Abstract
Recent studies in animal models for systemic lupus erythematosus (SLE) have shown that Toll-like receptors (TLR-7 and TLR-9) and interferon (IFN)-α are involved in the pathogenesis of murine lupus. Recent studies using flow cytometry have also shown increased expression of TLR-9 in peripheral blood mononuclear cells (PBMCs) from SLE patients. In this study, we performed quantitative real-time reverse transcription–polymerase chain reaction analyses of PBMCs from 21 SLE patients and 21 healthy subjects, to estimate TLR2, TLR3, TLR4, TLR5, TLR7, TLR8, TLR9, IFN-α and LY6E (a type I IFN-inducible gene) mRNA expression levels. Expression levels of TLR2, TLR7, TLR9, IFN-α and LY6E mRNAs in SLE patients were significantly higher than those in healthy controls. Expression levels of TLR7 and TLR9 mRNAs correlated with that of IFN-α mRNA in SLE patients. These results suggest that up-regulated expression of TLR7 and TLR9 mRNAs together with increased expression of IFN-α mRNA in PBMCs may also contribute to the pathogenesis of human lupus.
Keywords: interferon-α, peripheral blood mononuclear cells, real-time reverse transcription–polymerase chain reaction, systemic lupus erythematosus, Toll-like receptors
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease of generalized autoimmunity characterized by B cell hyperactivity, abnormally activated T cells and defects in the clearance of apoptotic cells and immune complexes [1,2]. Because these immune dysfunctions lead to the production of a wide range of autoantibodies and immune complex deposition in vital organs, autoantibody-producing B cells appear central to the pathogenesis of SLE. Several mechanisms have been proposed to explain the production of autoantibody-producing B cells: impaired survival/apoptosis signals preventing negative selection [3], dysfunctional complement or inhibitory Fc receptors [4,5], loss of peripheral tolerance through activation of myeloid dendritic cells induced by interferon (IFN)-α overproduction [6,7] and activation of Toll-like receptors (TLRs) in response to accumulation of apoptotic bodies [8,9].
The TLR family plays a critical role in the mammalian innate immune system, the first line of host defence against invading pathogens. To date, 12 members of the TLR family have been identified in mammals, and have been shown to bind to a variety of ligands, including lipids, proteins and nucleic acids derived from both microorganisms and endogenous tissues [10]. TLRs are now considered to have an important role in autoimmunity, because numerous in vitro studies have documented activation of both autoreactive B cells and plasmacytoid dendritic cells by TLR ligands [8]. Experimental evidence in animal models for SLE suggests a role for TLR-7 (a receptor for single-stranded RNA) and TLR-9 (a receptor for DNA) in the development of murine lupus [8,9]. Two recent studies using flow cytometry showed increased expression of TLR-9 in peripheral blood mononuclear cells (PBMCs) from human lupus patients [11,12]. However, TLR-7 was not examined in these studies.
In the present study, we examined TLR2, TLR3, TLR4, TLR5, TLR7, TLR8, TLR9, IFN-α and LY6E (a type I IFN-inducible gene [13]) mRNA expression levels in PBMCs from SLE patients, by quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR). Expression levels of TLR2, TLR7, TLR9, IFN-α and LY6E mRNAs in SLE patients were significantly higher than those in healthy subjects. Furthermore, expression levels of TLR7 and TLR9 mRNAs correlated with that of IFNα mRNA. These findings suggest a possible role for TLR-7, TLR-9 and IFN-α in the development of human lupus, as well as in murine lupus.
Materials and methods
Patients
We recruited 21 consecutive untreated patients entering out-patient clinics of Akita University Hospital and its affiliated hospitals who fulfilled the American College of Rheumatology 1997 revised criteria for SLE [14,15] for this study. Twenty-one sex- and age-matched healthy subjects were also included. SLE activity was assessed by the SLE Disease Activity Index (SLEDAI) score [16] at the onset of SLE. The protocol of this study was approved by the ethics committee of the institution involved, and informed consent for genetic studies was obtained from all the subjects. The characteristics of SLE patients and healthy subjects are summarized in Table 1.
