers did not inhibit the recovery of TER, which returned to baseline after 24 h of culture (data not shown). In contrast, when B. lactis was administered together with gliadin, the bacteria inhibited the effects of gliadin on TER dose-dependently (Fig. 1b). The lowest concentration of B. lactis (105 cfu/ml) did not provide protection against gliadin insult, while a concentration of 106 cfu/ml of B. lactis provided some protection and the highest tested concentration (107 cfu/ml) full protection against gliadin-induced changes in TER measured in Caco-2 cell monolayers.
Effect of probiotics on gliadin-induced membrane ruffle formation
Gliadin is reported to induce distinct membrane ruffling on the edges of Caco-2 cell clusters [21]. To determine the extent of this ruffling we measured the length of the edge of the cell cluster covered by membrane as a percentage of the total length of the cluster. This enabled us to study objectively the effects of gliadin and the probiotics on membrane ruffling.
As expected, PT-gliadin induced substantial membrane ruffle formation in Caco-2 cells (Fig. 2a). In control cultures without any supplementation, membrane ruffles covered 11·3% and in cultures with PT-BSA 23·2% of the total length of the cell cluster edge. PT-gliadin treatment increased the percentage of cell cluster edge covered by ruffles to 51·6%.
Fig. 2.
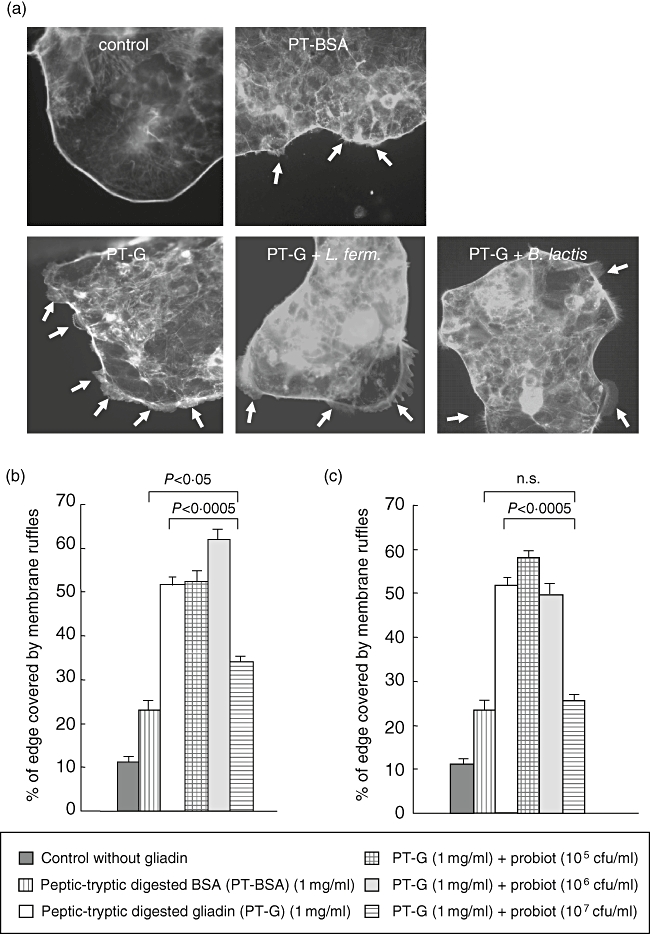
Membrane ruffle formation in Caco-2 cells in the presence of gliadin and the two different bacterial strains. (a) Caco-2 cells cultured without gliadin show a uniform cell cluster edge without any membrane protrusions while PT-BSA induced some ruffle formation. Caco-2 cell clusters which have received gliadin have large membrane ruffles on their edge. The addition of 107 colony-forming units (cfu)/ml of both Lactobacillus fermentum and Bifidobacterium lactis together with gliadin reduced the formation of the ruffles. Arrows point to membrane ruffles. (b) Addition of L. fermentum to Caco-2 cells cultures supplemented with gliadin at a lower concentration does not inhibit the membrane ruffle formation induced by gliadin. At a concentration of 107 cfu/ml L. fermentum reduced membrane ruffle formation from 51·6% to 34·1%. (c) The lowest concentrations of B. lactis do not protect from gliadin-induced membrane ruffle formation, while B. lactis at a concentration of 107 cfu/ml is able to lower the percentage of cellular edge covered by ruffles from 51·5% to 25·7%. The bars represent data measured from five pictures in duplicate samples performed three times. The error bars represent standard error of the mean. Different experimental settings are indicated in the box below. P-value < 0·05 is considered significant. PT-BSA, peptic-tryptic-digested bovine serum albumin; PT-G, peptic-tryptic-digested gliadin.