Table 1.
Characteristics of the studied subjects.
| Subjects | SLE (n = 21) | Healthy controls (n = 21) | P-value |
|---|---|---|---|
| Female/male | (14/7) | (13/8) | n.s. |
| Age mean (years) (range) | 30·4 (13–66) | 32·2 (23–55) | n.s. |
| Race | Japanese | Japanese | |
| SLEDAI score (range) | 16·4 (4–35) | ||
| Renal disease | 11 | ||
| Central nervous system disease | 0 | ||
| Serosistis | 0 | ||
| Haematological disease | 18 | ||
| Leucopenia | 13 | ||
| Thrombocytopenia | 11 | ||
| Polyarthritis | 11 | ||
| Skin involvement | 17 | ||
| Vasculitis | 4 | ||
| Fever | 8 | ||
| Treatment | |||
| None | 21 | ||
SLE, systemic lupus erythematosus; n.s., not significant; SLEDAI, SLE Disease Activity Index.
Laboratory assessments
We assessed laboratory data including serum levels of complements C3 and C4, CH50, circulating immune complexes determined by a C1q binding assay, and titres of anti-dsDNA, anti-RNP and anti-Sm antibodies determined by enzyme-linked immunosorbent assays in each SLE patient. We also assessed peripheral blood leucocyte, neutrophil, monocyte and lymphocyte counts.
Quantitative real-time RT–PCR
We quantified TLR2, TLR3, TLR4, TLR5, TLR7, TLR8, TLR9, IFN-α, and LY6E mRNA expression levels in PBMCs from SLE patients and healthy subjects. PBMCs were isolated by Ficoll-Conray (GE Healthcare Bio-Science AB, Uppsala, Sweden; Daiichi Seiyaku Corp., Tokyo, Japan) density gradient centrifugation.
Total RNA was prepared with an RNeasy kit (Qiagen, Hilden, Germany) and used for cDNA synthesis with an oligo(dT) primer (Amesham Biosciences, Piscataway, NJ, USA). PCR primers used in this study are shown in Table 2.
Table 2.
Primers.
| Tm (°C) | ||
|---|---|---|
| TLR-2-forward | GGC CAG CAA ATT ACC TGT GTG | 64 |
| TLR-2-reverse | AGG CGG ACA TCC TGA ACC T | |
| TLR-3-forward | CCT GGT TTG TTA ATT GGA TTA ACG A | 62 |
| TLR-3-reverse | TGA GGT GGA GTG TTG CAA AGG | |
| TLR-4-forward | CTG CAA TGG ATC AAG GAC CA | 64 |
| TLR-4-reverse | TTA TCT GAA GGT GTT GCA CAT TCC | |
| TLR-5-forward | TGC CTT GAA GCC TTC AGT TAT G | 62 |
| TLR-5-reverse | CCA ACC ACC ACC ATG ATG AG | |
| TLR-7-forward | TTA CCT GGA TGG AAA CCA GCT ACT | 64 |
| TLR-7-reverse | TCA AGG CTG AGA AGC TGT AAG CTA | |
| TLR-8-forward | CAG AAT AGC AGG CGT AAC ACA TCA | 62 |
| TLR-8-reverse | TGT CAA GGC GAT TGC CAC TGA | |
| TLR-9-forward | TGA AGA CTT CAG GCC CAA CTG | 64 |
| TLR-9-reverse | TGC ACG GTC ACC AGG TTG T | |
| IFN-α-forward | TGC TTT ACT GAT GGT CCT GGT | 60 |
| IFN-α-reverse | TCA TGT CTG TCC ATC AGA CAG | |
| LY6E-forward | ATC TTC TTG CCA GTG CTG CT | 60 |
| LY6E-reverse | AGT CAC GCA GTA GTT GTC CC | |
| GAPDH-forward | ATG GCT ATG ATG GAG GTC CAG | 60 |
| GAPDH-reverse | TTG TCC TGC ATC TGC TTC AGC |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN, interferon; TLR, Toll-like receptor; LY6E, lymphocyte antigen 6 complex, locus E.
Real-time RT–PCR reaction was carried out in a final volume of 20 μl containing 10 μl DNA Master Hybridization Probe 2× (Qiagen, Hilden, Germany), 1 μl of 10 pmol forward and reverse primers, 1 μl of cDNA and 7 μl of water, according to the manufacturer's instructions. After an initial denaturation step at 95°C for 900 s, temperature cycling was initiated. Each cycle consisted of denaturation at 95°C for 15 s, hybridization at suitable temperatures (Table 2) for 20 s and elongation at 72°C for 20 s, using a LightCycler (Roche Diagnostics, Mannheim, Germany). A total of 45 cycles were performed. Each sample was run in triplicate.
Quantitative real-time RT–PCR curves were analysed by LightCycler 3·5 software (Roche Diagnostics). For relative quantification of TLRs, IFN-α and LY6E mRNA expressions, the mRNA expression of GAPDH was used as a control.
Statistics
Differences in the mean age and sex distribution between SLE patients and healthy subjects were determined using the χ2 test. We compared TLRs, IFN-α and LY6E mRNA expression levels in PBMCs in SLE patients and healthy subjects, using the Mann–Whitney U-test. Relations between TLRs mRNA expression levels and IFN-α mRNA expression level, the SLEDAI scores and laboratory parameters were examined by the Spearman's correlation coefficient rank test. All analyses were performed using an Excel Statistical Software (Igakutosho Shuppan Corp, Tokyo, Japan). Data are expressed as mean ± standard deviation. A P-value less than 0·05 was regarded as statistically significant.
Results
Patients
The characteristics of 21 SLE patients are shown in Table 1. The SLEDAI score ranged from 4 to 35, with a mean of 16·4. Because all patients had more than four SLEDAI scores they were considered to have mild to very high activity [17]. Haematological disease, including leucopenia and thrombocytopenia, was the most common manifestation, which was found in 18 patients. Skin rash was observed in 17 patients. Renal disease, polyarthritis, fever and vasculitis were found in 11, 11, eight and four patients respectively.
Quantification of expression levels of TLRs, IFN-α and LY6E mRNAs in PBMCs from SLE patients and control subjects by real-time RT–PCR
We examined TLRs, IFN-α and LY6E mRNA expression levels in PBMCs from 21 untreated SLE patients and 21 control subjects by real-time RT–PCR. The results showed that relative TLR2, TLR7, TLR9, IFN-α and LY6E mRNA expression levels (TLR2/GAPDH, TLR7/GAPDH, TRR9/GAPDH, IFN-α/GAPDH and LY6E/GAPDH mRNAs) were significantly higher in SLE patients than in control subjects (Table 3). We compared TLRs mRNA expression levels with IFN-α and LY6E mRNA expression levels, SLEDAI scores and laboratory parameters in SLE patients. The results revealed that expression levels of IFN-α mRNA correlated significantly with those of TLR3, TLR5, TLR7, TLR8 and TLR9 mRNAs (Table 4). On the other hand, expression levels of LY6E mRNA did not correlate with those of TLRs and IFN-α mRNAs. Titres of anti-dsDNA antibodies correlated inversely with expression levels of TLR9 and IFN-α mRNAs (Figs 1 and 2). No significant differences were found between TLRs mRNA expression levels and SLEDAI scores, circulating immune complex levels or other laboratory parameters in SLE patients.
Table 3.
Relative quantification of Toll-like receptors (TLRs) mRNA expression [control versus systemic lupus erythematosus (SLE) patients].
| Control | SLE | P-value* | |
|---|---|---|---|
| TLR-2 | 0·082 ± 0·045 | 0·119 ± 0·058 | 0·019 |
| TLR-3 | 1·801 ± 2·137 | 2·609 ± 2·069 | 0·089 |
| TLR-4 | 3·035 ± 1·505 | 4·407 ± 2·239 | 0·051 |
| TLR-5 | 0·243 ± 0·184 | 0·269 ± 0·132 | 0·279 |
| TLR-7 | 1·365 ± 1·171 | 1·872 ± 0·756 | 0·011 |
| TLR-8 | 3·658 ± 1·854 | 3·473 ± 1·985 | 0·741 |
| TLR-9 | 1·477 ± 1·883 | 2·791 ± 1·914 | 0·007 |
| IFN-α | 0·037 ± 0·045 | 0·069 ± 0·059 | 0·033 |
| LY6E | 0·094 ± 0·044 | 0·343 ± 0·163 | 0·0000067 |
P-value was estimated by Mann–Whitney U-test. LY6E, lymphocyte antigen 6 complex, locus E; IFN, interferon.