Supplementation of L. fermentum alone to Caco-2 cells increased membrane ruffling with the two higher concentrations near to 20% (data not shown) which was not, however, statistically significant. The two lowest concentrations of L. fermentum (105 and 106 cfu/ml) did not inhibit the ruffling induced by gliadin administration. In contrast, the highest concentration (107 cfu/ml) had a significant inhibitory effect on the gliadin action (34·1%, P < 0·0005), although even at that concentration B. lactis failed to counteract entirely the gliadin-induced membrane ruffling when compared with PT-BSA treated cells (P < 0·05) (Fig. 2b).
Similarly, addition of B. lactis alone at all tested concentrations increased membrane ruffling only slightly in Caco-2 cells (data not shown). When administered together with gliadin, B. lactis showed a protective effect similar to that of L. fermentum (Fig. 2c). The two lowest concentrations (105 and 106 cfu/ml) of B. lactis were ineffective in inhibiting gliadin-induced membrane ruffle formation, while the highest concentration (107 cfu/ml) was significantly protective (P < 0·0005). B. lactis at a concentration of 107 cfu/ml was even more protective against gliadin-induced damage than L. fermentum at the same concentration, as only 25·7% of the cellular edge was covered by membrane ruffles, in contrast to 34·1% in L. fermentum-supplemented cells. Supplementation of the Caco-2 cell cultures with 107 cfu/ml of B. lactis was able to reduce the percentage of membrane ruffling to the level of the PT-BSA control (P = 0·5).
Tight junction protein ZO-1 expression in Caco-2 cells
The tight junction protein ZO-1 was analysed by immunofluorescence microscopy to establish whether the probiotics exert effects on the appearance of tight junctions. Tight junctions in control cells without any supplemetation were markedly curvy (Fig. 3a), as in cells cultured in the presence of PT-BSA (Fig. 3b), but after gliadin exposure straightened significantly and the cells seemed larger (Fig. 3c). Bacterial administration of either probiotic strain alone without PT-gliadin did not affect the tight junction arrangement (data not shown). When either L. fermentum or B. lactis were added to Caco-2 cells together with gliadin, the curvy junctions and the size of the cells appeared to be better preserved than in cells cultured in the presence of gliadin alone (Fig. 3d and e respectively). Nevertheless, none of the tested concentrations of either of the probiotic bacterial strains was able to conserve the appearance of the tight junction entirely.
Fig. 3.
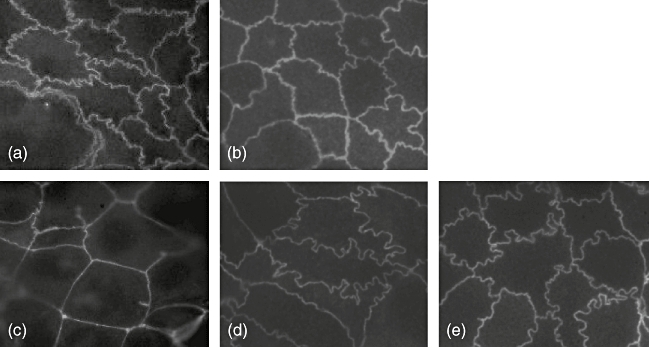
The appearance of tight junctions after gliadin and probiotic bacterial administration. In control cultures without any (a) and with peptic-tryptic-digested bovine serum albumin supplementation (b), the tight junctions as evinced by ZO-1 protein expression are curly. (c) Gliadin treatment straightens the tight junctions and the cells become larger. (d) The addition of 107 colony-forming units/ml of Lactobacillus fermentum preserves the tight junction to some extent similarly to the same concentration of Bifidobacterium lactis (e).
Discussion
In the present study we have shown for the first time that B. lactis probiotic bacteria are able to protect epithelial cells from cellular damage induced by gliadin administration. Addition of B. lactis to the cell culture medium together with gliadin was able to counteract the gliadin-induced inhibition of TER recovery, the formation of large membrane ruffles and the change in tight junctional protein ZO-1 expression.
Regardless of a different study setting, our results are in line with previous findings showing protective effects of certain probiotic bacterial strains against gluten/gliadin. The studies in question showed that when used in sourdough fermentation different probiotic bacterial strains are able to hydrolyze gluten with varying efficacy [15,23,24]. In addition, enzyme preparations [15], cell extracts [14] or intact probiotic preparations [24] have been shown to hydrolyze the gliadin peptides known to play a role in coeliac disease pathogenesis. Although the present study did not address the mechanism by which B. lactis inhibits gliadin-induced damage in Caco-2 cells, it can be hypothesized that they might do so by hydrolyzing PT-gliadin similarly to the live probiotic bacteria in the VSL3# probiotic preparation [24]. The fact that different probiotic bacterial strains have their characteristic set of peptidases, which may diverge from each other considerably and have variable substrate specificities, might explain why B. lactis was able to inhibit the gliadin-induced damage to Caco-2 cells more efficiently than L. fermentum. The peptidase repertoire of B. lactis could simply be more efficient than that of L. fermentum in breaking up gliadin into small harmless peptide products.