Table 4.
Correlation between IFN-α and TLRs mRNA expression levels in peripheral blood mononuclear cells from systemic lupus erythematosus patients.
| Results of the Spearman's correlation coefficient rank test | ||
|---|---|---|
| P-value | r | |
| TLR-2 | 0·089 | 0·381 |
| TLR-3 | 0·0000445 | 0·770 |
| TLR-4 | 0·129 | 0·342 |
| TLR-5 | 0·002 | 0·626 |
| TLR-7 | 0·014 | 0·528 |
| TLR-8 | 0·005 | 0·584 |
| TLR-9 | 0·001 | 0·645 |
TLR, Toll-like receptor.
Fig. 1.
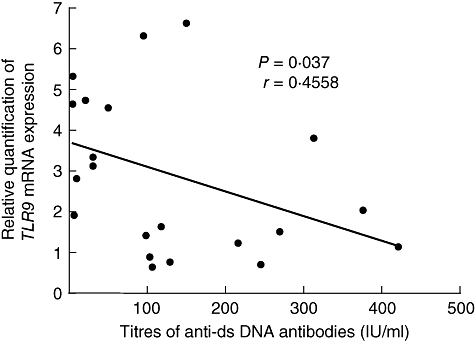
Inverse correlation between expression level of TLR9 mRNA in peripheral blood mononuclear cells and titres of anti-dsDNA antibodies for all systemic lupus erythematosus patients. TLR, Toll-like receptor.
Fig. 2.
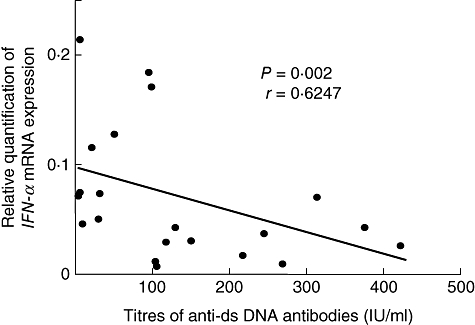
Inverse correlation between expression level of IFN-α mRNA in peripheral blood mononuclear cells and titres of anti-dsDNA antibodies for all systemic lupus erythematosus patients. IFN, interferon.
Discussion
In this study, we have demonstrated that TLR2, TLR7, TLR9, IFN-α and LY6E mRNA expression levels in PBMCs from SLE patients were significantly higher than those in healthy controls, using quantitative real-time RT–PCR. Expression levels of TLR7 and TLR9 mRNAs correlated significantly with that of IFN-α mRNA. Furthermore, titres of anti-dsDNA antibodies correlated inversely with expression levels of TLR9 and IFN-α mRNAs. Table 5 summarizes previously reported data of TLR expressions [11,12] and our results in SLE patients.
Table 5.
Expression of Toll-like receptors (TLRs) in peripheral blood mononuclear cells from patients.
| References | Methods | TLR-2 | TLR-3 | TLR-4 | TLR-5 | TLR-7 | TLR-8 | TLR-9 |
|---|---|---|---|---|---|---|---|---|
| [11] | Flow cytometry | → | → | → | n.d. | n.d. | n.d. | ↑ |
| [12] | Flow cytometry | ↓ | n.d. | → | n.d. | n.d. | n.d. | ↑ |
| Present study | Real-time RT–PCR | ↑ | → | → | → | ↑ | → | ↑ |
n.d., Not determined; RT–PCR, reverse transcription–polymerase chain reaction.
There is conclusive evidence of a role for TLR-9 in the pathogenesis of murine lupus models [8,9]. Recently, two groups examined the expression of TLRs in PBMCs from SLE patients by flow cytometry [11,12]. Papadimitraki et al. [11] demonstrated that the proportion of plasma cells and memory B cells expressing TLR-9 was increased in active SLE patients. Increased percentages of TLR-9-expressing B cells, but not monocytes, correlated with the presence of anti-dsDNA antibodies in their patients. Migita et al. [12] also showed that TLR-9 expression levels of CD19+ B cells were elevated significantly in SLE patients. There was no significant correlation between TLR-9 expression levels and clinical markers such as SLEDAI scores, CH50 and titres of anti-dsDNA antibodies in their patients.
Our results of real-time RT–PCR showed increased expression of TLR-9 in PBMCs from SLE patients. This supports the previous observations by Papadimitraki et al. [11] and Migita et al. [12]. In our patients, there was an inverse correlation between expression levels of TLR9 mRNA in PBMCs and titres of anti-dsDNA antibodies. Our finding is inconsistent with observations by Papadimitraki et al. [11]. This controversy might be due to differences in experimental procedures and/or background of patients. In studies by Papadimitraki et al. [11], B cells expressing TLR-9 were examined. Approximately one-third of their patients were receiving corticosteroids and approximately one-third of patients were receiving hydroxychloroquine, a potent inhibitor of TLR-9 signalling. Although they showed a correlation between increased percentages of TLR-9-expressing B cells and the presence of anti-dsDNA antibodies, they did not examine the relationship between percentages of TLR-9-expressing B cells and titres of anti-dsDNA antibodies. On the other hand, we used whole PBMCs including B cells, T cells and monocytes from patients before treatment in our study. In recent studies of murine lupus, there were also opposite findings regarding the role of TLR-9 in anti-DNA antibody formation [18–20]. Lartigue et al. [20] suggested that the discrepancy might be due to the different genetic background of mice used in these studies. The exact role of TLR9 in the pathogenesis of anti-DNA responses remains to be determined.
In our patients, there was an inverse correlation between expression levels of IFN-α mRNA in PBMCs and titres of anti-dsDNA antibodies. Christensen and Shlomchik [9] proposed a model for immune activation in SLE. Endogenous DNA- and RNA-containing antigens released by apoptotic cells activate TLRs within autoreactive B cells, allowing differentiation to autoantibody-secreting cells. Autoantibody immune complexes then activate plasmacytoid dendritic cells, inducing production of IFN-α. We consider that a large amount of DNA-containing antigens could be neutralized by anti-DNA antibodies and removed from the circulation in patients having high titres of the antibodies. As a result, it is possible that expression levels of IFN-α mRNA in PBMCs could be lower in such patients than in patients having low titres of anti-DNA antibodies.
In animal models, Berland et al. [21] reported TLR-7-dependent loss of B cell tolerance in pathogenic autoantibody knock-out mice. Christensen et al. [22] demonstrated that TLR-7-deficient lupus-prone mice had ameliorated disease, decreased lymphocyte activation and decreased serum IgG. Furthermore, mice with the Y-linked autoimmune accelerating locus mutation, leading a twofold increase in the expression of several genes including TLR-7, develop a severe form of murine lupus [23]. These findings suggest strongly that TLR-7 has an important role in the pathogenesis of murine lupus models. In the present study, we found up-regulated expression of TLR7 mRNA in PBMCs from SLE patients for the first time. This suggests that TLR-7 expression in PBMCs is also involved in the pathogenesis of human lupus.
It is known that repeated administration of recombinant IFN-α to patients with various malignancies or chronic viral infections could lead to the production of autoantibodies, sometimes to the development of clinical symptoms associated with SLE or other autoimmune diseases [24,25]. Recent studies have identified the IFN gene expression signature in PBMCs from active SLE patients [13,26,27]. These findings suggest that IFN-α can contribute to the pathogenesis of SLE through various mechanisms, including effects on antigen-presenting cells, B cells and T cells [8,9]. For example, Barrat et al. [28] showed that mammalian RNA and DNA, in the form of immune complexes, are potent self-antigens for TLR-7 and TLR-9 respectively, and induce IFN-α production by plasmacytoid dendritic cells. They suggested that TLR-7 and TLR-9 have a critical role in the promotion of lupus through the induction of INF-α by plasmacytoid dendritic cells. Our observations support this possibility. Expression levels of INF-α RNA in PBMCs from SLE patients were significantly higher than those from healthy controls. There was a correlation between expression level of INF-α RNA in PBMCs and those of TLR7 and TLR9 mRNAs in our patients.