Another conceivable mode of action for B. lactis is that they modulate directly the function of epithelial cells. It has been reported that different probiotic strains, including the VSL3# preparation, probiotic bacterial lysates or conditioned medium increase epithelial barrier function as measured with TER [25–27]. In addition, at least some probiotics stabilize tight junctions and induce mucin secretion in epithelial cells [25]. Furthermore, several probiotic bacterial strains have proved able to protect the epithelium, presumably by the above-mentioned mechanisms, from various insults including pathogenic bacteria [25,28] and inflammatory cytokines [29,30]. Thus B. lactis might protect the epithelium from the insult caused by gliadin by direct action on the cells. In fact, it has been shown that B. lactis, but not distinct Lactobacillus species, induce the expression of cyclooxygenase (Cox)-1 in Caco-2 cells and reduce simultaneously the expression of Cox-2 [31]. Cox-1 is considered responsible for the production of ‘housekeeping’ prostaglandins critical for the maintenance of normal mucosal integrity, while Cox-2 is associated with an inflammatory status [32]. Thus the potential of B. lactis, but not L. fermentum, to inhibit the damage caused by gliadin in our study might be explained by its ability to promote Cox-1 and reduce proinflammatory Cox-2 expression. However, further studies are needed to establish the precise mode of action of B. lactis.
In summary, the data presented in this paper suggest that B. lactis could, at least partially, inhibit the gluten/gliadin-induced damage in the small-intestinal mucosa. Previous studies have suggested that use of probiotic bacteria in sourdough fermentation could induce the hydrolysis of coeliac toxic gluten during food processing and thus be beneficial for coeliac disease patients. The present findings would suggest that the probiotic B. lactis could also be health-promoting as a dietary supplement. Inclusion of B. lactis in the diet might not allow coeliac disease patients to consume normal gluten-containing food permanently, but could be beneficial in cases with, for example, poor response to a gluten-free diet. Moreover, intake of B. lactis might speed up mucosal recovery after adoption of a gluten-free diet or provide protection to the small-intestinal mucosa against the traces of gluten in some supposedly gluten-free products. Thus consumption of B. lactis-containing products by coeliac disease patients could promote the small-intestinal mucosal health of the patient and lead to a general health gain.
Acknowledgments
The authors wish to thank Mr Jorma Kulmala for technical assistance as well as MSc Marjo Toivo and MSc Tarja Suomalainen at Raisio for providing the bacterial strains. The Coeliac Disease Study Group has been supported financially by the Research Council for Health, the Academy of Finland, the Paediatric Research Foundation, the Competitive Research Funding of the Pirkanmaa Hospital District, the Yrjö Jahnsson Foundation, the Finnish Medical Foundation and the European Commission (contract number MRTN-CT-2006-036032).
References
- 1.Howdle PR. Gliadin, glutenin or both? The search for the Holy grail in celiac disease. Eur J Gastroenterol Hepatol. 2006;18:703–6. doi: 10.1097/01.meg.0000221847.09792.34. [DOI] [PubMed] [Google Scholar]
- 2.Dewar DH, Amato M, Ellis HJ, et al. The toxicity of high molecular weight glutenin subunits of wheat to patients with celiac disease. Eur J Gastroenterol Hepatol. 2006;18:483–91. doi: 10.1097/00042737-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–9. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 4.Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol. 2002;283:G996–1003. doi: 10.1152/ajpgi.00136.2002. [DOI] [PubMed] [Google Scholar]
- 5.Sollid LM, Jabri B. Is celiac disease an autoimmune disorder? Curr Opin Immunol. 2005;17:1–6. doi: 10.1016/j.coi.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Wolters VM, Wijmenga C. Genetic background of celiac disease and its clinical implications. J Gastroenterol. 2007;102:1–6. doi: 10.1111/j.1572-0241.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- 7.Maiuri L, Ciacci C, Ricciardelli I, et al. Association between innate response to gliadin and activation of pathogenic T cells in celiac disease. Lancet. 2003;62:30–7. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 8.Tuckova L, Novotna J, Novak P, et al. Activation of macrophages by gliadin fragments: isolation and characterization of active peptide. J Leukoc Biol. 2002;71:625–31. [PubMed] [Google Scholar]
- 9.Nikulina M, Habich C, Flohe SB, Scott FW, Kolb H. Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol. 2004;173:1925–31. doi: 10.4049/jimmunol.173.3.1925. [DOI] [PubMed] [Google Scholar]