Previous studies showed up-regulation of various IFN-inducible genes in PBMCs from SLE patients by microarray analyses, although they failed to show increased expression of IFN-α mRNA [27,28]. We examined mRNA expression levels of LY6E, a type I IFN-inducible gene that is known to be up-regulated in SLE [13], in PBMCs from our subjects, and confirmed its significantly higher expression in SLE patients than in healthy controls. In our patients, expression level of LY6E mRNA did not correlate with those of TLRs and IFN-α mRNAs. Perhaps LY6E mRNA is expressed at a higher level in PBMCs compared with TLRs and IFN-α mRNAs. The discrepancy regarding IFN-α mRNA expression in PBMCs from SLE patients between previous microarray data [27,28] and our real-time RT–PCR data might be due to differences in experimental procedures and/or background of patients.
In conclusion, our real-time RT–PCR analyses confirmed the previous flow cytometry finding of increased expression of TLR-9 in PBMCs from SLE patients [11,12]. We also showed up-regulated expression of TLR7 mRNA together with increased expression of IFN-α mRNA in PBMCs from SLE patients. These results support the findings observed in murine lupus models [8,9], and suggest that TLR-7, TLR-9 and IFN-α may also contribute to the pathogenesis of human lupus.
Acknowledgments
This study was supported in part by the Global COE Program and a Grant-in Aid for Scientific Research (1759081 to A. K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–90. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Cruz DP, Khamashta MA, Hughes GRV. Systemic lupus erythematosus. Lancet. 2007;369:587–96. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 3.Mackay F, Schneider P, Rennert P, Browning J. BAFF and APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 4.Carroll MC. A protective role for innate immunity in systemic lupus erythematosus. Nat Rev Immunol. 2004;4:825–31. doi: 10.1038/nri1456. [DOI] [PubMed] [Google Scholar]
- 5.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–3. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 6.Koutouzov S, Mathian A, Dalloul A. Type-I interferons and systemic lupus erythematosus. Autoimmun Rev. 2006;5:554–62. doi: 10.1016/j.autrev.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–82. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen SR, Shlomchik MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007;19:11–23. doi: 10.1016/j.smim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S, Uematsu S, Takeuchi O. Pathogen reaction and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Papadimitraki ED, Choulaki C, Koutala E, et al. Expansion of toll-like receptor 9-expressing B cells in active systemic lupus erythematosus: implications for the induction and maintenance of the autoimmune process. Arthritis Rheum. 2006;54:3601–11. doi: 10.1002/art.22197. [DOI] [PubMed] [Google Scholar]
- 12.Migita K, Miyashita T, Maeda Y, et al. Toll-like receptor expression in lupus peripheral blood mononuclear cells. J Rheumatol. 2007;34:493–500. [PubMed] [Google Scholar]
- 13.Feng X, Wu H, Grossman JM, et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–62. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [Letter] [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI: a disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 17.Cook RJ, Gladman DD, Pericak D, Urowitz MB. Prediction of short term mortality in systemic lupus erythematosus with time dependent measures of disease activity. J Rheumatol. 2000;27:1892–5. [PubMed] [Google Scholar]
- 18.Christensen SR, Kashgarian M, Alexopoulou L, et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–31. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–42. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 20.Lartigue A, Courville P, Auquit I, et al. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–54. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 21.Berland R, Fernandez L, Kari E, et al. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–40. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian S, Tus K, Li Q-Z, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103:9970–5. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rönnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227:207–10. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 25.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36:511–18. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- 26.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–15. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrat FJ, Meeker T, Gregorio J, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–9. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]