- 10.Hue S, Mention J-J, Monteiro RC, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Sollid LM, Khosla C. Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:140–7. doi: 10.1038/ncpgasthep0111. [DOI] [PubMed] [Google Scholar]
- 12.Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endopeptidase in a gastrointestinal model: implications for celiac disease. Gut. 2008;57:25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- 13.Gass J, Bethune MT, Siegel M, Spencer A, Khosla C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007;133:472–80. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Rollan G, De Angelis M, Gobbetti M, de Valdez GF. Proteolytic activity and reduction of gliadin-like fractions by sourdough lactobacilli. J Appl Microbiol. 2005;99:1495–502. doi: 10.1111/j.1365-2672.2005.02730.x. [DOI] [PubMed] [Google Scholar]
- 15.Di Cagno R, De Angelis M, Lavermicco P, et al. Proteolysis by sourdough lactic acid bacteria: effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl Environ Microbiol. 2002;68:623–33. doi: 10.1128/AEM.68.2.623-633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Cagno R, De Angelis M, Auricchio S, et al. Sourdough bread made from wheat and nontoxic flours and strated with selected lactobacilli is tolerated in celiac sprue patients. Appl Environ Microbiol. 2004;70:1088–96. doi: 10.1128/AEM.70.2.1088-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerez CL, Rollan GC, de Valdez GF. Gluten breakdown by lactobacilli and pediococci strains isolated from sourdough. Lett Appl Microbiol. 2006;42:459–64. doi: 10.1111/j.1472-765X.2006.01889.x. [DOI] [PubMed] [Google Scholar]
- 18.Rizzello CG, De Angelis M, Di Cagno R, et al. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl Environ Microbiol. 2007;73:4499–507. doi: 10.1128/AEM.00260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sander GR, Cummins AG, Powell BC. Rapid distruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005;579:4851–5. doi: 10.1016/j.febslet.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 20.Maiuri L, Ciacci C, Ricciardelli I, et al. Unexpected role of surface transglutaminase type II in celiac disease. Gastroenterology. 2005;129:1400–13. doi: 10.1053/j.gastro.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 21.Barone MV, Gimigliono A, Castoria G, et al. Growth factor-like activity of gliadin, an alimentary protein: implications for celiac disease (CD) Gut. 2007;56:480–8. doi: 10.1136/gut.2005.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, DeMarco VG, West CM, Neu J. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem. 2003;14:947–9. doi: 10.1016/s0955-2863(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 23.Zotta T, Piraino P, Ricciardi A, McSweeney PL, Parente E. Proteolysis in model sourdough fermentation. J Agric Food Chem. 2006;54:2567–74. doi: 10.1021/jf052504s. [DOI] [PubMed] [Google Scholar]
- 24.De Angelis M, Rizzello CG, Fasano A, et al. VSL3# probiotic preparation has the capacity to hydrolyse gliadin polypeptides responsible for celiac sprue. Biochim Biophys Acta. 2005;1762:80–93. doi: 10.1016/j.bbadis.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Otte JM, Podolsky DK. Functional modulation of enterocytes by Gram-positive and Gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–26. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 26.Bai YH, Pak SC, Lee SH, et al. Assessment of a bioactive compound for its potential anti-inflammatory property by tight junction permeability. Phytoter Res. 2005;19:1009–12. doi: 10.1002/ptr.1772. [DOI] [PubMed] [Google Scholar]
- 27.Klingberg TD, Pedersen MH, Cencic A, Budde BB. Application of measurement of transepithelial electrical resistance of intestinal epithelial cell monolayers to evaluate probiotic activity. Appl Environ Microbiol. 2005;71:7528–30. doi: 10.1128/AEM.71.11.7528-7530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–97. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinque B, Di Marzio L, Della Riccia DN, et al. Effect of Bifidobacterium infantis on interferon-gamma-induced keratinocyte apoptosis: a potential therapeutic approach to skin immune abnormalities. Int J Immunopathol Pharmacol. 2006;19:775–86. doi: 10.1177/039463200601900407. [DOI] [PubMed] [Google Scholar]
- 30.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurmi JT, Puolakkainen PA, Rautonen NE. Bifidobacterium lactis sp420 up-regulates cyclooxygenase (Cox)-1 and downregulates Cox-2 gene expression in a Caco-2 cell culture model. Nutr Cancer. 2005;51:83–92. doi: 10.1207/s15327914nc5101_12. [DOI] [PubMed] [Google Scholar]
- 32.Iezzi A, Ferri C, Mezzetti A, Cipollone F. COX-2: friend or foe? Curr Pharm Des. 2007;13:1715–21. doi: 10.2174/138161207780831293. [DOI] [PubMed] [Google Scholar]


